Abstract
Epidemiologic studies have shown that foods rich in polyphenols, such as flavanols, can lower the risk of ischemic heart disease; however, the mechanism of protection has not been clearly established. In this study, we investigated whether epicatechin (EC), a flavanol in cocoa and tea, is protective against brain ischemic damage in mice. Wild-type mice pretreated orally with 5, 15, or 30 mg/kg EC before middle cerebral artery occlusion (MCAO) had significantly smaller brain infarcts and decreased neurologic deficit scores (NDS) than did the vehicle-treated group. Mice that were posttreated with 30 mg/kg of EC at 3.5 hours after MCAO also had significantly smaller brain infarcts and decreased NDS. Similarly, WT mice pretreated with 30 mg/kg of EC and subjected to N-methyl-aspartate (NMDA)-induced excitotoxicity had significantly smaller lesion volumes. Cell viability assays with neuronal cultures further confirmed that EC could protect neurons against oxidative insults. Interestingly, the EC-associated neuroprotection was mostly abolished in mice lacking the enzyme heme oxygenase 1 (HO1) or the transcriptional factor Nrf2, and in neurons derived from these knockout mice. These results suggest that EC exerts part of its beneficial effect through activation of Nrf2 and an increase in the neuroprotective HO1 enzyme.
Keywords: epicatechin, heme oxygenase 1, MCAO, Nrf2, stroke, NF-E2-related factor-2
Introduction
Numerous epidemiologic studies have revealed a strong inverse correlation between ischemic heart disease and consumption of red wine and certain fruits and vegetables that contain high levels of flavonoids and other polyphenols (Simonyi et al, 2005). Flavanols (e.g., epicatechin (EC) and catechin) and their monomers/oligomers/polymers, known as procyanidins, compose a major category of secondary polyphenolic plant metabolites. Mounting evidence suggests a protective role for various polyphenols and flavonoids in cerebral ischemia (Lee et al, 2004; Lee et al, 2000; Shah et al, 2005; Shin et al, 2006; Simonyi et al, 2005). It is noteworthy also that flavanols have been shown to improve vasorelaxation (Duarte et al, 1993; Karim et al, 2000). The simple chemical structure of flavanols may interact with specific cellular and molecular targets, thereby mediating a wide range of biologic activities.
Studies have revealed that induction of phase II antioxidant enzymes through transcriptional activation is mediated through transcriptional factor Nrf2 and antioxidant-response elements (AREs). In these paradigms, polyphenols or electrophylic agents can target a specific set of genes that encode phase II enzymes, which include heme oxygenase 1 (HO1), nicotinamide adenine dinucleotide phosphate (NADPH) quinone oxidoreductase 1, and γ-glutamyl cystein ligase. These enzymes provide protection by regulating and maintaining intracellular redox states (Gong et al, 2002; Itoh et al, 2004). Of these, HO1 has been reported to have the most AREs on its promoter, making it a highly effective therapeutic target for protection against neurodegenerative diseases. It offers protection in part by degrading its pro-oxidant substrate, heme, and generating the antioxidants biliverdin and bilirubin (Doré et al, 1999). The reaction also releases one molecule of iron from the core of the heme porphyrin rings. This iron may then increase the cellular levels of ferritin, which can prevent additional generation of free radicals. Carbon monoxide (CO), another by-product of this reaction, may also have vasodilatory actions along with reported antiapoptotic and antiinflammatory properties at low concentrations; we have recently shown it to offer beneficial effects in transient ischemia (Zeynalov and Doré, 2009).
Increasing evidence suggests that HO1 can be upregulated through the Nrf2/ARE-mediated pathway (Satoh et al, 2006; Zhao et al, 2006). We have shown that Nrf2, a key regulator of the HO1 gene, has an important role in protection against cerebral ischemic stroke (Shah et al, 2007). Considering the beneficial properties of polyphenols and the possible role of the Nrf2/HO1 pathway, in this study we used in vivo and in vitro ischemic paradigms to analyze the protective properties of the flavanol (−)-EC. We hypothesized that EC would provide neuroprotection against brain injury induced by transient middle cerebral artery occlusion (MCAO) or N-methyl-aspartate (NMDA), and that the protection would occur through activation of the Nrf2/HO1 pathway.
Materials and methods
Animals
All animal protocols were approved by the institutional animal care and use committee of Johns Hopkins University. HO1 knockout (HO1−/−) mice were originally generated by Drs Poss and Tonegawa (Poss and Tonegawa, 1997). Mouse genotype was assessed by PCR and additionally confirmed by standard Western blot analysis. Male HO1−/−, Nrf2−/−, and wild-type (WT) mice (20 to 25 g; 7 to 8 weeks old) had access to food and water ad libitum and were housed under controlled conditions (23°C±2°C; 12-h light/dark periods). Animals were provided Teklad Global 18% Protein Rodent Diet (Harlan Holding, Inc., Wilmington, DE, USA), formula 2018S, which is a fixed-formula, autoclavable pellet chow that contains no nitrosamines and a low level of natural phytoestrogens, with 18% protein (non-animal) and 5% fat for consistent growth, gestation, and lactation. All mice were randomly assigned to the different experimental groups.
Gavage Administration of Epicatechin
Epicatechin was administered orally (per kilogram of body weight) by gavage with efforts made to minimize stress to the mice. For pretreatment studies, one dose of EC (2.5, 5, 15, or 30 mg/kg) or distilled water (control) was administered 90 minutes before MCAO; in posttreatment experiments, 30 mg/kg EC or distilled water was administered at 3.5 or 6 hours after MCAO. We selected these doses based on previous findings in which a 30-mg daily dose resulted in 7.3 ng/mg tissue (wet weight) (−)EC and 16.0 ng/mg tissue (wet weight) 3′-O-methyl-(−)EC in brain tissues (Cuevas et al, 2009; van Praag et al, 2007). In addition, a 30-mg dose of EC was observed to be neuroprotective in hippocampal toxicity caused by β-amyloid in rats (Cuevas et al, 2009).
