Abstract
Brain accumulation of the amyloid-β peptide (Aβ) and oxidative stress underlie neuronal dysfunction and memory loss in Alzheimer's disease (AD). Hexokinase (HK), a key glycolytic enzyme, plays important pro-survival roles, reducing mitochondrial reactive oxygen species (ROS) generation and preventing apoptosis in neurons and other cell types. Brain isozyme HKI is mainly associated with mitochondria and HK release from mitochondria causes a significant decrease in enzyme activity and triggers oxidative damage. We here investigated the relationship between Aβ-induced oxidative stress and HK activity. We found that Aβ triggered HKI detachment from mitochondria decreasing HKI activity in cortical neurons. Aβ oligomers further impair energy metabolism by decreasing neuronal ATP levels. Aβ-induced HKI cellular redistribution was accompanied by excessive ROS generation and neuronal death. 2-deoxyglucose blocked Aβ-induced oxidative stress and neuronal death. Results suggest that Aβ-induced cellular redistribution and inactivation of neuronal HKI play important roles in oxidative stress and neurodegeneration in AD.
Introduction
Hexokinase (HK) catalyzes the first step of glycolysis, i.e., the ATP-dependent phosphorylation of glucose to glucose-6P (G6P), with concomitant generation of ADP. Although HK is generally known as a key glycolytic enzyme, in neurons and other cell types HK activity also regulates vital cellular processes, including ATP synthesis and apoptosis [1]–[3].
In the brain, HKI is the major isozyme present [4], being mainly (∼70–90%) associated with the outer mitochondrial membrane. Release of HK from mitochondria is known to cause a severe decrease in enzyme activity [5], [6]. Interestingly, mitochondrial-bound hexokinase I (m-HKI) activity in neurons has been shown to be neuroprotective, maintaining adequate glutathione levels, inducing neurite outgrowth, and preventing neuronal oxidative damage [6]–[8]. The activity and specific subcellular localization of neuronal m-HKI is of great significance to protect neurons from different insults.
We have previously demonstrated that neuronal m-HKI reduces hyperglycemia-induced generation of excessive ROS through an ADP recycling mechanism [8]. In the mitochondrial membrane, HKI is bound to the voltage-dependent anion channel (VDAC), which is associated with the ADP/ATP carrier. HKI benefits from preferential access to ATP produced in the mitochondria, while local ADP generation by HKI facilitates the exchange of ADP and ATP through the inner mitochondrial membrane [8], [9]. This enhances mitochondrial oxidative phosphorylation and reduces monoelectronic oxygen reduction that gives rise to excessive ROS generation. Thus, m-HK1 may play an important antioxidant role in the brain.
Several cellular features of the brain suggest that it is highly sensitive to oxidative stress [10]. Abnormally elevated ROS levels have been implicated in the age-related impairment of long-term potentiation (LTP), a well-known model for synaptic plasticity and learning [11]. It is known that excessive ROS levels are implicated in the molecular etiology of Alzheimer's disease (AD) [12]–[14]. Elevated ROS levels can be selectively dysfunctional in AD, a disease characterized by memory loss.
Previous investigations have shown that different aggregated forms of the amyloid-β peptide (Aβ) stimulate ROS production in neurons [14], [15]. Accumulation of Aβ in AD brains is thought to underlie neuronal dysfunction and memory loss, being centrally implicated in AD pathogenesis [16]. In particular, soluble protein oligomers are currently thought to be emerging toxins in Alzheimer's [17]–[19] and other amyloid diseases [18], [20].
We now report that Aβ triggers neuronal oxidative stress by interfering with m-HKI activity and subcellular localization. Exposure of mature cortical neurons to Aβ caused a decrease in m-HKI activity and its detachment from mitochondria. Aβ oligomers further induced mitochondrial dysfunction and caused a marked reduction in neuronal ATP levels, indicating an impairment of energy metabolism. By causing a cellular redistribution of HKI, Aβ instigates an abnormal increase in mitochondrial ROS generation that is prevented by 2-deoxyglucose (2-DOG). Results establish a novel cellular mechanism underlying oxidative stress and neurodegeneration in AD.
