Abstract
The FMR1 gene partially appears to control ovarian reserve, with a specific ovarian sub-genotype statistically associated with a polycystic ovary (PCO)- like phenotype. Some forms of PCO have been associated with autoimmunity. We, therefore, investigated in multiple regression analyses associations of ovary-specific FMR1 genotypes with autoimmunity and pregnancy chances (with in vitro fertilization, IVF) in 339 consecutive infertile women (455 IVF cycles), 75 with PCO-like phenotype, adjusted for age, race/ethnicity, medication dosage and number of oocytes retrieved. Patients included 183 (54.0%) with normal (norm) and 156 (46%) with heterozygous (het) FMR1 genotypes; 133 (39.2%) demonstrated laboratory evidence of autoimmunity: 51.1% of het-norm/low, 38.3% of norm and 24.2% het-norm/high genotype and sub-genotypes demonstrated autoimmunity (p = 0.003). Prevalence of autoimmunity increased further in PCO-like phenotype patients with het-norm/low genotype (83.3%), remained unchanged with norm (34.0%) and decreased in het-norm/high women (10.0%; P<0.0001). Pregnancy rates were significantly higher with norm (38.6%) than het-norm/low (22.2%, p = 0.001). FMR1 sub-genotype het-norm/low is strongly associated with autoimmunity and decreased pregnancy chances in IVF, reaffirming the importance of the distal long arm of the X chromosome (FMR1 maps at Xq27.3) for autoimmunity, ovarian function and, likely, pregnancy chance with IVF.
Introduction
Recent publications reported associations between number of triple CGG nucleotide repeats on the fragile X mental retardation 1 (FMR1) gene and risk towards premature ovarian senescence [1]–[8], leading in milder cases to so-called premature ovarian aging (POA) [2], [9], also called occult primary ovarian insufficiency (OPOI) [10], and at end stage to premature ovarian failure (POF), also called primary ovarian insufficiency (POI) [10]. We recently reported evidence that different FMR1 genotypes vary in rate of follicle recruitment and, therefore, at least partially, affect functional ovarian reserve, as assessed by anti-Müllerian hormone (AMH) [8].
A normal triple nucleotide (CGG) count range of 26 to 34 repeats (median 30), in respect to ovarian function, allows definition of distinct FMR1 genotypes, depending on whether both (normal), only one (heterozygous) or neither (homozygous) allele is in normal range [8], [11]. In a small pilot study a heterozygous-normal/low (het-norm/low) sub-genotype appeared associated with a lean polycystic ovary (PCO)-like phenotype with rapidly depleting ovarian reserve [12].
A PCO-like phenotype is integral to all definitions of the polycystic ovary syndrome (PCOS) [13]. The phenotype, however, solely denotes excessive follicle activity (reflected in high AMH) and, consequently, hyperactivity of ovarian function. PCO and PCOS, therefore, have distinctively different connotations.
Some authors have speculated about a possible autoimmune etiology for selected forms of PCOS [14]–[16]. Others implied such an association when histological demonstrating autoimmune oophoritis with polycystic aspects, accompanied by anti-ovarian antibodies [17]–[19].
At the other end of ovarian function, autoimmunity has been for decades implicated in POF/POI [20]. A very specific phenotype, characterized by a preserved pool of functional follicles, has recently been associated with steroidogenic cell autoimmunity [21]. Hypo-activity is, thus, well documented in association with autoimmunity, while hyperactivity of ovaries is not.
Considering the substantial evidence in support of autoimmune-associated suppression of ovarian function, we have speculated about autoimmune-induced ovarian stimulation in PCOS patients [22], which would mimic the independent duality of, for example, thyroid autoimmunity and function, both etiologically linked, interacting, yet relatively independent of each other [23].
Initial efforts at our center to demonstrate evidence for autoimmune activity in association with PCOS remained unsuccessful (Weghofer A, Gleicher N, unpublished data). We attributed our failure to phenotypical and etiologic variabilities of PCOS, as defined by current Rotterdam criteria [13]. Identifying above noted PCO-like phenotype with close association to the het-norm/low FMR1 sub-genotype offered, however, a unique opportunity to study a clinically homogenous PCO-like patient population. While widely suspected, close genetic markers of PCOS are still lacking [24].
This study confirms close associations between FMR1 genotypes and ovarian function but for the first time also associates FMR1 genotypes in infertile women with risk towards autoimmunity and with pregnancy chances in association with in vitro fertilization (IVF).
Methods
This study involved the retrospective review of medical records. All data utilized were extracted from medical charts and the Center's centralized, confidential electronic research data bank. Patients, at time of initial consultation, sign an informed consent, which permits such utilization of medical records for research purposes as long as the patients' identities remain undisclosed, medical records are untraceable and remain confidential. The Center's Institutional Review Board (IRB) allows such studies under expedited review.