Induction of Transient MCAO and Measurement of Infarct Size, Neurologic Deficits, Blood Gases, and Physiologic Parameters
Mice were subjected to MCAO as described previously (Shah et al, 2006). In brief, mice were anesthetized with halothane (3% initial, 1% to 1.5% maintenance) in O2 and air (80%:20%). Relative cerebral blood flow (CBF) was measured by laser-Doppler flowmetry (DRT4; Moor Instruments Ltd, Devon, UK) through a microfiber affixed to the skull over the area of parietal cortex approximately 6 mm lateral and 1 mm posterior of the bregma. Through a midline incision in the neck, a silicone-coated 7-0 Ethilon nylon filament (Ethicon, Inc., Somerville, NJ, USA) was advanced into the internal carotid artery through the severed external carotid artery to block the blood circulation to the middle cerebral artery, or Circle of Willis. A drop in CBF of ⩾80% was considered to be a successful occlusion. Mice not attaining the required decrease in CBF were excluded from the study. Cortical perfusion values were expressed as a percentage relative to baseline. Mice were moved to a 32°C humidity/temperature-controlled chamber to maintain a body temperature of 37°C during the 90-minute occlusion. With the mice anesthetized, reperfusion was initiated by withdrawing the filament. Mice were returned to the humidity/temperature-controlled chamber for 2 hours before being returned to their respective cages. The stroke was considered successful if no subarachnoid hemorrhage was observed, a lesion was produced, and the mouse survived to the required end point. To evaluate motor deficits, sensorimotor performance was evaluated on a 4-point neurologic deficit severity scale as described previously (Shah et al, 2006). Mice were assessed for neurologic deficits at 24 hours (pretreatment group) or 72 hours (posttreatment group) after occlusion by the following scale: 1, no deficit; 2, forelimb weakness; 3, inability to bear weight on the affected side; and 4, no spontaneous motor activity. After being tested, the mice were anesthetized, and their brains were removed and cut into 2-mm coronal sections, which were stained with 2,3,5-triphenyltetrazolium chloride (TTC; Sigma, St Louis, MO, USA). The sections were scanned individually by a video imaging system and analyzed using image analysis software (SigmaScan pro 4 and 5; Systat, Inc., Point Richmond, CA, USA). Measurements of pH, PaO2, PaCO2, and CBF were made in a separate cohort of mice.
Induction of NMDA-Induced Acute Excitotoxicity and Quantification of the Lesion Volume
Mice were administered a single oral dose of either saline or 30 mg/kg EC 90 minutes before unilateral intrastriatal NMDA injection. After body weight and rectal temperature were recorded, mice were anesthetized and placed on a stereotaxic stand. Then, 15 nmol of NMDA were injected slowly into the right striatum. The injection needle was slowly withdrawn, the hole was blocked with bone wax, and the skin was sutured. On recovery from anesthesia in a thermoregulated chamber, mice were transferred to their home cages. Throughout the experimental procedure, the rectal temperature of each mouse was monitored and maintained at 37.0°C±0.5°C. After 48 hours, mice were deeply anesthetized and transcardially perfused and fixed. Brains were equilibrated in 30% sucrose, frozen, and cut sequentially into 25-μm sections on a cryostat. The sections were stained with Cresyl Violet to estimate the lesion volume (Ahmad et al, 2006).
Neuronal Cell Cultures, Cell Survival, and Caspase Assays
Embryonic cortical neuronal cells were isolated from 17-day embryos of timed pregnant mice, and postnatal cortical neurons were obtained from 1- to 2-day-old mice, as previously detailed (Shah et al, 2007). Neurons (5 × 105 cells/well) were plated in serum-free Neurobasal medium supplemented with 1 mmol/L Glutamax (Invitrogen, Carlsbad, CA, USA) and B27 supplement onto 24-well plates coated with poly--lysine. Cells were maintained at 37°C in a 95% air and 5% CO2 humidified atmosphere. All experiments were performed after 14 days in vitro.
Neuronal survival was assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay, as described (Shah et al, 2007). Cell viability was also measured by the lactate dehydrogenase (LDH) assay, which assesses the membrane integrity of cells. After experimental treatment, the culture medium was collected and mixed with substrate, enzyme, and dye solution. After 30 minutes of incubation in the dark, the reaction was terminated by adding 1:10 volume of 1 N hydrochloric acid. Absorbance was measured at 490 nm.
The caspase 3/7 activity of neuronal samples was measured according to the manufacturer's instructions (Promega, Madison, WI, USA). Homogeneous caspase reagent was added to the cells, which were then incubated at room temperature for 18 hours in the dark. The fluorescence of each sample was measured at excitation and emission wavelengths of 485 and 530 nm, respectively. Experiments were repeated with at least three separate batches of cultures.
Western Blot Analysis
Cytosolic and nuclear fractions were isolated from the cortical neurons as described previously (Shah et al, 2007). Protein concentration was determined by a BCA kit (Pierce, Rockford, IL, USA). Equivalent amounts of protein per sample were resolved through sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% gels. The proteins were electrophoretically transferred to a nitrocellulose membrane, which was blocked for 1 hour at 22°C with 5% dried milk and then exposed to the primary antibody overnight at 4°C and to the secondary antibody in 5% dried milk for 1 hour at 22°C. Immunocomplexes were visualized by enhanced chemiluminescence detection (ECL; Amersham, Piscataway, NJ, USA). Each Western blot shown is a representative of at least three separate experiments.