Methods
Materials
Aβ1-42 and Aβ1-40 were purchased from Bachem (Torrance, CA). ADP, ATP, horseradish peroxidase, carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), 2-deoxyglucose (2-DOG), Leuconostoc Mesenteroid's glucose-6 phosphate dehydrogenase (G6PDH), Percoll, β-NAD+, bovine serum albumin and poly-L-lysine were from Sigma-Aldrich (St. Louis, MO). Neurobasal medium, B27 supplement, Live/Dead cell viability assay kit and CM-H2DCFDA were from Invitrogen Molecular Probes (Eugene, OR). Goat polyclonal anti-cytochrome c oxidase and monoclonal anti-hexokinase-1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-cyclophilin B rabbit polyclonal antibody was from Affinity Bio Reagents (Golden, CO). SuperSignal West Femto Maximum Sensitivity substrate was from Pierce (Rockford, IL). All other reagents were of the highest analytical grade available.
Aβ preparation
Aβ1-42 was freshly dissolved from the lyophilized powder in a 50% (v/v) solution of trifluoroethanol (TFE) in phosphate-buffered saline (PBS), as described [21]–[23]. Amyloid aggregation was triggered by dilution of small aliquots from this stock solution into PBS (resulting in ≤ 0.5% residual TFE) to yield final concentrations of 5 µM or 20 µM Aβ.
Alternatively, Aβ oligomers were prepared as previously described [14], [24], [25]. Briefly, the peptide was dissolved to 1 mM in hexafluoro-2-propanol and stored in aliquots as a dried film at −80°C after solvent evaporation. The film was resuspended in DMSO to a final concentration of 5 mM. The solution was diluted to 100 µM in ice-cold PBS and left at 4°C overnight. The solution was then centrifuged at 14,000×g for 10 min at 4°C and the supernatant was collected, transferred to a clean tube and stored at 4°C for a maximum of 24 hours until use. Aβ1-40 monomer solution was prepared in aliquots as a dried hexafluoro-2-propanol film and stored at -80°C. The peptide film was dissolved in undiluted, sterile DMSO to make a 5 mM solution. The solution was diluted to 100 µM with Ham's F-12 medium without glutamine and immediately centrifuged (14,000×g/10 min/4°C) to remove insoluble aggregates. The supernatant was transferred to a clean tube and used immediately.
Brain mitochondrial isolation, determination of HK activity and isolation of neuronal cytosolic and mitochondria fractions
Mitochondria were isolated from forebrains by conventional differential centrifugation as previously described [8]. m-HKI activity was determined using an enzymatic assay coupled to Leuconostoc Mesenteroid's G6PDH activity, monitoring hexokinase activity indirectly by NADH formation at λ = 340 nm [8]. Cytosolic and mitochondria-enriched fractions from primary cultures of rat cortical neurons were isolated as described [8].
Neuronal cultures and reactive oxygen species (ROS) formation
Cortices from 14-day-old rat embryos were dissected and cultured as previously described [22], [26] with minor modifications. Cells were plated on glass coverslips previously coated with 1.5 µg/ml poly-L-lysine in Neurobasal medium supplemented with B27 and cultures were maintained at 37°C in a humidified 5% CO2 atmosphere for 14–18 days prior to use. ROS formation was evaluated in live cortical neurons using 2 µM CM-H2DCFDA [14]. Probe fluorescence was analyzed using NIH Image J software [27] as described [14]. 16 images were analyzed in each experimental condition (carried out in triplicate wells in each of three independent experiments using different neuronal cultures) and were combined to allow quantitative estimates of changes in ROS levels.
Neuronal viability and MTT assays
Aβ1-42 (20 µM), in the absence or in the presence of 30 mM 2-DOG, was added to cultures and was kept in the medium for 48 hours. Control cultures consisting of neurons cultured in growth medium alone or in the presence of residual TFE (0.5% v/v) were also prepared. Cell viabilities in cultures were assessed using the Live/Dead kit. Live neurons were identified by green calcein fluorescence and dead neurons were identified by red propidium iodide fluorescence. Percentages of live neurons are expressed relative to the total number of neurons in each field. Five randomly chosen fields independent fields were imaged on a Nikon Eclipse TE300 microscope and analyzed in different experimental conditions (carried out in at least triplicate wells in each of three independent experiments using different cultures).
MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyl tetrazolium bromide) assay (Boehringer Mannheim, Indianapolis, IN) was used to evaluate the cellular metabolic redox activity. Nineteen-day old hippocampal neuronal cultures were used. Three independent experiments (each with 6 wells per experimental condition) were carried out with different neuronal cultures.
