Abstract
In this article, we examined theoretically the role of human cerebral glycogen in buffering the metabolic requirement of a 360-second brain stimulation, expanding our previous modeling study of neurometabolic coupling. We found that glycogen synthesis and degradation affects the relative amount of glucose taken up by neurons versus astrocytes. Under conditions of 175:115 mmol/L (∼1.5:1) neuronal versus astrocytic activation-induced Na+ influx ratio, ∼12% of astrocytic glycogen is mobilized. This results in the rapid increase of intracellular glucose-6-phosphate level on stimulation and nearly 40% mean decrease of glucose flow through hexokinase (HK) in astrocytes via product inhibition. The suppression of astrocytic glucose phosphorylation, in turn, favors the channeling of glucose from interstitium to nearby activated neurons, without a critical effect on the concurrent intercellular lactate trafficking. Under conditions of increased neuronal versus astrocytic activation-induced Na+ influx ratio to 190:65 mmol/L (∼3:1), glycogen is not significantly degraded and blood glucose is primarily taken up by neurons. These results support a role for astrocytic glycogen in preserving extracellular glucose for neuronal utilization, rather than providing lactate to neurons as is commonly accepted by the current ‘thinking paradigm'. This might be critical in subcellular domains during functional conditions associated with fast energetic demands.
Keywords: brain glycogen, lactate shuttle, mathematical modeling, neurometabolic coupling, neuronal activation
Introduction
Cerebral energetics is believed to be sustained via the complete oxidation of glucose (Bak et al, 2009). Nonetheless, regional glucose and oxygen utilization during focal brain stimulation exhibit a greater increase in glycolysis compared with oxidative metabolism, which is evidenced by a modest accumulation of lactate even in the presence of adequate oxygen tension in tissue (reviewed by Mangia et al, 2009a). Recently, it has been largely appreciated that the recruitment of brain cells in food-for-thought is not restricted to the neuronal electrical activity. Indeed, astrocytes are mandatory partners of neurons, and their metabolism is closely linked to synaptic activity (Hertz et al, 2007). The involvement of astrocytes in the coupling between glutamatergic neurotransmission and glucose utilization has posed into question the relative amount of neuronal versus astrocytic glucose uptake (Chih et al, 2001), and is at the basis of the astrocyte–neuron lactate shuttle hypothesis, which places astrocyte-derived lactate as a critical neuronal energy substrate (Pellerin and Magistretti, 1994). Conforming with this scenario, it has been suggested that energy transfer in the form of lactate from astrocytes to neurons is partly provided by glycogen metabolism (Brown, 2004).
In this study, we investigated the effect of glycogenolysis in buffering the high metabolic needs of the stimulated brain tissue by incorporating glycogen metabolism in mathematical models coupling brain electrical activity, metabolism, hemodynamics, and nutrients transport (Aubert and Costalat, 2005; Simpson et al, 2007; Mangia et al, 2009b). We specifically extended our previous integrative theoretical account (DiNuzzo et al, 2010), with a set of kinetic equations linking glycolysis to glycogen synthesis and breakdown in astrocytes. The main goal of this study was to evaluate the changes in cellular substrate availability, taking place when a fraction of the energy required by brain activation is supplied by stored carbohydrate. We found that the astrocytic support to neuronal function due to glycogen mobilization is identified by the indirect provision of glucose, not lactate to neurons (Swanson, 1992), contrary to the intuitive idea that an intermediary compound must be used by neurons for glycogen localization in astrocytes to be beneficial (Brown and Ransom, 2007). Specifically, glycogenolysis reduces astrocytic need for blood-borne interstitial glucose, thereby allowing its increased uptake into neurons. Our results are in agreement with the suggestion that, although the use of astrocytic glycogen is difficult to detect in vivo with standard methods for metabolic rates determination, it can make a substantial contribution to cerebral functional metabolism (Dienel et al, 2002, 2007).
Materials and methods
Kinetic modeling of cerebral glycogen metabolism was performed on top of several mathematical models of brain functional energetics (Aubert and Costalat, 2005; Simpson et al, 2007). In particular, we included a basic theoretical account for glycogen synthesis and mobilization to the key cell metabolic pathways of our previous integrative biochemical model (DiNuzzo et al, 2010). The model describes the time course of the main electrophysiological, metabolic, and vascular variables involved in neural activation. Briefly, nutrients traverse the blood–brain barrier and balance in cellular (neuron and astrocyte) and extracellular domains through diffusion or facilitative transport systems. Resting cell energy metabolism is regulated by basal energy consumption due to housekeeping processes plus activity of Na+/K+-ATPase after sodium leakage. Stimulation produces a rise in cerebral blood flow as well as inward Na+ currents in both neurons and astrocytes, which upregulate buffering of adenosine triphosphate (ATP) by adenylate and creatine kinases, and catabolism of glucose and lactate via glycolysis and respiration. We conformed to previous modeling works (Aubert and Costalat, 2005; DiNuzzo et al, 2010) by recasting all ATP-consuming processes as the gross cellular Na+ influx. Within astrocytes, in addition to Na+-glutamate cotransport, the modeled Na+ influx includes the effects of K+ sequestration via activation of Na+/K+-ATPase. Importantly, K+ sequestration is specifically relevant to the activation of glycogenolysis, as experimentally demonstrated in cell cultures (Dienel and Cruz, 2006, and references therein).