Immunocytofluorescence Staining
After being treated with EC, neurons were permeabilized for 2 minutes with 0.5% Triton X-100 and then fixed with 3% paraformaldehyde for 20 minutes. Cells were first incubated with normal goat serum to block nonspecific binding and then with primary antibodies to NeuN (a neuronal marker; Chemicon, Temecula, CA, USA) or Nrf2 (Sigma) for 30 minutes. Cells were washed and then incubated with rhodamine-conjugated, affinity-purified donkey anti-rat IgG (H+L) and fluorescein isothiocyanate (FITC)-conjugated, affinity-purified goat anti-rabbit IgG (H+L) (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) secondary antibodies for 30 minutes. Images were captured with a CoolSNAP HQ camera (Image Processing Solutions, Inc., North Reading, MA, USA) using OpenLab software (Improvision Inc., Boston, MA, USA).
Statistical Analysis
Data, expressed as mean±s.e.m., were analyzed using Student's t-test, analysis of variance (ANOVA), or Newman–Keuls multiple range test. Neurologic deficit scores were analyzed using the nonparametric Kruskal–Wallis analysis of ranks and are presented as medians with interquartile ranges (25th and 75th percentiles). Statistical significance was set at P<0.05.
Results
Protective Effect of Epicatechin Pretreatment in Transient MCAO
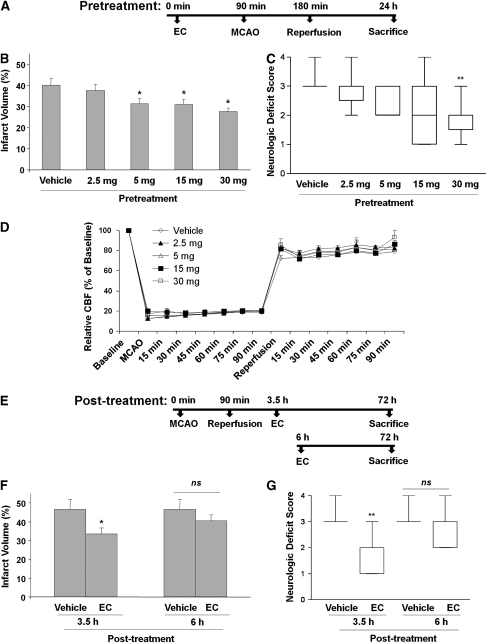
Pretreatment of WT mice with EC 90 minutes before MCAO significantly and dose-dependently protected against neurologic deficit and brain injury. Infarct volumes of mice pretreated with EC were significantly smaller than those of vehicle-treated mice. Infarct volumes were 40.0%±3.2% (n=10) for the vehicle-treated group, 31.3%±2.6% (n=10; P<0.05) for the 5 mg/kg group, 30.9%±2.3% (n=9; P<0.04) for the 15 mg/kg group, and 27.6%±1.9% (n=11; P<0.002) for the 30 mg/kg group. However, there was no significant difference in the infarct volume of the 2.5 mg/kg group (37.6%±3.2% n=12; Figure 1B). Similarly, NDS measured at 24 hours decreased with increasing concentration of EC. Vehicle-treated mice had a mean NDS of 3.1±0.1, whereas the NDS of mice that received 2.5, 5, 15, and 30 mg/kg averaged 2.8±0.2, 2.4±0.2, 2.2±0.4, and 1.8±0.2, respectively (Figure 1C). When the data were analyzed using the nonparametric Kruskal–Wallis analysis of ranks, we observed significant improvement of NDS only with 30 mg/kg pretreatment.
Figure 1.
(−)-Epicatechin (EC) is protective against transient middle cerebral artery occlusion (MCAO)-induced cerebral damage in wild-type (WT) mice. (A) A schematic diagram showing the pretreatment protocol of EC administration (90 minutes before MCAO) in WT mice. (B–D) EC was administered 90 minutes before 90-minute MCAO and 24 hours of reperfusion. (B) Infarct volumes were significantly smaller than those of vehicle-treated (control; n=10) mice at EC doses of 30 (n=10), 15 (n=9), and 5 (n=10), but not 2.5 mg/kg (n=12). (C) Neurologic deficit at 24 hours was significantly less severe in the mice that were administered 30 mg/kg EC than in the vehicle-treated mice. (D) No significant differences in cerebral blood flow (CBF), as monitored by laser-Doppler flowmetry, were observed among the mouse groups receiving vehicle or EC (n=5/group). (E) A schematic diagram showing the posttreatment protocol of EC administration (3.5 and 6 hours after MCAO) in WT mice. (F) WT mice were subjected to 90 minutes of MCAO, and EC (30 mg/kg) was administered orally after 3.5 hours (n=10) or 6 hours (n=8). Quantification showed that mice posttreated with EC at 3.5 hours had significantly smaller infarct volumes than did vehicle-treated mice (n=10), but no protection was observed in mice posttreated at 6 hours. (G) After 72 hours of reperfusion, neurologic deficit scores of 3.5-hour posttreated mice, but not of 6-hour posttreated mice, were significantly smaller than those of the vehicle-treated mice. Data represent means ± s.e.m.; *P<0.05; **P<0.01; NS, not significant.
Physiologic Parameters and Cerebral Blood Flow
In a separate cohort of mice also pretreated with vehicle or EC (2.5, 5, 15, and 30 mg/kg; n=5 per group), no differences in blood pH, PaO2, or PaCO2 were observed between any of the groups before, during, or after MCAO (data not shown). In this same group of mice, relative CBF was measured continuously from 30 minutes before occlusion through 1 hour of reperfusion. No significant differences were noted between the vehicle- and EC-treated groups (Figure 1D).
Protective Effect of Epicatechin Posttreatment in Transient MCAO
In mice posttreated with 30 mg/kg EC, no mortality was observed, but three mice in the vehicle-treated group died after 48 hours. The cause of the deaths appeared to be severe edema, and not a direct result of the surgical procedure. Mice that were posttreated with EC 3.5 hours after MCAO had significantly smaller infarct volumes (33.5%±3.0% n=10) at 72 hours than did the vehicle-treated group (46.6%±5.0% n=10; P<0.04; Figure 1F). Similarly, there was a significant difference in the NDS between EC-treated (2.4±0.3) and vehicle-treated (3.3±0.1) groups (P<0.05; Figure 1G). However, posttreatment at 6 hours after MCAO provided no significant protection in terms of infarct size or NDS (n=10 (vehicle) and 8 (30 mg/kg); Figures 1F and 1G).