Measurement of intracellular ATP levels
ATP contents in 19-day old rat cortical neurons were analyzed by ion-paired reverse phase liquid chromatography. Cultures were exposed to 500 nM ADDLs or vehicle for 12 hours. Medium was then removed and cells were washed twice with cold PBS. Liquid nitrogen was used to disrupt cells and stop cellular metabolism. The plates were kept in an ice bath until the liquid nitrogen evaporated completely. Cells were then homogenized with 6% trichloroacetic acid, neutralized by adding a small aliquot of a 1M Tris solution, and centrifuged at 20,800×g for 5 min at 4°C. Protein contents were determined using the BCA method. Aliquots were injected into an HPLC system using a Supelguard column (Supelco) coupled to a Supercosil C-18 carrier (particle size of 5 µm, Supelco) column. Isocratic elution was performed at a flow-rate of 1 mL/min at room temperature with 50 mM KH2PO4 buffer, pH 6.0/methanol (90/10) and 4 mM tetrabutyl ammonium bromide. ATP levels were measured by UV absorbance at 254 nm. ATP peaks were identified by co-injecting 2 nmols of ATP in an independent run. Results were expressed by normalizing peak areas by the total amount of protein obtained.
Determination of intracellular glucose-6P content
Aβ1-42 (5 µM), in the the presence of 30 mM 2-DOG, was added to cultures after 7 days in vitro and was kept in the medium for 24 hours. Cells were scraped in 200 µL of 6% (v/v) trichloroacetic acid per plate (35 mm2 dishes). The extract was neutralized by adding 80 µL of 1.0 M Tris solution. G6P levels were determined using an enzymatic assay coupled to G6PDH activity [28], [29]. Intracellular G6P levels were expressed as nmols/mg protein.
Western blot analysis
Aliquots from mitochondria-enriched and cytosolic cellular fractions were normalized for protein content (25 µg protein/lane) and separated on 12% SDS-PAGE gels followed by blotting onto nitrocellulose membranes. Hexokinase levels were determined from total, unfractionated neuronal extracts separated on 12% SDS-PAGE gels and blotted onto nitrocellulose membranes. Membranes were incubated with anti-HK1 (1∶500 dilution), anti-cytochrome c oxidase (1∶500 dilution, used as a loading control for mitochondria-enriched fractions) and anti-cyclophilin B (1∶4,000 dilution, used as a loading control for cytosolic fractions) antibodies. Protein bands were visualized by staining with horseradish peroxidase-labeled secondary antibody (1∶100,000) followed by enhanced chemiluminescence detection.
Results
Aβ induces inhibition of neuronal mitochondrial-bound hexokinase I (m-HKI) by interference with subcellular localization
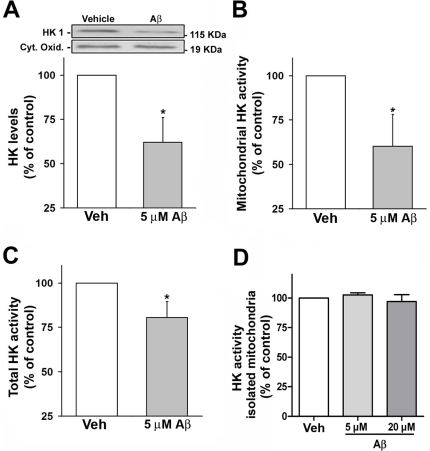
Brain HKI is mainly (∼70–90%) associated with the mitochondrial membrane and release of HK from mitochondria causes a significant decrease in enzyme activity [5], [6]. To test whether Aβ interferes with the subcellular localization of neuronal m-HKI, we measured HKI protein levels in mitochondrial fractions by western immunobloting. HKI levels were reduced by ∼40% in the mitochondrial fraction from Aβ-treated neurons (Fig. 1A). In agreement with the decrease in levels of mitochondrial-bound hexokinase, the activity determined in the mitochondria-enriched fraction from Aβ-treated neurons was significantly (∼40%) lower than the activity measured in mitochondria from vehicle-treated neurons (Fig. 1B). We also found that 5 µM Aβ induced ∼ 20% decrease in total neuronal HKI activity (Fig. 1C). These results show that Aβ interferes with the subcellular localization of HK, causes the release of HKI from mitochondria. In order to investigate the effect of Aβ on m-HKI activity, we have next proceeded checking whether Aβ directly inhibits HK. m-HKI activity was then measured in mitochondria isolated from adult rat brains incubated with Aβ. Aβ did not directly inhibit hexokinase, even at a high concentration (20 µM) (Fig. 1D). This suggests that Aβ only has an impact on HKI when the enzyme is associated with the mitochondrial membrane in the neuronal cellular context.