In the present extension of the model, we take into account brain glycogen storage and utilization during resting and stimulated conditions (Table 1). Glycogen is primarily located in astrocytes, so are the cellular enzymes that metabolize glycogen (Pfeiffer-Guglielmi et al, 2003). Therefore, we assumed that glycogen is entirely confined to astrocytes, with an equilibrium concentration of 14 mmol/L. Given an astrocytic element volume fraction of 0.25, this corresponds to a cerebral glycogen content of nearly 3.5 μmol/g, which is approximately the experimental value for the human brain (Oz et al, 2007), albeit somewhat lower compared with that found in rodent brain, where glucosyl units concentration has been found to range from 3 to 12 μmol/g depending on the extraction method and handling of the animal (Lowry et al, 1967; Cruz and Dienel, 2002). We ignored the subcellular distribution of glycogen in astrocytes, which can result in local concentration of up to 35 μmol/g in peripheral astrocytic processes (about 40% of astrocyte volume) (Hertz et al, 2007). It is noted that there is significant diurnal variation in total brain glycogen content, and the physiologic (activation or stress) as well as nutritional history of individual subjects may be important in defining the resting glycogen levels (Cruz and Dienel, 2002). We neglected the influence of organization and size of the glycogen particles on their metabolism. Because of the small changes in the glycogen level resulting from our simulations, we further assumed that the effective concentration of glucosyl units at glycogen outer branches is not considerably altered as glycogen size is reduced (Roach, 2002).
Table 1. Model kinetic equations linking glycolysis to glycogen metabolism*.
| Enzyme | Rate equation | Parameters | References |
|---|---|---|---|
| Hexokinase |  |
kHKn=0.031/s kHKa=0.017/s kI,G6P=0.017 mmol/L | Heinrich and Schuster (1996) Liu et al (1999) |
| Phosphofructokinase |  |
kPFKn=0.11/s kPFKa=0.06/s kI,ATP=1 mmol/L Km,G6P′=Km,F6P/qPGI=0.005 mmol/L n=4 | Heinrich and Schuster (1996) |
| Glycogen synthase |  |
kGSa=0.017 mmol/L per s [GLYa,0]=14 mmol/L | Nielsen and Richter (2003) |
| Glycogen phosphorylase |  |
Vmax,GPa=0.008 mmol/L per s Km,AMP=0.016 mmol/L h=1.5 | Buchbinder and Fletterick (1996) Sergienko and Srivastava (1997) |
AMP, adenosine monophosphate; G6P, glucose 6-phosphate; GP, glycogen phosphorylase; GS, glycogen synthase; HK, hexokinase; PFK, phosphofructokinase.
*Balance equations, rate equations, and parameter estimates of the complete model can be found in our previous theoretical account (DiNuzzo et al, 2010).
Note that hexokinase- and phosphofructokinase-catalyzed reactions were lumped together in our previous model. The maximum rates of these enzymes have been obtained by assuming a glucose 6-phosphate steady-state concentration of 0.1 mmol/L in both neurons and astrocytes, whereas rates of glycogen synthase- and phosphorylase-catalyzed reactions have been adjusted for experimental glycogen turnover and detection limit (see text).
In rate equations, x is either n (neuronal) or a (astrocytic).
The synthesis and degradation of glycogen are regulated through allosteric inhibitors and activators as well as via reversible phosphorylation–dephosphorylation cascades of glycogen synthase (GS) and glycogen phosphorylase (GP). The changes in phosphorylation status of both enzymes are under hormonal as well as energetic control (Roach, 2002). However, as we were interested in the stimulation-induced changes of glycogen metabolism, we considered stationary hormonal conditions. Moreover, we conformed to prior modeling works (Lambeth and Kushmerick, 2002; Dash et al, 2007) by incorporating the phosphorylation-induced modulation of enzyme activity in the regulatory effects of allosteric ligands. Specifically, adenosine monophosphate (AMP) has the predominant role in the allosteric activation of brain GP (Lowry et al, 1967; Dash et al, 2007; Walcott and Lehman, 2007). Accordingly, brain GP isozyme is more sensitive to control through changes in AMP concentration than through phosphorylation cascade system (Crerar et al, 1995; Pfeiffer-Guglielmi et al, 2003). As even small changes in cellular ATP level associated with increased energy demand result in large variation of the AMP level, the AMP-dependent mobilization of glycogen deposits is exquisitely sensitive to the cell energy status (see Roach, 2002). Because of the major role of AMP in GP control, we neglected the inhibition of the enzyme by several allosteric effectors including glucose, glucose-6-phosphate, ATP, purines, and AMP at nonphysiological concentrations (above 2 mmol/L) (Walcott and Lehman, 2007). Glycogen is mobilized as glucose 1-phosphate, which is in equilibrium with glucose-6-phosphate through a reaction catalyzed by phosphoglucomutase. Conversion of glucose 1-phosphate to glycogen requires the equivalent of one ATP due to the formation of uridine diphosphate glucose by uridine diphosphate-glucose pyrophosphorylase. We included these latter reactions into the GS-catalyzed synthesis of glycogen. In particular, the incorporation of glucose into glycogen can be greatly simplified by considering the tight inverse coupling between GS activity and tissue glycogen content, as clearly demonstrated in the skeletal muscle (Nielsen and Richter, 2003, and references therein), which is coherent with the fact the glycogenesis is a saturable process (Champe and Harvey, 1994). Given the similarity between muscle- and brain-type GS enzyme isoforms, we assumed that changes in brain glycogen content during activation have a primary role in the regulation of GS via a hyperbolic-type relationship (Nielsen and Richter, 2003). This simple dependence circumvents the incorporation of the overall metabolic network for glycogen synthesis.