Patients/cycles
We investigated 339 consecutive female infertility patients who presented to our center for initial diagnostic evaluation, which routinely includes limited genetic and immunologic testing. Amongst those, 75 women qualified as PCO-like phenotypes, based on large numbers of oocytes retrieved (≥12) in first IVF cycles, and/or high anti-Müllerian hormone (AMH, >4.0 ng/mL) at initial evaluations. These cut off values were chosen in consideration of the high prevalence of diminished ovarian reserve in our patient population [9] (see also ovarian reserve tests in Table 1).
Table 1. Patient characteristics*.
| Ovarian Phenotype | |||
| Normal | PCO-like | P-Value | |
| Number | 264 | 75 | |
| Age (years) | 38.8±4.8 | 34.8±4.3 | <0.0001 |
| FSH (mIU/MI, 95% CI) | 10.3 (9.72–11.03) | 7.35 (6.67–8.08) | <0.0001 |
| AMH (ng/mL, 95% CI) | 0.55 (0.48–0.62) | 2.21 (1.76–2.77) | <0.0001 |
| BMI | 22.7±13.5 | 23.3±13.2 | 0.74 (N.S.) |
| Oocytes retrieved (n) | 4.8±3.3 | 17.5±6.7 | <0.0001 |
| Embryos transferred (n) | 2.4±1.1 | 2.4±0.8 | 0.88 (N.S.) |
| Ethnicity/Race (n/%) | |||
| Caucasian | 157 (59.5) | 43 (57.3) | |
| African | 33 (12.5) | 12 (16.0) | |
| Asian | 38 (14.4) | 10 (13.3) | |
| Middle Eastern | 11 (4.2) | 2 (2.7) | |
| Ashkenazi Jewish | 15 (5.7) | 6 (8.0) | |
| Other | 10 (3.8) | 2 (2.7) | |
| Autoimmunity (n/%) | |||
| No | 162 (61.4) | 43 (57.3) | N.S. |
| Yes | 102 (38.6) | 32 (46.7) | |
*Ovarian reserve tests in this table reflect first patient evaluations. IVF outcome data reflect only first IVF cycles.
Data are shown with confidence intervals when log-transformed for analysis and with standard deviations where not. Positive autoimmunity denotes sum of all positive patients, demonstrating at least one abnormality in the immune panel tested (for further details, see Methods section).
The 339 patients underwent 455 IVF cycles, 116 thus representing repeat attempts. As Appendix S1, however, demonstrates, adding a covariate for number of IVF cycles experienced by each patient does in our patient population not affect the findings of an analysis based on per patient outcomes.
Genetic testing includes an assessment of triple CGG nucleotide repeats on the FMR1 gene, as reported before [5]–[8]. We previously also reported that, in regards to ovarian function, 26 to 34 repeats represent a normal range (median 30) [8], and counts below and above denote risk towards POA/OPOI [6]. Based on this normal range, normal (norm), heterozygous (het) and homozygous (hom) FMR1 genotypes can be described, which are reflective of distinct ovarian aging patterns [8]. They are defined by both alleles in normal range (norm), either one outside of range (het) or both alleles outside of range (hom). A het patient, in turn, can be either het-norm/low (<26 repeats) or het-norm/high (>34 repeats), while hom patients may be either high/high, high/low or low/low.
The het-norm/low FMR1 genotype has in a longitudinal and cross-sectional study been associated with very high ovarian reserve (based on AMH) at young ages, which, however, in the early 30 s, quickly depletes, resulting in rapid AMH declines at relatively young ages and relatively diminished age-dependent ovarian reserve thereafter [12]. Women with this genotype after ages 32–33 years, therefore, lose their PCO-like phenotype and, without FMR1 evaluations, based on AMH levels alone, would be perceived as either normal or suffering from prematurely diminished ovarian reserve.
Immunological testing involves, as previously reported [25], total immunoglobulin levels (IgG, IgM, IgA), antinuclear, anti-phospholipid and anti-thyroid antibody panels, as well as anti-ovarian and anti-adrenal antibodies. We previously demonstrated that this panel of immunological tests is sufficient in identifying even subclinical levels of autoimmunity that predisposes towards POA/OPOI [25], [26]. In utilizing this established screening method, a patient was defined as autoimmune-negative with absence of any abnormality and was considered autoimmune-positive with presence of even one, fully recognizing that such a definition increases sensitivity at expense of specificity and, therefore, weakens the discovery of potentially existing associations with autoimmunity. This definition of autoimmunity, therefore, consciously biases results against positive association and, thus, strengthens any detected associations.
IVF cycles were conducted in routine fashion. In principle, only two ovarian stimulation protocols were utilized: women with normal ovarian reserve were down-regulated with a gonadotropin releasing hormone agonist and stimulated with maximally 300 IU of gonadotropins daily. Patients with diminished ovarian reserve received a micro-dose agonist protocol with 450 to 600 IU of gonadotropins daily. Hormone assays for follicle stimulating hormone (FSH), estradiol and AMH were run in house as previously reported [27].
