Abstract
Temporary focal ischemia causes greater hemorrhagic transformation (HT) in diabetic Goto-Kakizaki (GK) rats, a model with increased cerebrovascular matrix metalloprotease (MMP) activity and tortuosity. The objective of the current study was to test the hypotheses that (1) diabetes-induced cerebrovascular remodeling is MMP dependent and (2) prevention of vascular remodeling by glucose control or MMP inhibition reduces HT in diabetic stroke. Control and GK rats were treated with vehicle, metformin, or minocycline for 4 weeks, and indices of remodeling including vascular tortuosity index, lumen diameter, number of collaterals, and middle cerebral artery (MCA) MMP activity were measured. Additional animals were subjected to 3 hours MCA occlusion/21 hours reperfusion, and infarct size and HT were evaluated as indices of neurovascular injury. All remodeling markers including MMP-9 activity were increased in diabetes. Infarct size was smaller in minocycline-treated animals. Both metformin and minocycline reduced vascular remodeling and severity of HT in diabetes. These results provide evidence that diabetes-mediated stimulation of MMP-9 activity promotes cerebrovascular remodeling, which contributes to greater HT in diabetes. Metformin and minocycline offer vascular protection, which has important clinical implications for diabetes patients who are at a fourfold to sixfold higher risk for stroke.
Keywords: diabetes, hemorrhagic transformation, MMP, stroke, vascular remodeling
Introduction
Diabetes is an increasingly growing epidemic affecting 21 million Americans over 65% of whom will eventually die of a macrovascular event such as stroke (Lloyd-Jones et al, 2009). As diabetic patients are at a higher risk of stroke and have poorer prognosis compared with the nondiabetic population, a better understanding of diabetes-induced vascular pathology and the underlying mechanisms is pivotal for developing better vascular protection strategies before and after an ischemic insult (Poppe et al, 2009).
Traditional vascular complications of diabetes are categorized as (1) microvascular (nephropathy, neuropathy, and retinopathy) and (2) macrovascular (stroke, coronary artery disease, and peripheral arterial disease) (Brownlee, 2005). In both cases, the vascular wall structure and function are affected by remodeling changes. These may include vascular smooth muscle cells proliferation, degeneration of endothelial cells, basement membrane thickening, and a state of coagulopathy (Gabbay, 1975). In established disease, there is vascular wall growth as a result of increased collagen deposition or vascular smooth muscle cell hypertrophy/hyperplasia (Endemann et al, 2004). The matrix metalloprotease (MMP) system is involved in restructuring of the vessels and the surrounding matrix by degrading as well as stimulating matrix deposition (Olszynski and Zimowska, 2009). The MMP-2 and MMP-9 expression and activity are elevated under hyperglycemic conditions (Signorelli et al, 2005). We previously showed increased tortuosity and MMP activity of the cerebral vessels as evidence of early vascular structure changes in diabetes (Ergul et al, 2007). We recently reported that there is increased cerebral angiogenesis and arteriogenesis associated with augmented microvessel and macrovessel MMP activity in diabetic animals (Li et al, 2009). We also showed that when diabetic animals are exposed to ischemic brain injury, they suffer greater hemorrhage and edema, suggesting that preexisting neovascularization exacerbates stroke injury as also seen in diabetic retinopathy (Ergul et al, 2007; Li et al, 2009). Given that MMP-2 and MMP-9 rise acutely after stroke leading to edema and hemorrhagic transformation (HT) that develop secondarily to prolonged ischemia (Alvarez-Sabin et al, 2004), the relative contribution of diabetes-induced MMP activation and vascular remodeling to ischemic brain injury remained to be determined.
Cerebral vasculature has autoregulatory properties to adjust myogenic tone, a critical functional aspect of the cerebral circulation for adequate blood flow under normal conditions and more so in ischemia/reperfusion injury. Cipolla et al (1997) reported that intrinsic myogenic tone of posterior cerebral arteries is diminished in response to increased glucose concentration in vitro. However, Zimmermann et al (1997) showed a constriction of middle cerebral arteries (MCAs) in diabetic rats. A third study reported that posterior cerebral artery tone is enhanced in diabetes (Jarajapu et al, 2008). Collectively, these studies point out to the importance of understanding more about cerebral vessel structure and function under physiologic circumstances and pathologic alterations that may cause deleterious outcomes. As discussed above, we reported increased cerebrovascular remodeling and neovascularization in diabetes. Whether and to what extent these changes influence vascular tone, integrity, and ultimately the magnitude of ischemia/reperfusion injury are yet to be determined. Taken together, our working hypothesis for the current study was that MMP-mediated cerebrovascular remodeling in diabetes augments vascular damage after ischemia/reperfusion injury. We also hypothesized that tight glycemic control or MMP inhibition serve as vascular protection strategies.
Materials and methods
Animals
The institutional animal care and use committee of the Medical College of Georgia approved all protocols used in animal work. Male Wistar and Goto-Kakizaki (GK) rats were purchased from Harlan (Indianapolis, ID, USA) and Taconic (Hudson, NY, USA) Laboratories, respectively. For all studies, weight-matched rats (270 to 310 g, 9 to 11 weeks) were used.