The connection between astrocytic glycogen metabolism and our previous biochemical model was formalized at the level of glucose-6-phosphate. To model glucose-6-phosphate concentration explicitly, which we assumed to have steady-state value of 0.1 mmol/L (Watanabe and Passonneau, 1973), we linked glycogen metabolism to glycolysis through the separation of the reactions catalyzed by hexokinase (HK) and phosphofructokinase (see Table 1). Rate equations and kinetic parameter estimates for these enzymes were obtained after analysis of metabolite concentration transients as well as resting and activation-induced glucose and lactate flow rates in absence of glycogenolysis. Specifically, we correctly reproduced our original simulation outcomes for glucose and lactate fluxes (DiNuzzo et al, 2010) with the present model—i.e., when glycogen metabolism is inactive. The resulting HK product-inhibition constant (KI,G6P=0.017 mmol/L) is consistent with the observation that glucose-6-phosphate severely inhibits the enzyme at micromolar concentrations (Liu et al, 1999, and references therein), and is in good agreement with early reports from the mouse brain (Lowry and Passonneau, 1964).
Glycogen phosphorylase is active only when high-energy phosphates are depleted (Lowry et al, 1967), whereas under resting conditions it is >85% inactive (Breckenridge and Norman, 1965). Furthermore, measured flux of glucose through GS is very small (<3 nmol/g per min) in awake resting conditions (Oz et al, 2003). Therefore, we considered exiguous basal activity of both GS and GP under steady-state resting conditions. As experimental data for maximal reaction rates of these enzymes in vivo are not available, they were adjusted to match the experimental stimulation-induced glycogen utilization and subsequent replenishment after activation (see ‘Results' section), keeping in mind that glycogen depletion depends on the metabolic rate of the tissue rather than on its initial concentration (Brown, 2004).
We additionally tested the model response after altering the relative neuronal versus astrocytic activation fraction from 1.5:1 to 3:1, matching the same conditions adopted in our previous modeling work (DiNuzzo et al, 2010). Cell activation was shown to have the largest modulatory effect on cellular substrate utilization, with a 1.5:1 ratio colliding with the limitation of astrocytic glucose transport capacity, thus suggesting a biochemical/transport basis for glycogen to be located in these cells (DiNuzzo et al, 2010). Although the 3:1 ratio was identified as a low-end estimate for a sustained neuronal versus astrocytic stimulation based on current literature (DiNuzzo et al, 2010), it is worth exploring higher levels of astrocytic activation as they might hold at a subcellular level or during rapid transients of the energetic demand likely occurring during the early phase of stimulation. Moreover, mobilization of glycogen in astrocytes might circumvent the failure of astrocytic glucose uptake, thus further rationalizing a potentially lower neuronal versus astrocytic stimulation ratio. To examine the intraparenchymal metabolite trafficking, we adopted the convention of expressing cell outward glucose and lactate fluxes as positive and cell inward fluxes as negative. Model simulations were performed by Rosenbrock-based integration algorithms using the software MATLAB (The Mathworks Inc., Natick, MA, USA; http://www.mathworks.com/) version 7.0.4 R14.
Results
First, we examined the activity of the enzymes involved in glycogen metabolism under conditions of stimulation using 1.5:1 neuronal/astrocytic activation ratio, taking into account the rate of brain glycogen synthesis and degradation observed experimentally (Dienel and Cruz, 2006; Oz et al, 2007; Swanson et al, 1992). Under these conditions, glycogenolysis is robustly activated during the stimulation period. Figure 1 shows the simulated velocity of GS- and GP-catalyzed reactions during a sustained 360-second activation, as well as the astrocytic glycogen level change. In agreement with previous findings, our data show that mobilization of the stored sugar is rapidly activated at the stimulation onset, whereas glycogen resynthesis is slower and protracted compared with the duration of the stimulus, which is also consistent with previous reports indicating that potential GP activity in brain far exceeds that of synthase (Dienel et al, 2002, 2007). Tissue glycogen concentration (inset in Figure 1) was found to decrease by ∼0.4 mmol/L (or 12%), thus resulting in a mean breakdown rate of 1.1 μmol/L per s (∼0.07 μmol/g per min), which is compatible with the experimental values ranging from 0.04 to 0.5 μmol/g per min obtained in animal studies and in cell cultures (Dienel and Cruz, 2006; Swanson et al, 1992). Furthermore, the simulated rate of functional glycogen utilization is almost within the experimental detection limit (20% in individual subjects) of the sole study, which examined the role of human brain glycogen during stimulation by 13C nuclear magnetic resonance spectroscopy (Oz et al, 2007). Our simulations showed that, although at rest simultaneous activation of GS and phosphorylase is prevented, brain activation promotes both glycogenolysis and glycogen synthesis at the same time (Dienel et al, 2007; Shulman et al, 2001; Walls et al, 2009). Accordingly, the analysis of net mean breakdown rate as a function of stimulation time suggests that prolonged stimulations are detrimental rather than advantageous to detect the functional changes in glycogen concentration induced by physiological activation (Figure 2). It is noted that Oz et al (2007) found no significant change in the 13C-labeled C1 glycogen signal measured before and after a 20-minute visual stimulation. However, turnover of glycogen outer layers induced ∼30% clearance of the label before stimulation (Figure 3 in Oz et al, 2007), which started several hours after cessation of glucose infusion. This is to be compared with the amount of glucosyl residues readily available to the phosphorylase in the glycogen outer tier, which is ∼35% (Melendez-Hevia et al, 1993). Therefore, the discrepancy between the study of Oz et al (2007) and our findings may be partly explained by the fact that labeled glucosyl residues were retained in the inner, less accessible tiers of the glycogen granules. The simulated net glycogen utilization after 20 minutes of nearly 0.67 μmol/g (inset in Figure 2) results in a 19% signal change, which is near or below the experimental detection limit if we assume concomitant synthesis and degradation within the same glycogen deposits.