Statistical Analysis
Patient characteristics and IVF cycle outcomes were evaluated in association with FMR1 genotypes and autoimmune status, as defined above. PCO-like phenotypes were distributed in 62 percent as norm, 25 percent as het-norm/low and 13 percent as het-norm/high and represented 22 percent of all patients. Women with normal ovarian phenotype were in same order distributed at 52, 28 and 20 percent, respectively and represented 78 percent of all patients.
This distribution corresponds to an effect size, w, of 0.091 and, equivalently, to a contingency coefficient (C) as well as a Cramer's phi coefficient (phi) of 0.091. With a sample size of 339 the study, thus, had only a power of 30.3% to yield statistically significant results. Assuming continuation of in this study observed proportions, a study with identical effect size (w = 0.091) would require a sample size of 1,164 patients to achieve an 80 percent power to detect a significant alpha (0.05, two-tailed).
Univariate comparison between women with PCO-like phenotype and control was performed using Chi Square and analysis of variance as appropriate. Variables in which the distribution of data did not conform to normality were first log transformed for analysis and then converted back to standard units for presentation. Where cycles served as study units, odds ratios were compared. Continuous variables are presented as either mean ± standard deviation (SD) or mean and 95 percent confidence interval (95% CI), as appropriate.
We constructed a general linear model with logit link function, testing the association of the PCO-like phenotype with FMR1, and autoimmunity and also for pregnancy. General linear models were run on SAS version 9.2 (GENMOD module).
Results
Table 1 summarizes patient characteristics for all 339 women, amongst those 264 (77.9%) representing a normal, non-PCO-like ovarian phenotype and 75 (22.1%) the defined PCO-like phenotype. Women with PCO were younger (34.8±4.3 vs. 38.8±4.8 years, P<0.0001), had lower FSH (8.0±4.0 vs. 11.9±6.8 mIU/mL, P<0.0001) and higher AMH levels (3.2±2.6 vs. 0.8±0.7 ng/mL P<0.0001) and produced larger oocyte yields (17.5±5.6 vs. 4.8±3.3, P<0.0001). Otherwise, the two groups did not differ significantly, including in ethnic/racial distribution and BMI, confirming the non-obese nature of the here investigated PCO-like phenotype.
FMR1 Genotypes
Table 2 summarizes FMR1 genotype distribution patterns in women with apparently normal and PCO-like ovaries. Amongst 264 women with normal ovaries 137(51.9%) demonstrated a norm FMR1 genotype; the remaining 127 (48.1%) were het (there were no hom patients in the study population), 52 (19.7%) were het-norm/high and 75 (28.4%) het-norm/low. This distribution did not significantly differ from the FMR1 genotype distribution amongst the 75 women with PCO-like phenotype, where norm were 46 (61.3%), het-norm/high 10 (13.3%) and het-norm/low 19 (25.3%). The previously noted age-dependency of the definition of the PCO-like phenotype [12] mandates caution in interpreting these results.
Table 2. FMR1 genotype distribution*.
| Phenotype | Norm | Het-Norm/High | Het-Norm/Low |
| PCO-like | 46 (61.3) | 10 (13.3) | 19 (25.3) |
| Normal | 137 (51.9) | 52 (19.7) | 75 (28.4) |
| Total | 183 (54.0) | 62 (18.3) | 94 (27.7) |
*Chi-Square 2.46, df = 2.0, P = 0.29.
Autoimmunity
Distribution of autoimmunity also did not differ between both patient groups (Table 1): In women with normal ovaries 162/264 (61.4%) showed no evidence of autoimmunity and 102 (38.6%) did, while in the PCO-like cohort 43 (57.3%) did not and 32 (46.7%) did.
If autoimmunity was, however, assessed in reference to FMR1 genotype (Figure 1), the het-norm/low genotype was most frequently associated with autoimmunity (51.1%), followed by norm patients (38.3%) and het-norm/high women (24.2%), a statistically significant difference in distribution (p = 0.003). These differences in distribution further strengthened in women with PCO-like phenotype: het-norm/low women demonstrated autoimmunity in 83.3 percent of cases, while het-norm/high genotypes in only 10.0 percent, almost categorically differentiating between these two het genotypes, while norm women held a middle ground with 34.0 percent prevalence (P<0.0001).
Figure 1. Prevalence of autoimmunity in reference to FMR1 genotype.
The prevalence of autoimmunity was in both patient groups the highest with het-norm/low FMR1 genotype and the lowest with het-norm/high genotype. This pattern, however, intensified in women with PCO-like phenotype. Gray bars represent women with normal ovarian reserve; white bars represent the PCO-like phenotype.