Protective Effect of Epicatechin Pretreatment in NMDA-Induced Acute Excitotoxicity
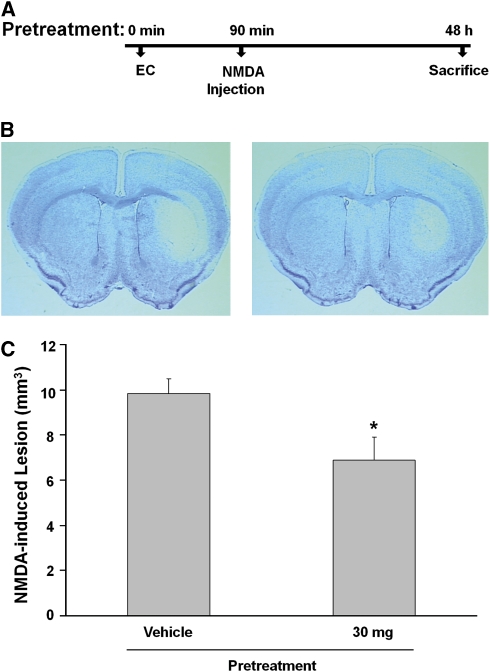
Unilateral injection with 15 nmol of NMDA produced brain damage in the mouse ipsilateral striatum (Figure 2B, left) that was significantly attenuated by pretreatment with 30 mg/kg EC (Figure 2B, right). At 48 hours, lesion volume of the EC-treated group (6.8%±1.0% n=12) was significantly smaller than that of the vehicle-treated group (9.8%±0.6% n=12; P<0.001; Figure 2C).
Figure 2.
Epicatechin (EC) treatment reduces N-methyl-aspartate (NMDA)-induced acute excitotoxicity in mice. (A) A schematic diagram showing the pretreatment protocol of EC administration in wild-type (WT) mice injected with NMDA. (B, C) Mice were pretreated with either vehicle or 30 mg/kg EC (n=12/group) before being administered a unilateral intrastriatal injection of 15 nmol NMDA. At 48 hours after NMDA injection, mice were killed and brains were processed for lesion volume analysis. (B) Representative photographs of Cresyl Violet-stained brain sections of mice that were pretreated with vehicle (left) or EC (right) followed by a unilateral intrastriatal NMDA injection. (C) Bar graph showing the volume of brain damage induced by NMDA. The brain lesion volume was attenuated in the EC-treated group compared with the vehicle-treated group. Values are reported as means ± s.e.m. *P<0.03 versus vehicle-treated group.
Neuroprotection of Epicatechin Against Oxidative Stress in Neuronal Cultures
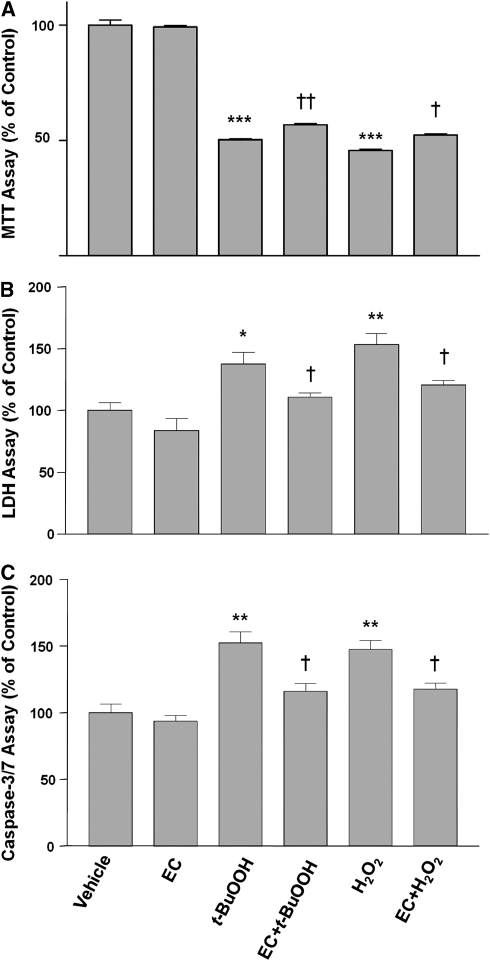
To study the EC-mediated neuroprotection in vitro, we used H2O2 and tert-butyl hydroperoxide (t-BuOOH) to simulate oxidative stresses that occur during ischemia–reperfusion injury in vivo. The oxidative species generated by H2O2 are hydroxyl radicals, whereas those generated by t-BuOOH are alkoxy or peroxyl radicals (Barr et al, 1996). Therefore, we compared the two kinds of oxidative stressors here to substantiate the protective ability of EC. On the basis of the results of the MTT cell survival assay, application of t-BuOOH (60 μmol/L) or H2O2 (60 μmol/L) to primary neuronal cultures significantly decreased neuronal viability after 24 hours compared with that of control neurons (Figure 3A). This decrease was abrogated by 100 μmol/L EC. EC alone had no significant effect on neuronal viability. Similar results were observed with LDH cytotoxicity assays (Figure 3B). To further substantiate the protection observed by EC treatment, we examined the activity of caspase-3/7, described as a terminal effector of the apoptotic-like cell death pathway. We found that t-BuOOH and H2O2 each induced a significant increase in caspase-3/7 activity (Figure 3C). EC had no effect on basal levels of caspase-3/7 activity but was able to prevent the increase evoked by both stressors (Figure 3C).
Figure 3.