Metformin was titrated to maintain euglycemia in GK rats (∼300 mg/kg per day based on blood glucose levels) and was given in drinking water artificially sweetened by noncaloric sweetener. Minocycline was also given in drinking water (5 mg/kg per day). Both treatments were chronic, starting with the onset of diabetes in GK rats (5 to 6 weeks of age) till the animals reached the weight range used for MCA occlusion (MCAO), which averaged about 5 weeks. Minocycline treatment was stopped 3 days before MCAO to allow for a wash-out period. Blood glucose levels were measured from the tail vein blood using a glucometer (Freestyle, Alameda, CA, USA).
Measurement of Remodeling Indices
Tortuosity index, collateral number, and diameter were measured as indices of remodeling in a masked manner. After being anesthetized with sodium pentobarbital (100 mg/kg), the animals were injected with 3 mL freshly prepared polyurethane elastomer PU4ii (vasQtec, Zuerich, Switzerland) through the aorta within a minute. PU4ii mixture was prepared by mixing the blue-stained ethylmethylketone (30% of the final mixture) and 0.8 g of PU4ii hardener shortly before casting as previously described (Krucker et al, 2006). The rats were decapitated immediately, and the brain was removed and immersed in 4% paraformaldehyde for 48 hours.
Stereomicroscopic images of the perfused brains were captured and actual vessel length of cortical branches of MCA starting from its origin was traced on a Wacom tablet 493-3 using Image J I-36 software. Each hemisphere was divided into six grids of equal area and the total number of collaterals between the anterior, posterior, and middle cerebral arteries were counted manually (Chalothorn et al, 2007). A collateral was defined as an anastomosis between MCA and anterior or posterior cerebral arteries. Arteriole-to-arteriole anastomoses between the MCA branches were also counted and defined as an intra-tree anastomosis. The diameter was measured at the midpoint of the collaterals. At least eight measurements of the diameters were taken on each hemisphere, and the average of all these measurements was reported as the average diameter of the collaterals. Tortuosity index was defined as the ratio of the vessel length over straight-line distance between two vessel ends. In each grid, two middle size vessels were traced, and the average of 12 measurements was used as the Tortuosity index per animal.
Evaluation of Middle Cerebral Artery Vascular Structure and Myogenic Tone
One of the MCAs collected immediately after decapitation was mounted on the pressurized arteriography (Living Systems Instrumentation, Burlington, VT, USA), to measure media thickness, lumen and outer diameters with a video dimension analyzer at different pressures ranging from 5 to 180 mm Hg at 20 mm Hg pressure increments. The system was equilibrated in Krebs-HEPES (4-(2-hydroxyethyl)-1-piperazinethananesulfonic acid) buffer free of calcium to obtain measurements under passive conditions and media thickness, lumen and outer diameters and vessel cross-sectional areas were determined as follows: wall thickness (WT, μm)=outer diameter − lumen diameter (i.e., OD−LD); (M/L) ratio = WT/LD; cross-sectional area (μm2)=outer vessel area − lumen area. Myogenic tone and stiffness of MCAs (β-coefficient) were calculated as follows: myogenic tone (% tone)=(1−(ODactive/ODpassive)) × 100; stiffness (β-coefficient) was obtained from the slope of the stress versus strain curve using the equation: y=aeβx (y=stress, x=strain, a=intercept, and β=slope) (Rigsby et al, 2007).
Isolation of Cerebral Vessels
The animals were subjected to ischemic brain injury as described below. At 24 hours, animals were killed and macrovessels were isolated immediately from ischemic and nonischemic side separately, snap frozen in liquid nitrogen and kept at −80°C for later protein work. Macrovessels are defined as basilar artery, MCA, circle of Willis, and ACA. Vessels were homogenized using RIPA (RadioImmunoPrecipitation Assay) buffer to extract MMPs and a standard Bradford protein assay was performed before running immunoblots or zymograms to determine the amount of loaded protein. In an additional group of animals treated with vehicle, metformin, or minocycline, macrovessels were isolated at the end of the treatment period without any ischemic injury.
Matrix Metalloprotease-9 Expression and Activity
The MMP-2 and MMP-9 expression was determined by immunoblotting. A measure of 50 μg proteins were directly loaded on SDS-PAGE gel and separated under reducing conditions. After electrophoresis, proteins were transferred to a nitrocellulose membrane. In all, 5% milk-TTBS (Tween Tris Buffered Saline) was used for blocking and band detection was performed using primary antibody against MMP-2 or MMP-9 (Calbiochem, San Diego, CA, USA) and a peroxidase-conjugated goat antimouse secondary antibody. The chemiluminescent signal was detected using (Alpha Imager, Santa Clara, CA, USA) and bands intensity quantified by image analysis using GelPro analyzer (Media Cybernetics, Bethesda, MD, USA).
The MMP-2 or MMP-9 activity was assessed by gelatin zymography. A measure of 30 μg protein was loaded on an SDS-PAGE gel containing 0.1% gelatin and separated under nonreducing conditions. After electrophoresis, the gel was washed in 2.5% ‘Triton X-100' for 20 minutes twice and incubated for 20 hours in a substrate buffer- 50 mmol Tris-HCl, 5 mmol CaCl2+0.02% NaN3− pH=7.5 at 37°C. A recombinant MMP-2 and MMP-9 standard was run as positive control. After incubation, the gel was stained overnight using ‘Coomassie blue' then destained. The zymogram was digitized and bands intensity was quantified by image analysis using GelPro.