Figure 1.
Time courses of glycogen synthase (GS) and glycogen phosphorylase (GP) reaction rates. Brain stimulation produces a rapid activation of GP because of increased energy demand, which effect glucosyl equivalents concentration in astrocytes (inset). The GP-catalyzed mobilization of glycogen decreases during the late phase of the stimulation period, and readily returns to basal level after the end of stimulation. The GS-catalyzed incorporation of glucose into glycogen is delayed and much slower than glycogen breakdown; however, it remains significantly elevated during the poststimulus period. Note that, although the resting activity of both GS and GP is very small, substantial synthesis and degradation of glycogen occur simultaneously during activation. The simulated neuron/astrocyte activation ratio is 1.5:1.
Figure 2.
Net brain glycogen breakdown as a function of time during the stimulation. The simulated net glycogen breakdown rate (difference between phosphorolysis rate and synthesis rate) shows a transient peak (up to 0.12 μmol/g per min) at 1 to 2 minutes and then reaches a steady-state value of 0.02 μmol/g per min after 6 minutes. Therefore, the mean net glycogen breakdown rate averaged over the stimulation period decreases with increasing duration of the stimulus. As shown in the inset, the amount of mobilized glycogen is 0.67 μmol/g in correspondence of a 20-minute stimulation. The simulated neuron/astrocyte activation ratio is 1.5:1.
Figure 3.
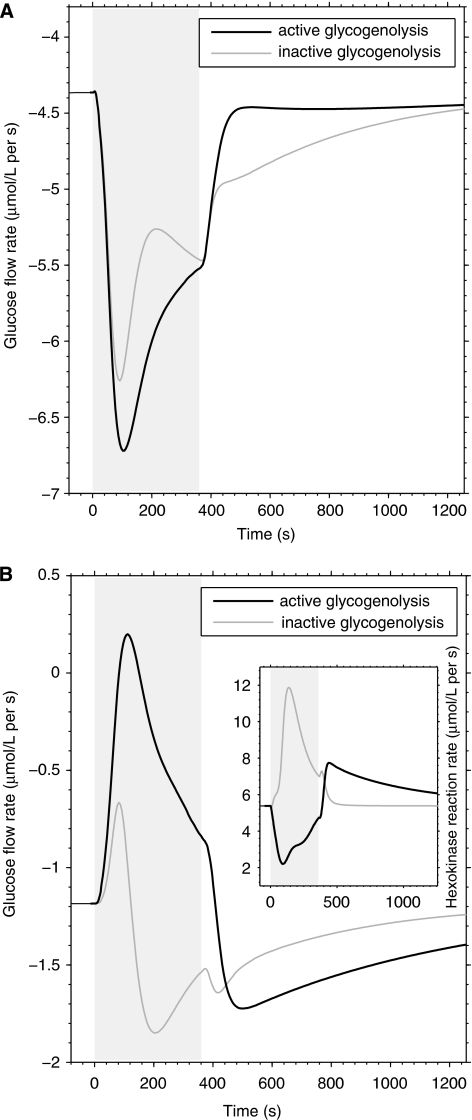
Time courses of neuron (A) and astrocyte (B) glucose flow rates (relative to interstitium) for active or inactive glycogenolysis. Under the same activation conditions, astrocytic glucose uptake from the extracellular space is significantly diminished when glycogen is mobilized. This is a result of glucose-6-phosphate-induced inhibition of hexokinase in astrocytes (inset). On average, substrate flow through astrocytic hexokinase is nearly 40% inhibited (65% peak inhibition, values relative to resting conditions), without any change in the activity of neuronal hexokinase (not shown). The increased availability of paracellularly diffused glucose into the interstitium results in increased amount of the sugar taken up by neurons. On the contrary, astrocytes diminish extracellular glucose uptake, which translates to a small, transient gradient-driven release of part of their glucose pool to the interstitium. The glycogenolysis-induced changes in neuronal and astrocytic glucose fluxes shown here are introduced by the breakdown of about 12% of total glycogen content. It is noted that only when hexokinase is inhibited by its product during active glycogenolysis, the rate of glycogen breakdown (see Figure 1) exceeds the rate of glycolysis during early stimulation, consistent with experimental evidence (Subbarao et al, 1995). The simulated neuron/astrocyte activation ratio is 1.5:1.
Figure 3 shows the time course of neuronal and astrocytic glucose flow rates at rest and during brain activation in correspondence of the presence or absence of glycogenolyis. The assumed energetic demand in astrocytes after stimulation was previously shown to rapidly deplete astrocytic glucose due to unbalanced supply and demand of glucose, suggesting a role for glycogen in supporting high activation of these cells (DiNuzzo et al, 2010). Accordingly, we found that the limited glucose transport capacity of astrocytes is circumvented when astrocytic energy production is partly derived from glycogenolysis, as glucose depletion in astrocytes is prevented by glycogen mobilization (data not shown). Nevertheless, the stimulation-induced glycogen breakdown reinforces the simulated glucose flux trends obtained in the absence of glycogen. Specifically, neuronal glucose uptake during activation is further increased by glycogenolysis (Figure 3A), whereas the transport of glucose in astrocytes drops (Figure 3B). Analysis of enzyme activity reveals that phosphorolysis of astrocytic glycogen by GP facilitates the rapid formation of glucose-6-phosphate compared with uptake and phosphorylation of glucose, producing on average an ∼40% substrate flow inhibition through the HK step during stimulation (65% peak value) as compared with resting conditions (inset in Figure 3B). Given an 85% resting HK product inhibition (calculated from Table 1), this translates to a total HK inhibition of ∼91% on average during stimulation (95% peak value), which is in agreement with previous reports for the level of HK product inhibition by glucose-6-phosphate (Lowry and Passonneau, 1964). The suppression of HK activity in turn reduces glucose utilization in astrocytes, and hence the glucose uptake by these cells is depressed due to the passive nature of glucose carrier proteins. These results are consistent with the observed supercompensatory increase in the utilization of blood glucose during activation but not rest when GP is inhibited (Dienel et al, 2007) (see also Table 2). The astrocytic glucose flow rate shows a small, transient glucose release during the early phase of stimulation, indicating that glucose phosphorylation inhibition is sufficient for astrocytes to export part of their own glucose pool. In the late phase of stimulation and after the end of stimulation, glucose is directed to glycogen first rather than directly entering glycolysis, thus bringing about increased astrocytic and decreased neuronal glucose uptake compared with inactive glycogenolysis.