Neuronal protection by epicatechin (EC). Neurons were grown for 24 hours in culture medium alone (vehicle) or in the presence of tert-butyl hydroperoxide (t-BuOOH; 60 μmol/L) or H2O2 (60 μmol/L) with or without EC (100 μmol/L). (A) Neuronal viability was assessed by the MTT assay; the absorbance at 570 nm is shown. (B) Neuronal cell death was assessed by the level of lactate dehydrogenase (LDH) released into the cell culture medium; the absorbance at 490 nm is shown. (C) Caspase-3/7 activity was determined by the amount of fluorescent substrate formed and is shown as percentage of control. *P<0.05, **P<0.01, ***P<0.001 versus vehicle control; †P<0.05 versus the corresponding t-BuOOH or H2O2 control. Values shown are the mean±s.e.m. of three batches of cells.
Induction of HO1 Expression and Nrf2 Translocation by Epicatechin in Neuronal Cultures
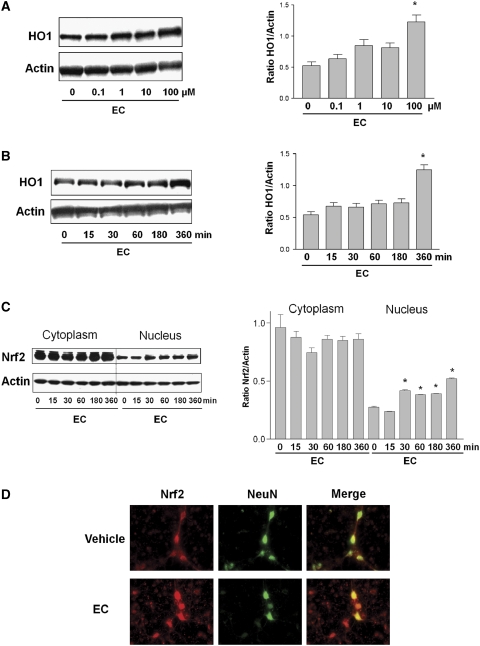
To analyze whether EC can induce HO1 protein expression, primary neuronal cultures were exposed to increasing concentrations of EC (0.1 to 100 μmol/L). Western blot analysis showed that EC increased HO1 protein expression in a dose-dependent manner (Figure 4A). Even at the highest concentration tested, 100 μmol/L, EC did not affect cell survival. Therefore, we used 100 μmol/L EC for the remainder of the in vitro study.
Figure 4.
Effects of epicatechin (EC) on heme oxygenase 1 (HO1) and Nrf2 protein expression and nuclear accumulation in cortical neurons. (A, B, left panels) Western blots showing HO1 and actin expression in (A) cortical neurons treated for 6 hours with Neurobasal medium alone or with serial concentrations of EC and (B) cortical neurons treated with 100 μmol/L EC for 15 minutes to 6 hours. The expression of actin (used as a loading control) was unaffected. (A, B, right panels) The bar graphs show the ratio of density captured from the HO1 bands to that of actin. Values shown are means±s.e.m. for three independent blots. (C, left panel) The effect of EC on primary cortical neurons incubated for the times shown (minutes) with serum-free B27 minus antioxidant-supplemented medium alone or that containing EC (100 μmol/L). Nuclear and cytoplasmic samples were analyzed by Western blot with antibodies to Nrf2 and actin. The actin expression level was unchanged. (C, right panel) The bar graph shows the ratio of density captured from the Nrf2 band to that of actin. *P<0.05 versus control. (D) Cortical neurons incubated under basal conditions or treated with EC for 30 minutes were stained with antibodies to Nrf2 (red) and neuronal marker NeuN (green). The merged image shows colocalization, depicting the presence of Nrf2 in the nucleus.
The protein expression of HO1 also increased with increased exposure time to EC (Figure 4B). If the transcriptional factor Nrf2 is partially responsible for the upregulation of HO1 by EC, one would expect Nrf2 to accumulate in the nucleus of cells as a result of EC exposure. To analyze whether this occurs, primary cultured cortical neurons were exposed to EC (100 μmol/L), and cytoplasmic and nuclear fractions were analyzed by Western blot. The presence of Nrf2 in the nuclear fraction increased in a time-dependent manner. It increased rapidly in the first 30 minutes and remained elevated throughout the full 6-hour time course (Figure 4C). The protein samples were also probed with Lamin B (a nuclear marker) to verify the enrichment of the nuclear extracts. On Western blots, bands were clearly observed in the nuclear extract but were undetectable in the cytoplasmic extract (data not shown).
Next, we used indirect immunofluorescence microscopy to show that EC regulates Nrf2 distribution in neurons. In the control group, Nrf2 (red fluorescence) was detected in the neuronal cell body and dendritic trees and axons (Figure 4D). The nuclear marker 4,6-diamidino-2-phenylindole (DAPI; blue fluorescence) was used to determine the nuclear area (data not shown). The neuronal marker NeuN (green fluorescence) was detected mainly in soma and to a lesser extent in the axon–dendritic compartment. The merged image revealed colocalization of Nrf2 with NeuN. After the neurons had been exposed to EC for 30 minutes, Nrf2 appeared to be concentrated within the nucleus, with less signal remaining in the cytoplasm or axon–dendritic compartments.