Middle Cerebral Artery Occlusion and Cerebral Perfusion Measurement
Three hours MCAO/21 hours reperfusion model was used. Animals were anesthetized using isoflurane in an induction chamber then maintained for about 15 minutes on 3% isoflurane for the procedure. A midline cervical incision was made to expose the common carotid artery. The external carotid artery separated, ligated, and severed. A rounded-tip, by heating, 4-0 nylon monofilament suture was inserted into the internal carotid artery to occlude the origin of MCA (Longa et al, 1989). The occlusion suture was secured with one-silk suture at the stump of external carotid artery, and the incision was closed. After 3 hours, the animals were reanesthetized, and the occlusion suture was removed to allow reperfusion. Laser Doppler (PIM-3, Perimed, Stockholm, Sweden) was used to confirm a consistent drop in perfusion among groups. Core body temperature was maintained using a heating pad and monitored through a rectal probe during the surgery and on a heating pad under the cage till the end of 24 hours. Animals were singly housed before and after MCAO with free access to food and water.
Evaluation of Infarct Size, Edema, and Hemorrhagic Transformation
At 24 hours after occlusion, cerebral blood perfusion was evaluated with PIM-3 again and the animal was immediately killed. Brains were removed and sliced in the coronal plane with 2 mm intervals, labeled A–F, front to back and were used to calculate infarct size, edema, and HT. Visual inspection of hemorrhage if present was reported as a qualitative score for the frequency of macroscopic bleeding. 2,3,5-triphenyltetrazolium chloride (Sigma Chemical Co., St Louis, MO, USA) was used to outline the infarct area. Images analysis was performed in a masked manner using the Image-J (NIH, Bethesda, MD, USA) software. After staining, hemispheres were separated; snap frozen at −80°C for later hemoglobin direct enzyme linked immunosorbent assay (ELISA) quantification (Hilali et al, 2004). Edema was expressed as a percentage of infarct hemisphere size to control hemisphere.
Neurobehavioral Assessment
Short-term neurobehavioral functional outcomes of ischemic injury was assessed by a battery of tests including Bederson, forepaw grasp, beam walk, hind limb retraction, and elevated body swing tests at 24 hours before the kill. Bederson test was scored for the presence of forelimb flexion, decreased resistance to push, and ipsilateral circling with each item given 1 point. A score of 3 is consistent with an MCAO. Bederson score was combined with beam walking ability, and bilateral forepaw grasp tests to determine a composite score (Li et al, 2009). Scores were given to each item from 0 to 3 for a total of 9 for maximal deficit. Bederson's score was also recorded at the end of 3 hours occlusion period to verify proper occlusion (Kozak et al, 2008).
Statistics
The distributions for the measures of stroke severity (infarct size, percent edema, and bleeding) as well as the measures of behavior and vascular remodeling were found to be skewed. The difference between stroke versus nonstroke side of the brain for MMP-2 and MMP-9 levels was calculated, and the difference was adjusted for the nonstroke value in the analysis. A rank transformation was used before the analysis of all measures. The analysis for the effect of minocycline on Wistar and GK rats was performed using a 2-disease (Wistar versus GK) × 2-treatment (vehicle versus minocycline) analysis of variance. An interaction between disease and treatment would indicate a differential effect of minocycline treatment that is dependent on disease status. The analysis for the effects of minocycline and metformin on GK rats was performed using a 3-treatment (vehicle, metformin, and minocycline) one-way analysis of variance. A Tukey's test was used to adjust for multiple comparisons when determining mean differences for significant analysis of variance effects.
Results
Diabetes Promotes Vascular Remodeling
Blood glucose levels (mg/dL) were 110.0±11 and 106.8±7 in control and control+minocycline groups, respectively (Table 1). In diabetic animals, metformin treatment provided glycemic control and blood glucose levels were 212.0±19, 102.2±8, and 177.4±21 in vehicle, metformin, and minocycline groups, respectively. Cerebrovascular structure was evaluated by two different methods: (1) visualization and evaluation of cerebral pial vessels by PU4ii injection and (2) pressurized arteriographic assessment of isolated MCAs. Tortuosity index, the number of collateral and anastomoses were significantly higher in diabetic rats than in control group. Both metformin and minocycline treatments in diabetes reduced them significantly to control values (Figures 1A to 1D). The collateral inner diameter (mm) was greater in diabetic GK rats and minocycline reduced it significantly in both control and diabetic animals (Figure 1E). Glycemic control with metformin also reduced inner diameter in GK rats.
Table 1. Baseline metabolic parameters.
| Control | Diabetes | Diabetes+ metformin | Control+ minocycline | Diabetes+ minocycline | |
|---|---|---|---|---|---|
| BW (g) | 293.6±9 | 279.2±10 | 256.0±4 | 316.3±4 | 270.9±3 |
| BG (mg/dL) | 110.0±11* | 212.0±19 | 106.8±7* | 102.2±8* | 177.4±21 |
BG, blood glucose; BW, body weight.
*P<0.001 versus diabetes.
Figure 1.