Table 2. Mean substrate flow rate changes as a function of cell stimulation and glycogen metabolism.
| Stimulationa | Na+ influxb | Glycogenolysis | Neuronal flow ratec | Astrocytic flow ratec | ||
|---|---|---|---|---|---|---|
| Glucose | Lactate | Glucose | Lactate | |||
| Rest | Active/inactive | −4.36 | 0.21 | −1.34 | 0.07 | |
| High astrocytic activation | 175:155 (1.5:1) | Active | −5.32 | 0.29 | −0.90 | 0.09 |
| Inactive | −5.06 | 0.23 | −1.54 | 0.18 | ||
| Low astrocytic activation | 190:65 (3:1) | Active | −5.52 | 0.67 | −0.98 | –0.30 |
| Inactive | −5.53 | 0.66 | −1.01 | –0.32 | ||
As neuronal versus astrocytic Na+ influx ratio. Flow rates for steady-state resting condition apply for both 1.5:1 and 3:1 stimulations.
As mmol/L of Na+ entry into cells induced by the stimulation. The actual activation ratio is enclosed in parenthesis.
As glucose equivalents. Positive (negative) values mean release (uptake) from (to) interstitium for neurons, and interstitium plus basal lamina for astrocytes.
Note that the overall glucose uptake by the brain (i.e., sum of neuronal and astrocytic glucose uptake) during high astrocytic activation is greater when glycogenolysis is inactive.
All values are expressed in units μmol/L per s.
Next, we examined the contribution of glycogen mobilization in astrocytes for the provision of energy substrate for neurons in the form of lactate. Figure 4 shows the time course of the resting and stimulation-induced neuronal and astrocytic lactate flow rates in correspondence of the presence or absence of glycogenolysis. Simulations show negligible differences in intercellular lactate flux induced by the metabolism of glycogen, especially at the onset of increased activity (prevalence of glycogenolysis) relative to the late and poststimulation periods (prevalence of glycogenesis), both in neurons (Figure 4A) and astrocytes (Figure 4B). This indicates that glycogen-derived pyruvate is consumed by astrocytes rather than transferred to neurons as lactate, supporting the hypothesis that shuttled lactate might have a minor role as a cell-to-cell energy substrate (DiNuzzo et al, 2010). In addition, we found that the rise in lactate concentration induced by stimulation is independent of glycogen breakdown (data not shown), consistent with experimental findings (Caesar et al, 2008). This further suggests that lactate accumulation is governed essentially by energy demand and that substrate availability is unrestricted for both neurons and astrocytes.
Figure 4.
Time courses of neuron (A) and astrocyte (B) lactate flow rates (relative to interstitium) for active or inactive glycogenolysis. Glycogenolysis affects marginally the lactate trafficking between neurons and astrocytes. In particular, flux changes during the initial phase of stimulation are negligible for both cell types. During the late stimulation period, astrocytes divert glucose to the replenishment of glycogen and thus reduce extracellular lactate release for aerobic energy production. In terms of glucose equivalents, the mean variations of cellular lactate flow rates between active and inactive glycogen metabolism represents only 12% and 20% of the concurrent changes of neuronal and astrocytic glucose flow rates, respectively (see Table 2). The simulated neuron/astrocyte activation ratio is 1.5:1.
Finally, to analyze the role of glycogenolysis in astrocytes during different levels activation of these cells, we calculated the mean glucose and lactate flux as a function of the neuronal versus astrocytic stimulation (Table 2). A neuronal versus astrocytic stimulation-induced Na+ influx ratio of 190:65 mmol/L (i.e., condition of ‘low' astrocytic activation, or 3:1 neuronal/astrocytic activation ratio) was found to result in glycogen-insensitive substrate utilization for both neurons and astrocytes, which reflects a smaller increase of intracellular AMP concentration after astrocytic energy consumption and a consequently reduced glycogen breakdown. On the contrary, for a neuronal versus astrocytic stimulation-induced Na+ influx ratio of 175:115 mmol/L (i.e, condition of ‘high' astrocytic activation, or 1.5:1 neuronal/astrocytic activation ratio), glycogen mobilization induces a mean 0.64 μmol/L per s decrease in astrocytic glucose uptake, which accompanies a 0.26 μmol/L per s increase in glucose routing to neurons. Simulations showed also the flux of intermediary metabolite as lactate depends on the level of stimulation of neurons compared with astrocytes. In particular, under low activation conditions of astrocytes, neuronal lactate release is 2.5-fold higher relative to conditions of high astrocytic stimulation. Accordingly, astrocytic glycogen-derived carbons are partly replaced by lactate taken up (flow rate of 0.30 μmol/L per s) from the extracellular space—that is, shuttled from neurons to astrocytes—which is in agreement with previous theoretical reports (Simpson et al, 2007; Mangia et al, 2009b). In this condition, glucose equivalents as transferred lactate represents ∼30% of astrocytic glucose uptake, whereas high activation of astrocytes compared with neurons results in lactate release by both cell types. Taken together, these simulations indicate that glucose is preserved for neuronal utilization regardless of the engagement of astrocytes, with lactate acting as a versatile (albeit secondary) substrate for either utilization (i.e., oxidation) or disposal (washout by the bloodstream) (DiNuzzo et al, 2010).