IVF Pregnancy Rates
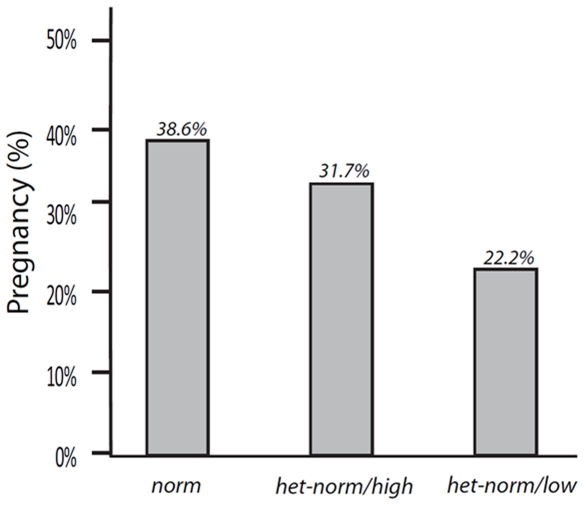
Pregnancy outcomes in 455 consecutive IVF cycles are summarized in Figure 2. As the figure demonstrates, norm women experienced the highest pregnancy rates (38.6%), a rate significantly higher than in het-norm/low patients [22.2%; OR 0.84 (95% CI 0.74 to 0.96; Wald 6.9; df = 1; P = 0.009)]. Women with het-norm/high genotype had intermediate pregnancy rates at 31.7 percent. Adjustment for age maintained the disadvantage in pregnancy rate for het-norm/low women (OR 0.43; 0.22 to 0.86, p = 0.017).
Figure 2. Pregnancy rates in IVF based on FMR1 genotype.
Pregnancy rates were the highest with norm FMR1 genotype and the lowest with het-norm/low genotype.
Discussion
This study demonstrates that in consecutive patients presenting for infertility treatments the prevalence of autoimmunity varies significantly with FMR1 genotype, with het-norm/low presenting with most and het-norm/high with least autoimmunity. This distribution is further strengthened in infertile women with the lean PCO-like phenotype, we previously described in a pilot study associated with the het-norm/low FMR1 genotype and with relatively rapidly depleting ovarian reserve [12]. Such patients almost guarantee positive autoimmune laboratory findings (83.3% prevalence, Figure 1), while a het-norm/high genotype is practically protective against autoimmunity (10.0% prevalence). Women with norm FMR1 genotype in both patient populations take up a middle ground with 38.3% and 34.0% prevalence, respectively.
The close association between the het-norm/low genotype and autoimmunity is further supported by the fact that the PCO-like phenotype was significantly younger (P <0.0001, Table 1), while autoimmunity actually increases in prevalence with advancing female age [28]. PCO-like phenotypes, thus, demonstrated significantly more autoimmunity, despite significantly younger ages.
The statistical clarity of here reported results is, however, especially remarkable, considering that patient selection criteria in this study strongly biased against discovery of such statistical associations. As already previously noted, the definition of positive autoimmunity consciously was based on improving sensitivity at the expense of specificity. Women defined as autoimmune, therefore, likely included a few without real polyclonal autoimmune activation.
Even more significantly, however, we noted earlier the time line for premature declines in ovarian reserve in women with the het-norm/low FMR1 genotype [12]. Even though the here investigated group of patients with PCO-like phenotype were significantly younger (P<0.0001, Table 1), it appears likely that older women with apparently normal ovarian phenotype must include at least some who at younger ages actually did demonstrate a PCO-like phenotypes.
Patients with PCO-like phenotype, in this study defined by 12 or more oocytes retrieved and/or an AMH above 4.0 ng/mL, represented 75 (22.1%) of all patients investigated. The definition of the PCO-like phenotype in this study was purely clinical, meant to identify a patient population with disproportionally high ovarian reserve, as documented by high oocyte yields and AMH values. Considering that the here investigated patient population included, as also previously reported [9], a disproportionate number of women with significantly diminished ovarian reserve (confirmed by elevated FSH, low AMH and oocyte yields in women with normal ovarian phenotype, Table 1), here chosen cut offs, defining a PCO-like phenotype for study purposes, appear appropriate. Twelve or more oocytes in such patients are above expected averages, as even women under age 35 years at our center produce only an average of 8.2±5.8 oocytes [29]. Similarly, an AMH above 4.0 ng/mL exceeds the 95% confidence interval (CI) of AMH levels at our center in women as young as age 26 years [30].
Here reported autoimmune laboratory findings in slightly above one third of women correspond well to prevalence numbers for infertility populations, reported in the literature [31], [32]. This not only validates the selected study population but also reaffirms the immune profile used in this, and prior studies [25], [26], to define presence of subclinical levels of autoimmunity. Though not reaching significance, prevalence of autoimmunity further increased (38.6% to 46.7%) from normal ovarian to PCO-like phenotypes. As further discussed below, this finding appears primarily the consequence of the strong association between het-norm/low FMR1 genotype and autoimmunity.