Diabetes promotes remodeling of cerebral vessels. (A) Representative images of the pial vessels after PU4ii injection to visualize middle, anterior, and posterior cerebral arteries trees in control (C), diabetes (D), diabetes+metformin (D+Me), control+minocycline (C+M), or diabetes+minocycline (D+M) groups. Tortuosity index (B), number of collaterals (C), number of anastomoses (D), and collateral diameter (E) were significantly higher in diabetes all of which were prevented by metformin or minocycline treatments. Mean±s.e.m., n=7 to 14, *P<0.0001 versus C, **P<0.001 versus D, ***P<0.0002 versus C.
Wall remodeling indices obtained from pressure arteriography included MCA inner and outer diameters (μm), cross-sectional area (μm2), and M/L ratio. In addition, myogenic tone and stiffness were assessed as a measure of vascular function and mechanics at an intraluminal pressure of 80 mm Hg, which represents the estimated pressure experienced by MCA in vivo. There was a disease and treatment effect on inner (P=0.0023) and outer diameters (P=0.01) such that minocycline increased these parameters in controls but reduced them in diabetes. There was no difference in cross-sectional area or M/L ratio between groups (Figure 2). Myogenic tone was significantly higher in diabetic rats than in controls at 80 mm Hg pressure and both metformin and minocycline significantly reduced it to less than control levels (Figure 3A). There was a disease by treatment effect on vascular stiffness. Minocycline reduced stiffness in controls but increased it in diabetes (Figure 3B). Metformin had no effect on any of these parameters.
Figure 2.
Diabetes does not increase wall thickness or M/L ratio in diabetes. Middle cerebral artery (MCA) wall remodeling parameters including (A) inner diameter, (B) cross-sectional area (CSA), (C) outer diameter, and (D) M/L ratio were measured by pressure arteriography at 80 mm Hg. Mean±s.e.m., n=6 to 9, *P=0.0023 and **P=0.01, disease by treatment interaction. M/L, media/lumen.
Figure 3.
Diabetes increases myogenic tone of isolated middle cerebral arteries (MCAs). (A) The MCA myogenic tone was increased in diabetic animals and both metformin and minocycline reduced the tone. (B) There was a disease and treatment interaction such that minocycline decreased stiffness in controls but increased it in diabetic rats. Mean±s.e.m., n=7 to 14, *P<0.05 versus C, **P=0.011 versus D, ***P=0.0046 versus C; ****P<0.0001. C, control; D, diabetes.
Diabetes Augments Stroke-Induced Increase in Matrix Metalloprotease Expression and Activity
The MMP-9 is involved in the regulation of vascular remodeling and blood–brain barrier breakdown after ischemic injury. Thus, MMP-9 activity was measured in macrovessel homogenates prepared from ischemic and nonischemic hemispheres after MCAO. When nonischemic hemispheres were compared as an indicator of baseline macrovascular MMP-9 activity, diabetic rats displayed greater enzyme activity than controls and glycemic control with metformin prevented this increase (Figure 4A). Ischemic injury increased MMP-9 activity in both control and diabetic animals and metformin treatment prevented ischemia-induced MMP-9 activity in diabetic animals. Minocycline treatment abolished gelatinolytic activity in both control and diabetic animals. There was no difference in MMP-2 activity among the study groups or between ischemic and nonischemic macrovessels (Figure 4B). To ensure that MCAO procedure itself is not affecting MMP levels on the nonischemic side, macrovascular MMP expression/activity was determined at baseline without any exposure to ischemia in an additional group of animals treated with vehicle, metformin, or minocycline. Both MMP-2 and MMP-9 activity were greater in the diabetic animals than in controls (Figure 4C). Metformin treatment reduced MMP-2 but not MMP-9 activity in diabetic animals. Minocycline treatment lowered MMP-9 but not MMP-2 activity in both control and diabetic rats. The MMP-2 protein levels followed the same pattern seen in activity results (Figure 4D). The MMP-9 protein levels were higher in diabetic vessels but treatment with either metformin or minocycline did not have an effect.
Figure 4.
Diabetes augments ischemia-induced stimulation of MMP-9 activity in isolated cerebral vessels. (A) The MMP-9 activity in macrovessels isolated from ischemic (I) and nonischemic (NI) hemispheres of animals subjected to middle cerebral artery occlusion (MCAO) was measured using gelatin zymography. The MMP-9 activity was greater in both the NI and I hemispheres in diabetes indicating increased baseline and ischemia-induced augmentation of MMP-9 activity. Both metformin and minocycline caused a dramatic reduction in enzyme activity. (B) The MMP-2 activity of the same groups did not show any difference. (C) When lytic activity was assessed in the same cerebral macrovessels isolated from animals not subjected to MCAO, both MMP-2 and MMP-9 baseline activities were greater in diabetes. (D) Protein levels of MMP-2 and MMP-9 were increased in diabetes. Metformin and minocycline treatments significantly reduced MMP-2 level but not MMP-9. Mean±s.e.m., n=5 to 8, *P<0.01 versus NI, **P=0.05 versus C, ***P=0.012 versus D, ****P<0.0001 versus vehicle C or D, ςP=0.031 versus D, ΨP=0.003 versus D. C, control; D, diabetes; MMP, matrix metalloprotease.