Discussion
On the basis of the results of this study, we propose a novel contribution to the functions of brain glycogen under physiological conditions. First, as generally accepted, glycogen buffers the provision of glucose-6-phosphate to provide energy substrate for astrocytic glycolysis, and possibly precursor for pentose phosphate pathway (see below). Second, increased glucose-6-phosphate level would reduce the astrocytic use of glucose delivered by blood during glycogenolysis due to inhibition of HK, thus allowing neurons greater access to glucose provided by blood. Third, some of the unmetabolized intracellular glucose in astrocytes could now diffuse to neurons as supplementary fuel.
Therefore, the proposed mechanism for the suppression of astrocytic glucose consumption identifies glycogen as a modulator of the changes in cellular glucose availability during stimulation. Specifically, reduction in the amount of glucose taken up and metabolized by astrocytes is realized via the inhibitory feedback on HK exerted by glucose-6-phosphate, which increases substantially as a result of stimulation-induced glycogenolysis (Figure 1). Noteworthy, even the mobilization of 0.4 mmol/L glucosyl residues is found to produce an ∼40% HK flux inhibition (relative to rest, averaged over the entire stimulation period) in astrocytes, with a consequent routing of glucose to neurons (Figure 3). Interestingly enough, intercellularly shuttled lactate is found to be fairly independent on glycogen utilization (Figure 4; Table 2), indicating that brain metabolism is efficient in relocating blood-borne glucose to satisfy cellular energy demand, with only secondary dependence on carbon equivalents derived from transferred lactate. Simulations showed that glycogen mobilization can induce partial release of the astrocytic glucose pool (Figure 3B), which is contrary to the notion that astrocytes cannot release glucose because glucose-6-phosphatase activity in the brain is very low (Brown, 2004). Accordingly, glucose-6-phosphatase is not included in the present model, as no significant dephosphorylation of glucose-6-phosphate is found in the brain in vivo (Nelson et al, 1985). Nonetheless, diffusion of intracellular glucose from astrocytes to neurons has been observed directly in adult rat brain slices under conditions of astrocytic glucose concentration high enough (20 mmol/L) to overwhelm its metabolism by HK (Ghandi et al, 2009). The modeling conclusions of this study indicate that astrocyte-to-neuron glucose trafficking at physiologic glucose level is likewise possible if HK in astrocytes is sufficiently inhibited and if neuronal glucose level is reduced. In particular, the mobilization of glycogen in astrocytes transiently reduces glucose consumption by these cells; therefore, the decrease in glucose concentration induced by activation is smaller in astrocytes compared with neurons. This situation creates a concentration gradient that causes passive uptake of glucose into neurons from interstitial fluid and nearby astrocytes. The intracellular glucose concentration (about 1.2 mmol/L for both neurons and astrocytes, see DiNuzzo et al, 2010) is in greater excess to the activation-induced glucose demand of astrocytes (0.2 mmol/L) compared with neurons (0.8 mmol/L) for the 360-second stimulation interval (calculated from Table 2). Therefore, in the presence of glycogenolysis, a fraction of cytosolyc glucose in astrocytes can be available for release to the interstitium without impairing astrocytic metabolism. Overall, neurons appear to use not only glucose present in the interstitium, but also part of the glucose taken up from blood and not metabolized by astrocytes; in the latter scenario, astrocytic glucose might represent a reservoir of carbons for neurons without involving any glucose-6-phosphatase activity in astrocytes.
Our results suggest that glycogen acts as a buffer within astrocytic metabolism to avoid the competition for glucose between neurons and astrocytes, thereby reducing the impact on neuronal metabolism by varying astrocytic energy demand. Accordingly, the assumption about cell stimulation ratio becomes much less critical on model outcomes (Table 2, compare with the results obtained in DiNuzzo et al, 2010), which suggests a role for glycogen in reducing the sensitivity of the brain metabolic response on the potential cellular heterogeneity. It should be realized that, although the simulated increase in glucose availability to neurons after glycogenolysis in astrocytes is relatively modest (from Table 2 it is calculated as a 0.2 mmol/L decrease and 0.1 mmol/L increase of astrocytic and neuronal glucose uptake, respectively, for the entire stimulation interval), the effect can become important in situ as the involved subcellular volume fraction can be very small compared with cell volume. The initiation of glycogen resynthesis before the end of stimulation, together with the small activation-induced decrease in brain glycogen is compatible with the observation that glycogen is not significantly degraded after protracted stimulations (Dienel et al, 2002), consistent with experiments showing only 20% brain glycogen decrease after somatosensory stimulation in rats (Swanson et al, 1992) or undetectable changes after visual stimulation in humans (Oz et al, 2007). Therefore, despite the experimental outcomes of these studies may be strongly dependent on the labeling method and experimental protocol, they support the argument that glycogen retention is important for the brain, likely to preserve the accessible glycogenolytic response, and that only a moderate fraction of brain glycogen is mobilized during the metabolic stress induced by activation (Lowry et al, 1967).