To define autoimmunity at subclinical levels is difficult to impossible, and is the reason why clinical diagnoses of autoimmune conditions in prodromal stages often are difficult [28]. Autoimmunity is, however, typically associated with a polyclonal activation of the immune system, which can be detected by broadly based laboratory evaluations [33], [34]. While such screens are not specific enough for diagnoses of autoimmune diseases, they appear sensitive enough in defining evidence of autoimmune activity [25], [26].
This study, once again, reaffirms this by demonstrating a surprisingly close association between autoimmunity and the het-norm/low FMR1 genotype. We already previously associated the het-norm/low genotype in a pilot study with a PCO-like phenotype, with rapidly depleting ovarian reserve [12]. Women with this genotype present at young ages with a PCO phenotype. Because of rapid follicle depletion (i.e., rapidly diminishing ovarian reserve), they then at older ages demonstrate normal to abnormally low AMH levels, reflecting relative or outright diminished ovarian reserve.
Since the FMR1 gene appears closely involved with regulation of follicle recruitment and, therefore, ovarian reserve [1]–[8], the association between het-norm/low FMR1 genotype and PCO-like phenotype does not surprise. The extremely close association between het-norm/low genotype and autoimmunity was, however, completely unanticipated. This association appears, indeed, so close that in a PCO-like population a het-norm/low genotype virtually predicts autoimmunity, while women with the het-norm/high genotype appear protected from autoimmunity.
These associations also translate into clinical significance for infertile women since the FMR1 genotype appears predictive of pregnancy chances with IVF. Women experience best pregnancy chances with norm, intermediate with het-norm/high and lowest rates with het-norm/low genotypes (Figure 2).
Whether a PCO-like phenotype, alone, affects pregnancy chances in IVF has remained controversial [35]. Lower [36] and similar [37] pregnancy rates have been suggested in PCOS in comparison to other infertile patients undergoing IVF. Multiple underlying etiologies for PCOS [13], different ovarian stimulation protocols and variability in genetic definitions of study populations easily explain such discrepancies.
The same kind of controversy surrounds the association of autoimmunity and pregnancy success in IVF. Many authorities have categorically denied an association [38]–[40], while others have pointed at considerable evidence [41], [42]. Here presented data suggest a possible explanation for these contradictory opinions since, like PCOS, most studies on autoimmunity have been performed in genetically and etiologically heterogenic patient populations. At least in a genetically homogenous population of women with the het-norm/low FMR1 genotype, autoimmunity, indeed, appears negatively associated with pregnancy chances in IVF.
Autoimmunity is abnormally high in practically all X-linked disorders [43]. If defective, a MHC-paralogue on the long arm of the X chromosome renders individuals immunologically less efficient [44]. With the FMR1 gene mapping to Xq27.3 [45], it appears to occupy the cross roads between ovarian function (ovarian recruitment and ovarian reserve) and autoimmunity [43], [44], [46].
Both autoimmunity and abnormalities in ovarian reserve are closely associated with X chromosome defects: A good example is Turner syndrome, in which Xq21 terminal deletions are common, often large and characterized by primary as well as secondary amenorrhea [46], [47]. Xq21 or further distal deletions usually present with secondary amenorrhea [47], a classical clinical presentation of premutation range FMR1 (fragile X) carriers who present with POF/POI [10], [48]. In contrast Turner syndrome with normal fertility generally involves more proximal Xq deletions [49].
POF/POI, indeed, demonstrates a 4MB locus exactly at Xq27-q28 [46], with the FMR1 gene mapping to Xq27.3 [45]. Small deletions in Xq27-q28 have variable phenotypes, some with early menopause but are usually able to reproduce until experiencing full POF/POI [46]. Similar correlations with ovarian function are also observed in balanced translocations, where only Xq23-q27 deletions are associated with POF/POI [46].
Turner syndrome is not only characterized by above noted abnormalities in ovarian function but, like most X-linked disorders, also by excessive autoimmunity. Both autoantibodies and autoimmune diseases are significantly increased [44]. The close association between X-linked disorders and autoimmunity led to the suggestion that the latter may be the consequence of genes and/or mutations on the long arm of the X chromosome [43], potentially explaining the increased prevalence of autoimmunity in women in comparison to males [50].
A PCO-like phenotype with strongly associated autoimmunity and specific FMR1 genotype (het-norm/low), and the protective effect of another FMR1 genotype (het-norm/high), support the notion we made earlier [8] that the FMR1 gene plays a role in regulating ovarian reserve. Now it appears that the same gene may also be involved in determining risk/protection of/from autoimmunity.
These observations also raise the intriguing possibility that a PCO-like phenotype may be associated with an autoimmune etiology. This has previously been suggested [14]–[19], [22] but, in contrast to cases of POF/POI [20], [21], PCO and/or PCOS have never been established as autoimmune in nature. Like POF/POI in its various forms [10], [25], [26], [51], PCOS is multifactorial in etiology [13], with PCO being a unifying phenotypical presentation of an, otherwise, still very controversial syndrome [52].