Neurovascular Injury Is Exacerbated in Diabetes
The drop in perfusion after MCAO was consistent among groups (45% to 55%, data not shown). The control group did not differ from diabetic animals with regard to mean arterial blood pressure, pH, pCO2, and pO2. Infarct size was significantly smaller in diabetic rats as reported by our group before (Figures 5A and 5B). Treatment with metformin did not have an effect on infarct size but minocycline reduced infarct in both control and diabetic animals.
Figure 5.
Infarct size is smaller in diabetes. (A) Representative images of 2,3,5-triphenyltetrazolium chloride-stained coronal sections of the brain after middle cerebral artery occlusion (MCAO). (B) Quantitative analysis of infarct size indicated smaller infarcts in diabetes. Although metformin had no effect on infarct size minocycline reduced infarct in both groups. Mean±s.e.m., n=6 to 9, *P<0.0001 versus C, **P=0.05 versus vehicle C or D. C, control; D, diabetes.
Edema and HT were evaluated as indices of ischemia-induced vascular damage. The HT was assessed qualitatively by measuring the incidence of intracerebral bleeding and quantitatively by a previously validated hemoglobin ELISA. Diabetes caused greater vascular damage in diabetic rats than controls as shown by higher incidence of bleeding (Figure 6A) and greater HT severity (Figure 6B). There was a disease and treatment interaction such that minocycline significantly reduced HT incidence and severity in diabetes but not in control rats. Metformin treatment also reduced HT severity. Edema was bigger in diabetes (Figure 6C). Metformin reduced it and there was a disease and treatment interaction where minocycline significantly reduced it in diabetes.
Figure 6.
Vascular injury is greater in diabetes. (A) Occurrence of macroscopic intracerebral bleeding was significantly higher in diabetes compared with control and was reduced by chronic minocycline treatment. (B) ELISA measurements of hemoglobin show significant increase in hemorrhagic transformation (HT) in diabetes and a vasoprotective effect of minocycline and metformin. There was a disease and treatment interaction showing minocycline preventing HT in diabetes but no effect on control animals. (C) Edema was significantly higher in diabetes compared with control and both metformin and minocycline reduced it. Minocycline had no effect on edema in control animals, indicating a disease/treatment interaction. Mean±s.e.m., n=6 to 11, *P<0.05 versus C, **P=0.001 versus D. ***P=0.0009 and ****P=0.0024 are disease/treatment interaction for minocycline. C, control; D, diabetes; ELISA, enzyme linked immunosorbent assay.
Neurologic Outcome Is Worsened in Diabetes
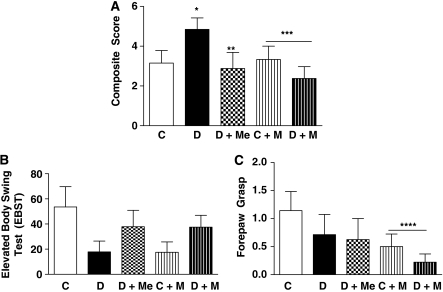
Multiple neurobehavioral tests were used to assess the short-term functional outcome of ischemia/reperfusion injury at 24 hours. The composite neurodeficit score was significantly higher in diabetic animals indicating worse functional outcome (Figure 7A). Treatment with metformin improved functional outcome. Minocycline treatment showed a disease–drug interaction with no effect on control animals but reduced deficit in diabetic rats. There was no significant difference in elevated body swing test between groups (Figure 7B). Minocycline improved forepaw grasp in both control and diabetic animals (Figure 7C).
Figure 7.
Short-term (24 hours) neurologic outcomes after middle cerebral artery occlusion (MCAO) in all treatment groups. (A) A composite score for multiple neurobehavioral tests shows worse functional recovery in diabetes compared with control. Both metformin and minocycline treatment improved the score significantly. Minocycline showed a differential effect indicated by no change in control animals but improvement of score in diabetic animals. Although there was no difference in individual scores of elevated body swing test (B) and forepaw grasp (C), forepaw grasp was improved in minocycline-treated animals. Mean±s.e.m., n=6 to 13, *P<0.05 versus C, **P=0.05 versus D, ***P=0.035 disease/treatment interaction for minocycline, ****P=0.0031 versus vehicle C or D. C, control; D, diabetes.
Discussion
The current study was designed to address the following important questions: (1) Is diabetic remodeling of the cerebrovasculature MMP dependent? (2) Does MMP-mediated cerebrovascular remodeling contribute to the augmented stroke injury seen in diabetes? and (3) Does glycemic control prevent MMP activation/remodeling and reduce neurovascular damage after ischemic brain injury? Our findings provide evidence that even after a short duration of relatively mild hyperglycemia, there are structural changes in the cerebral vessels as indicated by increased tortuosity, number of collaterals, and collateral diameter all of which can be prevented by MMP inhibition. When these structural alterations are inhibited by minocycline treatment, vascular damage ensuing ischemic brain injury is significantly reduced. Glycemic control prevents remodeling, reduces bleeding, and improves short-term functional outcome in diabetes.