The outcomes of the present theoretical study support the notion that during the early phase after brain stimulation, the properties and the regulations of cellular metabolic and transport competence favor the channeling of blood-borne glucose, rather than glycogen-derived lactate to activated neurons. Notably, the assumption that astrocytes release glycogen-derived lactate (Brown, 2004; Pellerin et al, 2007) is based on findings obtained in cultured cells often exposed to extreme stimulation paradigms or nonphysiological conditions (low or zero glucose concentration) (Brown and Ransom, 2007; Dringen et al, 1993), which might upregulate the reduction of glycogen-derived pyruvate to lactate compared with oxidation in the tricarboxylic acid cycle. Therefore, the correlation between the rate of lactate release and glycogen breakdown observed in these studies with possibly altered metabolic demand is not in contradiction with our modeling conclusions.
Although there is no thermodynamic energetic benefit for astrocytes to mobilize glycogen when glucose is available as a substrate, mobilization of glycogen has the clear kinetic advantage of rapidly providing energy for the fast energetic needs of astrocytes, such as K+ sequestration after neuronal action potentials. In fact, K+ was found to robustly stimulate astrocytic glycogenolysis (Dienel and Cruz, 2006, and references therein). Moreover, glycogen may sustain the net synthesis of glutamine from glycogen (Gibbs et al, 2008) via stimulation of the anaplerotic pyruvate carboxylation pathway in astrocytes, as well as the generation in the pentose phosphate pathway of the NADPH needed for the detoxification of reactive oxygen species (Murin et al, 2009, and references therein). The latter point applies both to neurons, which can divert a larger fraction of glucose to pentose phosphate pathway during activation, and to astrocytes, as involvement of glycogen for disposal of peroxides was directly demonstrated in astrocytic preparations (Rahman et al, 2000). On the other hand, our results suggest that part of the functional significance of brain glycogen is identified in the energetic benefit for neurons (i.e., supporting neuronal glycolysis to proceed). This is perhaps necessary to maintain neuronal processes depending on transmembrane sodium gradient, which are thought to use preferentially glycolytic energy (Ames, 2000). Accordingly, glucose is essential for the maintenance of neural activity in cultured neurons (Bak et al, 2009) and in rapidly prepared brain slices regardless of the ATP concentration (Yamane et al, 2000). Notably, the different dependence of brain glycogen utilization on the degree of astrocytic activation adds another level of complexity to the composite character of the metabolic response of the brain to stimulation. Although definite experimental proof about the physiologic significance of preserving extracellular glucose for neuronal use remains elusive, we conclude that the theoretical findings of this study support the importance of glucose as neuronal energy substrate during increased neuronal activity.
Acknowledgments
The authors thank Gulin Oz for her helpful comments on the paper.
The authors declare no conflict of interest.
References
- Ames A. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34:42–68. doi: 10.1016/s0165-0173(00)00038-2. [DOI] [PubMed] [Google Scholar]
- Aubert A, Costalat R. Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J Cereb Blood Flow Metab. 2005;25:1476–1490. doi: 10.1038/sj.jcbfm.9600144. [DOI] [PubMed] [Google Scholar]
- Bak LK, Walls AB, Schousboe A, Ring A, Sonnewald U, Waagapetersen HS. Neuronal glucose but not lactate utilization is positively correlated with NMDA-induced neurotransmission and fluctuations in cytosolic Ca2+ levels. J Neurochem. 2009;109 (Suppl 1:87–93. doi: 10.1111/j.1471-4159.2009.05943.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge BM, Norman JH. The conversion of phosphorylase b to phosphorylase a in brain. J Neurochem. 1965;12:51–57. doi: 10.1111/j.1471-4159.1965.tb10251.x. [DOI] [PubMed] [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89 (3:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55:1263–1271. doi: 10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Buchbinder JL, Fletterick RJ. Role of the active site gate of glycogen phosphorylase in allosteric inhibition and substrate binding. J Biol Chem. 1996;271:22305–22309. doi: 10.1074/jbc.271.37.22305. [DOI] [PubMed] [Google Scholar]
- Caesar K, Hashemi P, Douhou A, Bonvento G, Boutelle MG, Walls AB, Lauritzen M. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol. 2008;586:1337–1349. doi: 10.1113/jphysiol.2007.144154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champe PC, Harvey RA. Lippincott's Illustrated Reviews: Biochemistry. Lippincott Williams and Wilkins: Philadelphia; 1994. [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose. Trends Neurosci. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Crerar MM, Karlsson O, Fletterick RJ, Hwang PK. Chimeric muscle and brain glycogen phosphorylase define protein domains governing isozyme-specific responses to allosteric activation. J Biol Chem. 1995;270:13748–13756. doi: 10.1074/jbc.270.23.13748. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Dienel DA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. J Cereb Blood Flow Metab. 2002;22:1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. [DOI] [PubMed] [Google Scholar]