HO1 Is at Least Partially Required for the Neuroprotective Effects of Epicatechin In Vivo and In Vitro
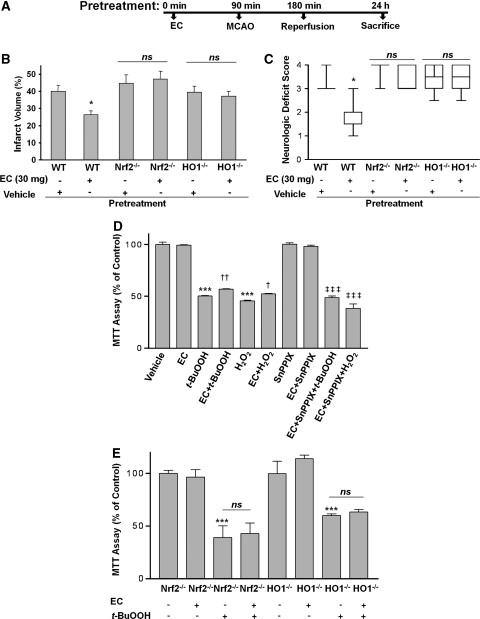
To test whether HO1 might mediate some of the protection associated with EC, EC was administered to HO1−/− mice to determine whether protection against transient ischemia-induced brain injury was still apparent. HO1−/− mice pretreated with 30 mg/kg EC 90 minutes before MCAO developed infarcts (37.1%±3.0% n=7) that were no different in size from those of mice pretreated with vehicle (39.5%±3.3% n=7; Figure 5B). Furthermore, NDS were not significantly different between the vehicle- and drug-treated mice (Figure 5C). These findings suggest that the protective effect of EC is dependent on the presence of HO1 protein.
Figure 5.
The protective effect of epicatechin (EC) is lost in Nrf2−/− and HO1−/− mice. (A) A schematic diagram showing the pretreatment protocol of EC administration (90 minutes before middle cerebral artery occlusion (MCAO)) in Nrf2−/− and HO1−/− mice. (B, C) Nrf2−/− and HO1−/− mice and their wild-type (WT) littermates were treated with vehicle (n=10 WT, 7 HO1−/−, and 8 Nrf2−/−) or 30 mg/kg EC (n=11 WT, 7 HO1−/−, and 8 Nrf2−/−) 90 minutes before MCAO. At 24 hours after reperfusion, mice were tested for neurologic deficits and killed; their brains were collected and sectioned for TTC staining (note that the values for the WT mice are those from Figure 1 and are shown again here for comparison). (B) Quantification analysis revealed that the infarct volumes of EC-treated WT mice were significantly smaller than those of vehicle-treated WT mice. The infarct volumes of EC-treated Nrf2−/− and HO1−/− mice were not significantly different from those of their vehicle-treated counterparts, but were significantly higher than those of the EC-treated WT mice. (C) Similarly, the neurologic deficit scores of EC-treated Nrf2−/− and HO1−/− mice at 24 hours after MCAO were significantly higher than those of the EC-treated WT mice and not significantly different from those of their vehicle-treated counterparts. *P<0.05 compared with EC-treated WT controls; NS, not significant. (D) Neurons were grown for 24 hours in serum-free medium with B27 supplement minus antioxidant in the presence and absence of tert-butyl hydroperoxide (t-BuOOH; 60 μmol/L) or H2O2 (60 μmol/L), with or without EC (100 μmol/L), SnPPIX (10 μmol/L), or combinations thereof. Cell viability was assessed with the MTT assay. ***P<0.001 versus vehicle; †P<0.05, ††P<0.01 versus t-BuOOH or H2O2; ‡‡‡P<0.001 versus t-BuOOH+EC or H2O2+EC. Values shown are the means ± s.e.m. of three batches of cells. (E) Primary postnatal cortical neurons isolated from HO1−/− or Nrf2−/− mice were incubated for 24 hours in Neurobasal medium alone (control) or in the presence or absence of t-BuOOH (60 μmol/L), EC (100 μmol/L), or combinations thereof. Cell viability was assessed with the MTT assay. ***P<0.001 versus control; NS, not significantly different versus t-BuOOH.
To further support the concept that HO1 is needed for EC protection of neuronal cells during oxidative stress caused by H2O2 or t-BuOOH, we compared the protective effects of EC on postnatal neurons isolated from WT and HO1−/− mice. We found that t-BuOOH (60 μmol/L) significantly increased neuronal cell death in neurons from both WT and HO1−/− mice (Figures 5C and 5D). Importantly, EC significantly reduced the neuronal cell death induced by H2O2 and t-BuOOH (Figure 5D) in WT neurons but did not protect the HO1−/− neurons (Figure 5E). The heme analog/HO-specific inhibitor SnPPIX was used to verify that HO activity contributes to the neuronal survival mechanisms. Under our experimental conditions, SnPPIX (10 μmol/L) had no effect on baseline neuronal viability but was able to significantly block the protection provided by EC against t-BuOOH- and H2O2-induced neuronal cell death (Figure 5D). Similar findings were observed in LDH assays and caspase activity measurements (data not shown). Overall, these data indicate that EC contributes to neuronal cell protection against oxidative stress from reactive oxygen species through a pathway that includes HO1 induction.
Nrf2 Is Required for the Neuroprotective Effect of Epicatechin In Vivo and In Vitro
To better elucidate the mechanism by which EC effects neuroprotection and further substantiate the pathway of HO1 regulation, we investigated the events upstream. Evidence in the literature suggests that HO1 is upregulated through the Nrf2 cascade (Satoh et al, 2006; Shih et al, 2005). To determine whether EC works through this interdependent pathway, we pretreated Nrf2−/− mice with vehicle or 30 mg/kg EC and then subjected them to the 90-minute MCAO protocol. After 24 hours of reperfusion, mice were evaluated for NDS and then killed. Nrf2−/− mice pretreated with 30 mg/kg EC 90 minutes before MCAO developed infarcts (40.1%±4.5% n=8) that were no different in size from those of mice pretreated with vehicle (44.9±4.9% n=8; Figure 5B). Similarly, NDS in the EC-treated Nrf2−/− mice were not significantly different from those of the vehicle-treated Nrf2−/− group (Figure 5C). Furthermore, the rescue effect of EC against neuronal cell death induced by t-BuOOH was significantly abolished in the Nrf2−/− neuronal cultures (Figure 5E). These data indicate that Nrf2 is important in the EC-associated neuroprotection.