Clinical data have shown remarkably worse outcomes, slower short- and long-term functional recovery, and higher mortality in diabetic patients after stroke compared with the nondiabetic population (Idris et al, 2006). Experimental studies in streptozotocin-induced model of diabetes with very high blood glucose levels also showed greater infarct development (de Courten-Myers et al, 1992). Our understanding of the mechanisms involved in augmented ischemic injury in diabetes is limited. Although much emphasis is focused on neuronal damage after stroke, it is becoming clear that vasculature has an important role not only in the pathophysiology but also the recovery of ischemic brain injury (Li et al, 2009). We recently extended studies on diabetic stroke to a lean model of diabetes that presents with glucose levels (∼200 mg/dL) that are comparable to levels seen in most acute ischemic stroke patients enrolled in various clinical trials (Bruno et al, 2008). Our studies have shown that cerebrovasculature undergoes extensive remodeling leading to increased tortuosity and neovascularization that is associated with increased MMP activity in early diabetes (Li et al, 2009). We also reported that when these animals are subjected to temporary focal ischemia, the occurrence rate of overt HT increases significantly but infarcts are smaller (Elewa et al, 2009; Ergul et al, 2007). Numerous reports also documented the importance of MMPs especially during the acute phase of ischemia in damaging the neurovascular unit (Lee et al, 2007; Lo, 2008). The resulting loss of its crucial barrier function leads to edema and extravasations of red blood cells into brain parenchyma particularly with prolonged periods of ischemia. If the MMP system is dysregulated as it occurs in diabetes, vascular wall integrity may be weakened setting the stage for an aggravated damage in case of stroke. The results of the current study show that chronic inhibition of MMPs by minocycline starting at the onset of diabetes prevents cerebrovascular remodeling and reduces HT incidence and severity. As acute activation of MMPs during ischemia is important for brain injury, in the current study we stopped minocycline treatment 3 days before surgery to allow for a wash-out period to separate the effect of acute MMP inhibition on ischemic injury from that on vascular remodeling and neovascularization. Although there was no change in MMP-9 protein levels in diabetes with chronic minocycline treatment, enzymatic activity on the ischemic and nonischemic hemispheres were abolished compared with untreated diabetic rats. A possible explanation is that 3-day withdrawal is not sufficient to eliminate the inhibitory effect of minocycline on MMPs. Further studies are needed to clarify this issue. Another interesting finding was that MMP-2 and MMP-9 activity patterns measured in the nonischemic side of stroked animals were different than those detected in nonstroked animals. We first compared MMP activity in the nonischemic hemispheres of control and diabetic animals to determine baseline differences, which showed higher MMP-9 but not MMP-2 activity in diabetes. However, when the same experiments were repeated in control and diabetic rats not subjected to MCAO, both MMP-2 and MMP-9 activities were increased in diabetic rats. These results suggest that MCAO procedure itself may affect the expression/activity patterns. These findings also highlight the possibility that the use of nonischemic side as a control may be limiting in stroke studies and sham animals will be a better control.
Minocycline, although a nonspecific MMPs inhibitor, was previously shown to inhibit cerebral MMP activity efficiently in experimental stroke (Lee et al, 2007; Machado et al, 2006). We also used it as it is a generic drug with a well-known safety profile and because there is an ongoing clinical trial Minocycline to Improve Neurologic Outcome to evaluate its use as a neurovascular protective agent. In addition to its MMP inhibitory effects, minocycline has antiinflammatory, antiapoptotic, and neuroprotective properties (Li and McCullough, 2009; Yrjanheikki et al, 1999). In the current study, minocycline-treated animals showed a small but significant decrease in infarct sizes as compared with vehicle-treated control and diabetic animals. Thus, in the ischemia model used in this study, minocycline was both neuroprotective and vasoprotective. It is possible that either reduction of bleeding because of MMP inhibition decreases infarct size or direct neuroprotective effects contribute to this finding.
An important feature of the cerebral circulation is the ability to regulate blood flow within a wide pressure range to maintain the nutrient and oxygen supply to the brain. The mechanism behind this autoregulatory capacity is the myogenic reactivity of vascular smooth muscle cell (Schubert and Mulvany, 1999). We wanted to study the effects of diabetes on this important functional feature of cerebral vessels. This is perceivable taking into account that structural wall alterations may affect the vascular wall function as well. This is especially true as previous studies showed different myogenic reactivity in different diabetes models (Cipolla et al, 1997; Jarajapu et al, 2008; Zimmermann et al, 1997). In the current study, the myogenic reactivity across the pressure range (40 to 120 mm Hg) was preserved in diabetic animals. Although there was an increase in myogenic tone at 80 mm Hg pressure, given that the lumen diameter is not different between control and diabetic rats at this pressure, it is unlikely to affect cerebral blood flow under normoxic conditions. However, it has to be recognized that we only measured myogenic reactivity and not neuronal or endocrine mechanisms that are involved in the regulation of vessel diameter. In addition, whether vascular reactivity is altered under hypoxic conditions remains to be determined. Unexpectedly, both metformin and minocycline treatment reduced the tone significantly compared with vehicle-treated animals, which deserves further investigation.