Only very recently, Hefter Frischmuth and associates reported serological evidence for elevated autoimmunity in women with PCOS [16]. The authors, like also in this study, investigated non-organ specific (antihistone and anti-dsDNA) antibodies. Another Austrian group, however, even more recently, reported a statistical association between levels of anti-thyroid peroxidase antibodies and treatment response in women with PCOS, suggesting that organ specific thyroid antibodies may also be associated with PCOS [53]. Thyroid autoimmunity, of course, is well known to be closely associated with ovarian autoimmunity [20].
These reports and here presented data support the hypothesis that, like other endocrine organs, ovaries may be subject to suppressive and stimulatory autoimmune influences, possibly mediated by autoantibodies, a hypothesis we previously proposed after Baroni and associates reported stimulatory antibodies (to platelet derived growth factor) in systemic sclerosis [54]. Building on their findings, we speculated that “functional” autoantibodies may represent a universal paradigm of autoimmunity [22].
In regards to ovarian function such a concept would suggest inhibitory autoantibodies as cause of selected cases of POA/OCPOI and POF/POI, as recently suggested by La Marca and associates [21], and stimulatory autoantibodies as promoters of follicular activity, resulting in at least one PCO-like phenotype. Here reported strong positive and negative associations with both het-FMR1 genotypes further suggest a possible role of the FMR1 gene in regulation of these “functional” autoantibodies and, therefore, possibly, in contributing to the well established higher prevalence of autoimmunity in females in comparison to males [42].
Despite the substantial size of the here reported population of infertile women, this study's principal weakness lies in its relatively limited power. As, however, previously noted, to achieve 80 percent power for a significant alpha under observe proportions, close to 1,200 patients would be required. While we are continuing to accumulate patients, this is a difficult goal to obtain in a single center study.
Supporting Information
Pregnancy analysis of only 339 1st IVF cycles*.
(DOC)
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: NG and DHB are listed as co-inventors on a number of pending United States patents and one already granted patent, which, peripherally, relate to this manuscript. Those patents claim: (1) Therapeutic benefits from supplementation of women with DOR with dehydroepiandrosterone (DHEA). Since some patients in this study were DHEA supplemented, this may have relevance. (2) Diagnostic benefits from determining triple CGG nucleotide repeats on the FMR1 gene in attempts to assess and predict ovarian reserve. NG is owner of the Center for Human Reproduction (CHR), where this study was conducted. The authors confirm that these conflicts do not alter our adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by the Foundation for Reproductive Medicine and by intramural funds from the Center for Human Reproduction. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hundscheid RDL, Braat DDM, Kiemaney LALM, Smits APT, Thomas CMG. Increased serum FSH in female fragile X premutation carriers with either regular menstrual cycles or on oral contraceptives. Hum Reprod. 2001;16:457–462. doi: 10.1093/humrep/16.3.457. [DOI] [PubMed] [Google Scholar]
- 2.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–4574. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 3.Bretherick KL, Fluker MR, Robinson WP. FMR1 repeat sizes in the gray zone and high end of normal range are associated with premature ovarian failure. Hum Genet. 2005;117:376–382. doi: 10.1007/s00439-005-1326-8. [DOI] [PubMed] [Google Scholar]
- 4.Bodega B, Bione S, Dalprà L, Toniolo D, Ornaghi F, et al. Influence of intermediate and uninterrupted FMR1 CGG expansionsin premature ovarian failure manifestations. Hum Reprod. 2006;21:952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- 5.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence. I. Correlation of triple CGG repeats on the FMR1 gene to ovarian reserve parameters FSH and anti-Müllerian hormone. Fertil Steril. 2009;91:1700–1706. doi: 10.1016/j.fertnstert.2008.01.098. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Weghofer A, Oktay K, Barad DH. Relevance of low triple CGG repeats on the FMR1 gene to ovarian reserve. RBMOnline; 2009;19:385–390. doi: 10.1016/s1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- 7.Gleicher N, Weghofer A, Oktay K, Barad DH. Correlation of triple repeats on the FMR1 (fragile X) gene to ovarian reserve: a new infertility test? Acta Obstet Gynecol Scand. 2009;88:1024–1030. doi: 10.1080/00016340903171058. [DOI] [PubMed] [Google Scholar]