Discussion
In this study, we tested the hypothesis that EC is neuroprotective against brain injury induced by transient MCAO or NMDA through the upregulation of HO1 and Nrf2. We found that EC protects the brain from transient ischemia-induced injury when administered 90 minutes before or 3.5 hours after MCAO, a finding that correlated with significant functional recovery after the onset of ischemia. The acute excitotoxicity induced by NMDA was also attenuated in mice pretreated with EC. However, treatment of HO1−/− and Nrf2−/− mice with EC failed to protect against the damage induced by brain ischemia. Our in vitro assays showed that EC significantly increased HO1 expression at 6 hours and Nrf2 translocation at 30 minutes, changes that were found to be essential for the EC-mediated neuronal protection pathway. These results suggest that EC regulates Nrf2, thereby enhancing the protective defense mechanisms through the HO1 neuroprotective pathway.
Several studies have revealed that a therapeutic window of approximately 6 hours exists between the onset of ischemia and irreversible neuronal death (Williams et al, 2004, 2003; Xu et al, 2006). Therefore, it is desirable that neuroprotective interventions should be attempted before a stroke occurs or very soon afterward. Interventions that have the potential to reverse the cascades of injury should target mechanisms underlying delayed ischemia-induced neuronal injury, especially in the penumbra region. We followed the guidelines set by the Stroke Therapy Academic Industry Roundtable (STAIR) for stroke preclinical studies (Stroke Therapy Academic Industry Roundtable, 2001), which include assessing functional outcomes, therapeutic window, and long-term effects. We found that EC significantly and dose-dependently improved the outcome after MCAO when administered before ischemia or 3.5 hours after ischemia, but 30 mg/kg administered 6 hours after MCAO offered no significant protection, suggesting that acute posttreatment is required for significant therapeutic efficacy. Another criterion of STAIR is that the drug must cross the blood–brain barrier. Available evidence indicates that EC does cross the blood–brain barrier (Abd El Mohsen et al, 2002), as epicatechin glucuronide and 3′-O-methyl epicatechin glucuronide have been observed in rat brain for up to 10 days after oral administration of EC (van Praag et al, 2007). Furthermore, after transient ischemia, the blood–brain barrier is likely to be more permeable, especially in the surrounding ischemic region. Body weight and mortality rates are also important parameters to be considered in animal models when testing neuroprotective agents in ischemia. Compared with controls, we found higher survival rates in groups pre- and post-treated with EC, but no differences were observed in body weights (data not shown). We did not observe any differences in CBF at the time of ischemia and reperfusion, but this absence of effect might be because only a single dose of EC was used. In a small clinical study, participants who received 46 mg of EC daily for over a period of 2 weeks showed endothelium-dependant vasodilation (Engler et al, 2004). It is possible that chronic treatment rather than a single dose is required to observe differences in CBF.
The literature provides evidence that EC is protective in many disease states (Natsume et al, 2004). For example, epidemiologic data have shown that in Kuna Indians, chronic consumption of an EC-rich cocoa drink has cardiovascular benefits that result from augmented levels of nitric oxide (Schroeter et al, 2006). In addition, it has been reported that EC consumption enhances cognition and spatial memory by increasing angiogenesis and neuronal spine density in the dentate gyrus of the hippocampus (van Praag et al, 2007). Our current results reveal that HO1 is at least partially required for EC activity. A number of cerebral ischemia studies indicate that neuroprotective agents exert an effect through the Nrf2 detoxifying pathway (Satoh et al, 2006; Shih et al, 2005; Zhao et al, 2006). This pathway has also been suggested to be at least partially mediated through HO1 induction by electrophiles (Gong et al, 2002; Itoh et al, 2004; Loboda et al, 2008). Our finding that Nrf2−/− mice were not protected against ischemic damage provides additional evidence that the protective effect of EC is mediated by activating the Nrf2/HO1 pathway. The data support our previous study, in which we showed that HO1 protects against NMDA-induced acute brain damage in mice (Ahmad et al, 2006). In addition, HO proteins likely have intrinsic cytoprotective properties (Hori et al, 2002; Kim and Doré, 2005). HO1 catalyzes the cleavage of heme (a pro-oxidant) to form iron (which can then increase ferritin levels), carbon monoxide (a vasodilator and anti-apoptotic agent), and biliverdin, which is rapidly reduced to bilirubin (an antioxidant) (Doré, 2002). Increased synthesis of HO1 leads to the degradation of heme molecules, producing the potent antioxidants biliverdin and bilirubin that are most likely responsible for the neuroprotective actions of HO1, and, therefore, of EC.
In neuronal viability assays, EC reduced neuronal cell death triggered by t-BuOOH or H2O2. The fact that EC could inhibit the increase in caspase 3 induced by t-BuOOH and H2O2 also shows that EC may have the ability to rescue cells from programmed cell death. These in vitro data support our in vivo results and show that EC promotes antioxidative and perhaps anti-cell-death activity. The antiapoptotic-like ability of EC has not yet been fully investigated, but Heo and Lee (2005) showed that EC and catechin both inhibit β-amyloid (Aβ25−35)-induced apoptosis in PC12 cells, and Cuevas et al (2009) recently showed that a 30-mg dose of EC limited the hippocampal toxicity caused by Aβ25−35 in rats.
In conclusion, we observed that the flavanol EC dose-dependently protected transient ischemia-induced brain injury in both pre- and post-treatment paradigms. The protective mechanism is likely mediated at least in part by the Nrf2/HO1 pathway. These results provide a better understanding of the mechanism by which dietary flavanols can be beneficial and may help promote the design of improved neuroprotective agents for use in stroke.
Acknowledgments
The authors thank Claire Levine for her assistance in the preparation of the paper and all Doré lab members for their insightful contributions.
The authors declare no conflict of interests.