The chronic hyperglycemia present in diabetes ultimately leads to both microvascular and macrovascular remodeling changes. One of the goals behind glycemic control in diabetes is to prevent both these changes and hence prevent and reduce diabetes-dependant vascular events. It is well established that early and good glycemic control reduces the microvascular complications in both types of diabetes. However, the relation between glycemic control and macrovascular events prevention has been only proven in type 1 diabetes (Akalin et al, 2009). The most recent major prospective randomized controlled clinical trial Action to Control Cardiovascular Risk in Diabetes was prematurely stopped because of the increased macrovascular events (Akalin et al, 2009; Dhar, 2009; Karalliedde and Gnudi, 2008; Kravetz and Federman, 2009; Lebovitz, 2008; Schatz, 2009; Skyler et al, 2009). However, ADVANCE (Action in Diabetes and Vascular Disease: Pretrax and Diamicron MR Controlled Evaluation) and VADT (Veterans Affairs Diabetes Trial) did not replicate the same findings (Buse et al, 2007; Chalmers et al, 2006; Duckworth et al, 2009). The latest consensus statement by the American Diabetes Association highlights that the long-term follow-up of DCCT and UKPDS showed that early glycemic control is associated with long-term reduction in macrovascular events and supports the overall benefits of glycemic control in reducing vascular events in addition to the importance of controlling other comorbidities (Skyler et al, 2009). Previous work from our group showed that glycemic control prevents microvascular remodeling in mesenteric bed (Sachidanandam et al, 2009). Yet, impact of glycemic control on cerebrovascular complications of diabetes remained unclear and the current study addressed this gap. It has to be recognized that metformin possesses some neuroprotective properties independent of its blood glucose lowering effect through its antioxidant and AMP-activated protein kinase stimulatory properties, which may have contributed to our findings (Correia et al, 2008; Poels et al, 2009).
In summary, our results provide evidence that diabetes-induced MMP-9 activity upregulation promotes cerebrovascular remodeling and affects vascular myogenic reactivity as well. This remodeling is associated with higher vascular damage after ischemia/reperfusion injury, which may explain at least in part why diabetic patients have a worsened injury compared with the nondiabetic population. Glycemic control is important to reduce vascular damage associated with ischemic brain injury. The clinical application of minocycline to reduce incidence and severity of HT as well as edema and infarct makes it a neurovasculoprotective agent. This is a safe and feasible strategy that can be used to benefit patients with diabetes who are at higher odds of ischemia/reperfusion injury.
Acknowledgments
The authors thank Dr Kamakshi Sachidanandam for technical assistance with arteriography and Dr James Faber, University of North Carolina, for his guidance with the PU4ii studies.
References
- Akalin S, Berntorp K, Ceriello A, Das AK, Kilpatrick ES, Koblik T, Munichoodappa CS, Pan CY, Rosenthall W, Shestakova M, Wolnik B, Woo V, Yang WY, Yilmaz MT. Intensive glucose therapy and clinical implications of recent data: a consensus statement from the Global Task Force on Glycaemic Control. Int J Clin Pract. 2009;63:1421–1425. doi: 10.1111/j.1742-1241.2009.02165.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J, Ribo M, Santamarina E, Quintana M, Monasterio J, Montaner J. Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke. 2004;35:1316–1322. doi: 10.1161/01.STR.0000126827.69286.90. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams LS. Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke. 2008;39:384–389. doi: 10.1161/STROKEAHA.107.493544. [DOI] [PubMed] [Google Scholar]
- Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, Genuth S, Gerstein HC, Ginsberg HN, Goff DC, Jr, Grimm RH, Jr, Margolis KL, Probstfield JL, Simons-Morton DG, Sullivan MD. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. 2007;99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Chalmers J, Perkovic V, Joshi R, Patel A. ADVANCE: breaking new ground in type 2 diabetes. J Hypertens Suppl. 2006;24:S22–S28. doi: 10.1097/01.hjh.0000240043.50838.28. [DOI] [PubMed] [Google Scholar]
- Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30:179–191. doi: 10.1152/physiolgenomics.00047.2007. [DOI] [PubMed] [Google Scholar]
- Cipolla MJ, Porter JM, Osol G.1997High glucose concentrations dilate cerebral arteries and diminish myogenic tone through an endothelial mechanism Stroke 28405–410.discussion 10–11 [DOI] [PubMed] [Google Scholar]
- Correia S, Carvalho C, Santos MS, Proenca T, Nunes E, Duarte AI, Monteiro P, Seica R, Oliveira CR, Moreira PI. Metformin protects the brain against the oxidative imbalance promoted by type 2 diabetes. Med Chem. 2008;4:358–364. doi: 10.2174/157340608784872299. [DOI] [PubMed] [Google Scholar]
- de Courten-Myers GM, Kleinholz M, Holm P, DeVoe G, Schmitt G, Wagner KR, Myers RE. Hemorrhagic infarct conversion in experimental stroke. Ann Emerg Med. 1992;21:120–126. doi: 10.1016/s0196-0644(05)80144-1. [DOI] [PubMed] [Google Scholar]