- 8.Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian ageing. RBMOnline 2010. 2010 doi: 10.1016/j.rbmo.2010.02.020. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Barad DH, Weghofer A, Gleicher N. Age-specific levels of basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109:1404–1410. doi: 10.1097/01.AOG.0000264065.37661.a0. [DOI] [PubMed] [Google Scholar]
- 10.Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleicher N, Weghofer A, Barad DH. Effect of race/ethnicity on triple CGG counts in the FMR1 gene in infertile women and egg donors. RBMOnline. 2010;20:485–491. doi: 10.1016/j.rbmo.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Barad DH, Weghofer A, Goyal A, Gleicher N. Further refinement in defining the effect of heterozygous-abnormal CGG counts on the FMR1 (Fragile X) gene: definition of a distinct subgroup of PCOS patients, based on normal/low genotype. Fertil Steril. 2009;92(Suppl):S105. [Google Scholar]
- 13.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group 2004. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 14.Fénichel P, Gobert B, Carré Y, Barbarino-Monnier P, Hiéronimus S. Polycystic ovary syndrome in in autoimmune diseases. Lancet. 1999;353:2210. doi: 10.1016/S0140-6736(99)00256-1. [DOI] [PubMed] [Google Scholar]
- 15.Van Gelderen CJ, Gomes dos Santos MI. Polycystic ovarian syndrome. Evidence for an autoimmune mechanism in some cases. J Reprod Med. 1993;38:381–386. [PubMed] [Google Scholar]
- 16.Hefler Frischmuth K, Walch K, Huebl W, Baummehlner K, Tempfer C, et al. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil Steril. 2010;93:2291–2294. doi: 10.1016/j.fertnstert.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Bannatyne P, Russell P, Shearman RP. Autoimmune oophoritis: a clinic-pathologic assessment of 12 cases. Int J Gynecol Pathol. 1990;9:191–207. doi: 10.1097/00004347-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Londsdale RN, Roberts PF, Trowell JE. Autoimmune oophoritis associated with polycystic ovaries. Histopathology. 1991;19:77–81. doi: 10.1111/j.1365-2559.1991.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 19.Ehrman DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 20.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–134. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 21.La Marca A, Marzotti S, Brozzetti A, Stabile G, Carducci Artenisio A, et al. on behalf of the Italian Addison Network. (2009) Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles. J Clin Endocrinol Metab. 94:3816–3823. doi: 10.1210/jc.2009-0817. [DOI] [PubMed] [Google Scholar]
- 22.Gleicher N, Barad D, Weghofer A. Functional autoantibodies, a new paradigm in autoimmunity? Autoimmune Rev. 2007;7:42–45. doi: 10.1016/j.autrev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Wall JR, Lahooti H. Pathogenesis of thyroid eye disease – does autoimmunity against the TSH receptor explain all cases? Endocrynol Pol. 2010;61:222–227. [PubMed] [Google Scholar]
- 24.Segars JH, DeCherney AH. Is there a genetic basis for polycystic ovary syndrome. J Clin Endocrinol Metab. 2010;95:2058–2060. doi: 10.1210/jc.2010-0518. [DOI] [PubMed] [Google Scholar]
- 25.Gleicher N, Weghofer A, Barad DH. A pilot study of premature ovarian senescence. II.Different gentotype and phenotype for genetic and autoimmune etiologies. Fertil Steril 91. 2009:1707–1711. doi: 10.1016/j.fertnstert.2008.01.099. [DOI] [PubMed] [Google Scholar]
- 26.Gleicher N, Weghofer A, Oktay K, Barad DH. Is the immunological noise of abnormal autoimmunity an independent risk factor for premature ovarian aging? Menopause. 2009;16:760–764. doi: 10.1097/gme.0b013e318193c48b. [DOI] [PubMed] [Google Scholar]
- 27.Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertile Steril. 2009;91:1553–1555. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 28.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 29.Singer T, Barad DH, Weghofer A, Gleicher N. Correlation of antimüllerian hormone and baseline follicle-stimulating hormone levels. Fertile Steril. 2009;91:2616–2619. doi: 10.1016/j.fertnstert.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 30.Barad DH, Weghofer A, Goyal A, Gleicher N. Age-specific anti-Müllerian hormone (AMH): Utility of AMH at various ages. RBMOnline; 2010 In press; (doi number not yet available) [Google Scholar]
- 31.Gleicher N, Pratt D, Dudkiewicz A. What do we really know about autoantibody abnormalities and reproductive failure: a critical review. Autoimmunity. 1993;16:115–140. doi: 10.3109/08916939308993318. [DOI] [PubMed] [Google Scholar]
- 32.Geva E, Amit A, Lerner-GevaL, Lessing JB. Autoimmunity and reproduction. Fertil Steril. 1997;67:599–611. doi: 10.1016/s0015-0282(97)81351-9. [DOI] [PubMed] [Google Scholar]