- Dash RK, DiBella JA, II, Cabrera ME. A computational model of skeletal muscle metabolism linking cellular adaptations induced by altered loading states to metabolic responses during exercise. Biomed Eng Online. 2007;6:14. doi: 10.1186/1475-925X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem. 2007;102:466–478. doi: 10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Astrocyte activation in working brain: energy supplied by minor substrates. Neurochem Int. 2006;48:586–595. doi: 10.1016/j.neuint.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. J Cereb Blood Flow Metab. 2002;22:1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Changes in glucose uptake rather than lactate shuttle take center stage in subserving neuroenergetics: evidence from mathematical modeling. J Cereb Blood Flow Metab. 2010;30:586–602. doi: 10.1038/jcbfm.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Ghandi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009;111:522–536. doi: 10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs ME, Bowser DN, Hutchinson DS, Loiacono RE, Summers RJ. Memory processing in the avian hippocampus involves interactions between beta-adrenoceptors, glutamate receptors, and metabolism. Neuropsychopharmacology. 2008;33:2831–2846. doi: 10.1038/npp.2008.5. [DOI] [PubMed] [Google Scholar]
- Heinrich R, Schuster S. The Regulation of Cellular Systems. Chapman & Hall: New York; 1996. [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Lambeth MJ, Kushmerick MJ. A computational model for glycogenolysis in skeletal muscle. Ann Biomed Eng. 2002;30:808–827. doi: 10.1114/1.1492813. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CS, Kurbanov FT, Honzatko RB, Fromm HJ. Dual mechanisms for glucose 6-phosphate inhibition of human brain hexokinase. J Biol Chem. 1999;274:31155–31159. doi: 10.1074/jbc.274.44.31155. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. The relationship between substrates and enzymes of glycolysis in brain. J Biol Chem. 1964;239:31–42. [PubMed] [Google Scholar]
- Lowry OH, Schulz DW, Passonneau JV. The kinetics of glycogen phosphorylases from brain and muscle. J Biol Chem. 1967;242:271–280. [PubMed] [Google Scholar]
- Mangia S, Giove F, Tkac I, Logothetis NK, Henry P-G, Olman CA, Maraviglia B, Di Salle F, Ugurbil K. Metabolic and hemodynamic events after changes in neuronal activity: current hypotheses, theoretical predictions and in vivo nmr experimental findings. J Cereb Blood Flow Metab. 2009a;29:441–463. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangia S, Simpson IA, Vannucci SJ, Carruthers A. The in vivo neuron-to-astrocyte lactate shuttle in human brain: evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009b;109 (Suppl 1:55–62. doi: 10.1111/j.1471-4159.2009.06003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez-Hevia E, Waddell TG, Shelton ED. Optimization of molecular design in the evolution of metabolism: the glycogen molecule. Biochem J. 1993;295:477–483. doi: 10.1042/bj2950477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murin R, Cesar M, Kowtharapu BS, Verleysdonk S, Hamprecht B. Expression of pyruvate carboxylase in cultured oligodendroglial, microglial and ependymal cells. Neurochem Res. 2009;34:480–489. doi: 10.1007/s11064-008-9806-6. [DOI] [PubMed] [Google Scholar]
- Nelson T, Lucignani G, Atlas S, Crane AM, Dienel GA, Sokoloff L. Reexamination of glucose-6-phosphatase activity in the brain in vivo: no evidence for a futile cycle. Science. 1985;229:60–62. doi: 10.1126/science.2990038. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Richter EA. Regulation of glycogen synthase in skeletal muscle during exercise. Acta Physiol Scand. 2003;178:309–318. doi: 10.1046/j.1365-201X.2003.01165.x. [DOI] [PubMed] [Google Scholar]
- Oz G, Henry PG, Seaquist ER, Gruetter R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem Int. 2003;43:323–329. doi: 10.1016/s0197-0186(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Oz G, Seaquist ER, Kumar A, Criego AB, Benedict LE, Rao JP, Henry PG, Van De Moortele PF, Gruetter R. Human brain glycogen content and metabolism: implications on its role in brain energy metabolism. Am J Physiol Endocrinol Metab. 2007;292:E946–E951. doi: 10.1152/ajpendo.00424.2006. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer-Guglielmi B, Fleckenstein B, Jung G, Hamprecht B. Immunocytochemical localization of glycogen phosphorylase isozymes in rat nervous tissues by using isozyme-specific antibodies. J Neurochem. 2003;85:73–81. doi: 10.1046/j.1471-4159.2003.01644.x. [DOI] [PubMed] [Google Scholar]
- Rahman B, Kussmaul L, Hamprecht B, Dringen R. Glycogen is mobilized during the disposal of peroxides by cultured astroglial cells from rat brain. Neurosci Lett. 2000;290:169–172. doi: 10.1016/s0304-3940(00)01369-0. [DOI] [PubMed] [Google Scholar]
- Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002;2:101–120. doi: 10.2174/1566524024605761. [DOI] [PubMed] [Google Scholar]
- Sergienko EA, Srivastava DK. Kinetic mechanism of the glycogen-phosphorylase-catalysed reaction in the direction of glycogen synthesis: co-operative interactions of AMP and glucose 1-phosphate during catalysis. Biochem J. 1997;328:83–91. doi: 10.1042/bj3280083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci USA. 2001;98:6417–6422. doi: 10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao KV, Stolzenburg JU, Hertz L. Pharmacological characteristics of potassium-induced, glycogenolysis in astrocytes. Neurosci Lett. 1995;196:45–48. doi: 10.1016/0304-3940(95)11834-j. [DOI] [PubMed] [Google Scholar]
- Swanson RA. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol. 1992;70 (Suppl:S138–S144. doi: 10.1139/y92-255. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51:451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Walcott S, Lehman SL. Enzyme kinetics of muscle glycogen phosphorylase b. Biochemistry. 2007;46:11957–11968. doi: 10.1021/bi7005527. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimburger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158:284–292. doi: 10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Passonneau JV. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973;20:1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Yamane K, Yokono K, Okada Y. Anaerobic glycolysis is crucial for the maintenance of neural activity in guinea pig hippocampal slices. J Neurosci Methods. 2000;103:163–171. doi: 10.1016/s0165-0270(00)00312-5. [DOI] [PubMed] [Google Scholar]