References
- Abd El Mohsen MM, Kuhnle G, Rechner AR, Schroeter H, Rose S, Jenner P, Rice-Evans CA. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic Biol Med. 2002;33:1693–1702. doi: 10.1016/s0891-5849(02)01137-1. [DOI] [PubMed] [Google Scholar]
- Ahmad AS, Zhuang H, Doré S. Heme oxygenase-1 protects brain from acute excitotoxicity. Neuroscience. 2006;141:1703–1708. doi: 10.1016/j.neuroscience.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Barr DP, Gunther MR, Deterding LJ, Tomer KB, Mason RP. ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. J Biol Chem. 1996;271:15498–15503. doi: 10.1074/jbc.271.26.15498. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Limon D, Perez-Severiano F, Diaz A, Ortega L, Zenteno E, Guevara J. Antioxidant effects of epicatechin on the hippocampal toxicity caused by amyloid-beta 25-35 in rats. Eur J Pharmacol. 2009;616:122–127. doi: 10.1016/j.ejphar.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Doré S. Decreased activity of the antioxidant heme oxygenase enzyme: implications in ischemia and in Alzheimer's disease. Free Radic Biol Med. 2002;32:1276–1282. doi: 10.1016/s0891-5849(02)00805-5. [DOI] [PubMed] [Google Scholar]
- Doré S, Takahashi M, Ferris CD, Hester LD, Guastella D, Snyder SH. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc Natl Acad Sci USA. 1999;96:2445–2450. doi: 10.1073/pnas.96.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J, Perez Vizcaino F, Utrilla P, Jimenez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- Engler MB, Engler MM, Chen CY, Malloy MJ, Browne A, Chiu EY, Kwak HK, Milbury P, Paul SM, Blumberg J, Mietus-Snyder ML. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- Gong P, Stewart D, Hu B, Li N, Cook J, Nel A, Alam J. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Delta(12,14)-prostaglandin J2 is mediated by the stress response elements and transcription factor Nrf2. Antioxid Redox Signal. 2002;4:249–257. doi: 10.1089/152308602753666307. [DOI] [PubMed] [Google Scholar]
- Heo HJ, Lee CY. Epicatechin and catechin in cocoa inhibit amyloid beta protein induced apoptosis. J Agric Food Chem. 2005;53:1445–1448. doi: 10.1021/jf048989m. [DOI] [PubMed] [Google Scholar]
- Hori R, Kashiba M, Toma T, Yachie A, Goda N, Makino N, Soejima A, Nagasawa T, Nakabayashi K, Suematsu M. Gene transfection of H25A mutant heme oxygenase-1 protects cells against hydroperoxide-induced cytotoxicity. J Biol Chem. 2002;277:10712–10718. doi: 10.1074/jbc.M107749200. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin J2. Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr. 2000;130:2105S–2108S. doi: 10.1093/jn/130.8.2105S. [DOI] [PubMed] [Google Scholar]
- Kim YS, Doré S. Catalytically inactive heme oxygenase-2 mutant is cytoprotective. Free Radic Biol Med. 2005;39:558–564. doi: 10.1016/j.freeradbiomed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res. 2004;77:892–900. doi: 10.1002/jnr.20193. [DOI] [PubMed] [Google Scholar]
- Lee S, Suh S, Kim S. Protective effects of the green tea polyphenol (−)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- Natsume M, Osakabe N, Yasuda A, Baba S, Tokunaga T, Kondo K, Osawa T, Terao J. In vitro antioxidative activity of (−)-epicatechin glucuronide metabolites present in human and rat plasma. Free Radic Res. 2004;38:1341–1348. doi: 10.1080/10715760400022087. [DOI] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Okamoto SI, Cui J, Watanabe Y, Furuta K, Suzuki M, Tohyama K, Lipton SA. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic [correction of electrophillic] phase II inducers. Proc Natl Acad Sci USA. 2006;103:768–773. doi: 10.1073/pnas.0505723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Gilani RA, Sharma P, Vohora SB. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol. 2005;101:299–307. doi: 10.1016/j.jep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S, Doré S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience. 2007;147:53–59. doi: 10.1016/j.neuroscience.2007.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Doré S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–138. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci. 2005;25:10321–10335. doi: 10.1523/JNEUROSCI.4014-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DH, Bae YC, Kim-Han JS, Lee JH, Choi IY, Son KH, Kang SS, Kim WK, Han BH. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J Neurochem. 2006;96:561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable Recommendations for clinical trial evaluation of acute stroke therapies. Stroke. 2001;32:1598–1606. doi: 10.1161/01.str.32.7.1598. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, Berti R, Dave JR, Elliot PJ, Adams J, Tortella FC. Delayed treatment of ischemia/reperfusion brain injury: extended therapeutic window with the proteosome inhibitor MLN519. Stroke. 2004;35:1186–1191. doi: 10.1161/01.STR.0000125721.10606.dc. [DOI] [PubMed] [Google Scholar]
- Williams AJ, Hale SL, Moffett JR, Dave JR, Elliott PJ, Adams J, Tortella FC. Delayed treatment with MLN519 reduces infarction and associated neurologic deficit caused by focal ischemic brain injury in rats via antiinflammatory mechanisms involving nuclear factor-kappaB activation, gliosis, and leukocyte infiltration. J Cereb Blood Flow Metab. 2003;23:75–87. doi: 10.1097/01.WCB.0000039285.37737.C2. [DOI] [PubMed] [Google Scholar]
- Xu Z, Croslan DR, Harris AE, Ford GD, Ford BD. Extended therapeutic window and functional recovery after intraarterial administration of neuregulin-1 after focal ischemic stroke. J Cereb Blood Flow Metab. 2006;26:527–535. doi: 10.1038/sj.jcbfm.9600212. [DOI] [PubMed] [Google Scholar]
- Zeynalov E, Doré S. Low doses of carbon monoxide protect against experimental focal brain ischemia. Neurotox Res. 2009;15:133–137. doi: 10.1007/s12640-009-9014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Kobori N, Aronowski J, Dash PK. Sulforaphane reduces infarct volume following focal cerebral ischemia in rodents. Neurosci Lett. 2006;393:108–112. doi: 10.1016/j.neulet.2005.09.065. [DOI] [PubMed] [Google Scholar]