- Dhar GC. Intensive glycemic control: implications of the accord, advance, and VADT trials for family physicians. Can Fam Physician. 2009;55:803–804. [PMC free article] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- Elewa HF, Kozak A, El-Remessy AB, Frye RF, Johnson MH, Ergul A, Fagan SC. Early atorvastatin reduces hemorrhage after acute cerebral ischemia in diabetic rats. J Pharmacol Exp Ther. 2009;330:532–540. doi: 10.1124/jpet.108.146951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann DH, Pu Q, De Ciuceis C, Savoia C, Virdis A, Neves MF, Touyz RM, Schiffrin EL. Persistent remodeling of resistance arteries in type 2 diabetic patients on antihypertensive treatment. Hypertension. 2004;43:399–404. doi: 10.1161/01.HYP.0000112029.03691.e7. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay KH. Hyperglycemia, polyol metabolism, and complications of diabetes mellitus. Annu Rev Med. 1975;26:521–536. doi: 10.1146/annurev.me.26.020175.002513. [DOI] [PubMed] [Google Scholar]
- Hilali HM, Simpkins AN, Hill WD, Waller JL, Knight RA, Fagan SC. Single slice method for quantification of hemorrhagic transformation using direct ELISA. Neurol Res. 2004;26:93–98. doi: 10.1179/016164104773026606. [DOI] [PubMed] [Google Scholar]
- Idris I, Thomson GA, Sharma JC. Diabetes mellitus and stroke. Int J Clin Pract. 2006;60:48–56. doi: 10.1111/j.1368-5031.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- Jarajapu YP, Guberski DL, Grant MB, Knot HJ. Myogenic tone and reactivity of cerebral arteries in type II diabetic BBZDR/Wor rat. Eur J Pharmacol. 2008;579:298–307. doi: 10.1016/j.ejphar.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalliedde J, Gnudi L. ACCORD and ADVANCE: a tale of two studies on the merits of glycaemic control in type 2 diabetic patients. Nephrol Dial Transplant. 2008;23:1796–1798. doi: 10.1093/ndt/gfn200. [DOI] [PubMed] [Google Scholar]
- Kozak W, Kozak A, Johnson MH, Elewa HF, Fagan SC. Vascular protection with candesartan after experimental acute stroke in hypertensive rats: a dose-response study. J Pharmacol Exp Ther. 2008;326:773–782. doi: 10.1124/jpet.108.139618. [DOI] [PubMed] [Google Scholar]
- Kravetz JD, Federman DG. Implications of new diabetes treatment trials: should current clinical practice be altered. Postgrad Med. 2009;121:67–72. doi: 10.3810/pgm.2009.05.2004. [DOI] [PubMed] [Google Scholar]
- Krucker T, Lang A, Meyer EP. New polyurethane-based material for vascular corrosion casting with improved physical and imaging characteristics. Microsc Res Tech. 2006;69:138–147. doi: 10.1002/jemt.20263. [DOI] [PubMed] [Google Scholar]
- Lebovitz HE. Glycaemic control and vascular complications in type 2 diabetes: new observations and clinical significance. J Indian Med Assoc. 2008;106:724–726. [PubMed] [Google Scholar]
- Lee CZ, Xue Z, Zhu Y, Yang GY, Young WL. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke. 2007;38:2563–2568. doi: 10.1161/STROKEAHA.106.481515. [DOI] [PubMed] [Google Scholar]
- Li J, McCullough LD. Sex differences in minocycline-induced neuroprotection after experimental stroke. J Cereb Blood Flow Metab. 2009;29:670–674. doi: 10.1038/jcbfm.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2009;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O′Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Machado LS, Kozak A, Ergul A, Hess DC, Borlongan CV, Fagan SC. Delayed minocycline inhibits ischemia-activated matrix metalloproteinases 2 and 9 after experimental stroke. BMC Neurosci. 2006;7:56. doi: 10.1186/1471-2202-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszynski K, Zimowska M. Structure and function of matrix metalloproteinases. Postepy Biochem. 2009;55:76–84. [PubMed] [Google Scholar]
- Poels J, Spasic MR, Callaerts P, Norga KK. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009;31:944–952. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigsby CS, Burch AE, Ogbi S, Pollock DM, Dorrance AM. Intact female stroke-prone hypertensive rats lack responsiveness to mineralocorticoid receptor antagonists. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1754–R1763. doi: 10.1152/ajpregu.00145.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandam K, Hutchinson JR, Elgebaly MM, Mezzetti EM, Dorrance AM, Motamed K, Ergul A. Glycemic control prevents microvascular remodeling and increased tone in type 2 diabetes: link to endothelin-1. Am J Physiol Regul Integr Comp Physiol. 2009;296:R952–R959. doi: 10.1152/ajpregu.90537.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz H. 2008—The year of the big studies about the therapy of type-2-diabetes. ACCORD, ADVANCE, VADT, and the UKPDS 10-year follow-up data. MMW Fortschr Med. 2009;151:42–43. [PubMed] [Google Scholar]
- Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond) 1999;96:313–326. [PubMed] [Google Scholar]
- Signorelli SS, Malaponte G, Libra M, Di Pino L, Celotta G, Bevelacqua V, Petrina M, Nicotra GS, Indelicato M, Navolanic PM, Pennisi G, Mazzarino MC. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc Med. 2005;10:1–6. doi: 10.1191/1358863x05vm582oa. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, Howard BV, Kirkman MS, Kosiborod M, Reaven P, Sherwin RS. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci USA. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K+ channel openers in cerebral arteries from diabetic rats. Circ Res. 1997;81:996–1004. doi: 10.1161/01.res.81.6.996. [DOI] [PubMed] [Google Scholar]