- 33.Singh RR, Hahn BH, Tsao BP, Ebling FM. Evidence for multiple mechanisms of polyclonal T cell activation in murine lupus. J Clin Invest. 1998;102:1841–1849. doi: 10.1172/JCI3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sutmuller M, Baelde JJ, Madaio MP, Bruijn JA, De Heer E. Idiotype usage by polyclonal activated B cells in experimental autoimmunity and infection. Clin Exp Immunol. 1999;115:275–280. doi: 10.1046/j.1365-2249.1999.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim YJ, Ku SY, Jee BC, Suh CS, Kim SH, et al. A comparative study on the outcomes of in vitro fertilization between women with polycystic ovary syndrome and those with sonographic polycystic ovary-only in GnRH antagonist cycles. Arch Gynecol Obstet 2010 [Epub ahead of print] 2010 doi: 10.1007/s00404-010-1401-9. [DOI] [PubMed] [Google Scholar]
- 36.Kuivasaari-Pirinen P, Hippeläinen M, Hakkarainen H, Randell K, Heinonen S. Cumulative baby take-home rate amongwomen with PCOS treated by IVF. Gynecol Endocrinol 2010 [Epub ahead of print] 2010 doi: 10.3109/09513591003632043. [DOI] [PubMed] [Google Scholar]
- 37.Urman B, Tiras B, Yakin K. Assisted reproduction in the treatment of polycystic ovarian syndrome. RBMOnline. 2004;8:419–430. doi: 10.1016/s1472-6483(10)60926-1. [DOI] [PubMed] [Google Scholar]
- 38.ASRM Practice Committee Report. AL, USA: American Society for Reproductive Medicine; 1999. Antiphospholipid Antibodies Do Not Affect IVF Success. [Google Scholar]
- 39.Hornstein MD. Antiphospholipid antibodies in patients undergoing IVF: the data do not support testing. Fertil Steril. 2000;74:635–636. doi: 10.1016/s0015-0282(00)01529-6. [DOI] [PubMed] [Google Scholar]
- 40.Hill GA, Scott RT., Jr Immunologic tests and IVF: “please enough already”. Fertil Steril. 2000;74:439–442. doi: 10.1016/s0015-0282(00)00705-6. [DOI] [PubMed] [Google Scholar]
- 41.ASRI Antiphospholipid Antibody Committee. A rational basis for antiphospholipid antibody testing and selective immunotherapy in assisted reproduction: a rebuttal to the American Society for Reproductive Medicine Practice Committee Opinion. Fertil Steril. 2000;74:631–634. [PubMed] [Google Scholar]
- 42.Gleicher N. Reproductive failure prior to the onset of clinical autoimmune disease. Rheumatology. 1999;38:485–487. doi: 10.1093/rheumatology/38.6.485. [DOI] [PubMed] [Google Scholar]
- 43.Pessach IM, Notarangelo LD. X-linked primary immunodefficiencies as a bridge to better understanding X-chromosome related autoimmunity. J Autoimmunity. 2009;33:17–24. doi: 10.1016/j.jaut.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Larizza D, Calcaterra V, Martinetti M. Autoimmune stigmata in Turner syndrome: When lacks an X chromosome. J Autoimmunity. 2009;33:25–30. doi: 10.1016/j.jaut.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Entrez Gene. Available: http://www.ncbi.nlm.nih.gov/sites/entrez?Db=gene&Cmd=retrieve&dopt=full_report&list. Accessed 2010 Dec 02. [Google Scholar]
- 46.Persani L, Rossetti R, Cacciatore C, Bonomi M. Primary ovarian insufficiency: X chromosome defects and autoimmunity. J Autoimmunity. 2009;33:35–41. doi: 10.1016/j.jaut.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Marozzi A, Manfredini E, Tibiletti MG, Furlan D, Villa N, et al. Molecular definition of Xq common-deleted region in patients affected by premature ovarian failure. Hum Genet. 2000;107:304–311. doi: 10.1007/s004390000364. [DOI] [PubMed] [Google Scholar]
- 48.Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Cremer FP, van de Pol DJ, Diergaarde PJ, Wieringa B, Nussbaum RL, et al. Physical fine mapping of the choroideremia locus using Xq21 deletions associated with complex syndromes. Genomics. 1989;4:41–46. doi: 10.1016/0888-7543(89)90312-1. [DOI] [PubMed] [Google Scholar]
- 50.Gleicher N, Barad DH. Gender as risk factor for autoimmune disease. J Autoimmunity. 2007;28:1–6. doi: 10.1016/j.jaut.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Gleicher N, Weghofer A, Oktay K, Barad D. Do etiologies of premature ovarian aging (POA) mimic those of premature ovarian failure (POF)? Hum Reprod. 2009;24:2395–2400. doi: 10.1093/humrep/dep256. [DOI] [PubMed] [Google Scholar]
- 52.Aziz R. Diagnosis of polycystic ovarian syndrome: The Rotterdam Criteria are premature. J Clin Endocrinol Metab. 2006;91:781–785. doi: 10.1210/jc.2005-2153. [DOI] [PubMed] [Google Scholar]
- 53.Ott J, Aust S, Kurz C, Nouri K, Wirth S, et al. Elevate anti-thyroid peroxidase antibodies indicating Hashimoto's thyroiditis are associated with treatment response in infertile women with polycystic ovary syndrome. Fertil Steril; 2010. In press; (doi number not yet available) [DOI] [PubMed]
- 54.Baroni SS, Santillo M, Bavilacqua F, Luchetti M, Spadoni T, et al. Stimulating autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–2676. doi: 10.1056/NEJMoa052955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pregnancy analysis of only 339 1st IVF cycles*.
(DOC)