Figure 1. Aβ inhibits HKI by triggering the release of m-HKI from mitochondria.
Panel A: Western immunoblot analysis of HKI levels in mitochondrial fractions from cultured cortical neurons exposed to 5 µM Aβ for 24 hours. Cytochrome c oxidase was used as a loading control. Bars correspond to means ± SE from at least three independent experiments carried out in duplicate. Panel B: HK activity as measured in mitochondrial enriched fractions from cultured cortical neurons exposed to 5 µM Aβ for 24 hours. Bars correspond to means ± S.D. from three independent experiments carried out in duplicate. Panel C: HK1 activity as measured in cortical neurons exposed to 5 µM Aβ for 24 hours. Bars represent the means ± SD from three independent experiments carried out in duplicate. (*) indicates a statistically significant (p<0.005) difference relative to control (vehicle-treated) cultures. Panel D: HK activity in mitochondria isolated from adult rat brains was measured in the absence or in the presence of Aβ (5 µM or 20 µM; 1 hour). Values represent means ± SD of the activity measured in two independent experiments carried out in triplicate.
Oligomers of the Aβ peptide decrease mitochondrial-bound HKI and impair neuronal energy metabolism
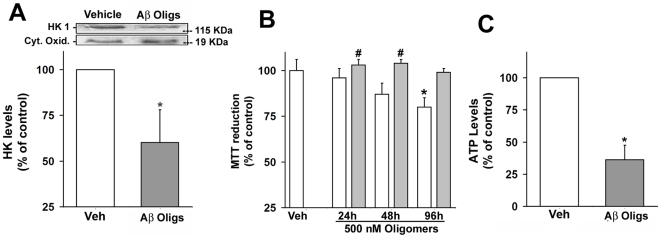
Because soluble Aβ oligomers (also known as ADDLs) have recently been recognized as the proximal neurotoxins in AD [17]–[19], we next investigated whether a defined preparation of soluble Aβ oligomers would instigate HKI release from mitochondria. Indeed, HKI levels were 40% reduced in the mitochondrial fraction from neurons treated with 500 nM ADDLs for 24 hours (Fig. 2A). Results demonstrate that Aβ oligomers instigate neuronal HKI release from mitochondria.
Figure 2. Effects of Aβ oligomers on neuronal m-HKI and ATP levels.
Panel A: Aβ oligomers triggers the release of m-HKI from mitochondria. Western immunoblot analysis of HKI levels in mitochondrial fractions from cultured cortical neurons exposed to 500 nM Aβ oligomers (ADDLs) (D) for 24 hours. Cytochrome c oxidase was used as a loading control. Bars correspond to means ± SE from at least three independent experiments carried out in duplicate. Panel B: Time-dependent toxicity of ADDLs (white bars) or Aβ monomers (gray bars) to mature hippocampal neurons measured using the MTT reduction assay. Values represent the means ± SD of the relative absorbance measured using 6 wells for each experimental condition for three independent experiments. (*) indicates a statistically significant (p<0.005) difference relative to control cultures. Panel C: Aβ oligomers decrease ATP levels in mature cortical neurons. Cultures were exposed to vehicle or 500 nM ADDLs for 12 hours. Values represent means ± SD of the relative levels measured in five independent experiments.
The impact of Aβ oligomers on mitochondrial redox activity was further evaluated using the MTT reduction assay in our model of mature hippocampal neuronal cultures. Neurons were exposed to ADDLs or monomers for 24, 48 or 96 h with ADDLs (Fig. 2B). After 24 h, no significant toxicity was observed in the presence of ADDLs or monomers, used as a control. Only at longer incubation times (48 and 96 h), ADDLs caused a progressive decrease in MTT reduction, while Aβ monomers had no effect (Fig. 2B).
ATP is essential for the excitability and survival of neurons, as well as for protein phosphorylation reactions that mediate synaptic signaling and associated changes in neuronal structure and function [28]. Because the results presented above have indicated mitochondrial impairment in ADDL-treated neurons, we next measured ATP levels in cortical neurons treated with 500 nM ADDLs for 12 hours, well before an effect on mitochondrial redox activity could be observed (Fig. 2B). Results show a marked decrease in ATP levels in ADDL-treated neurons (Fig. 2C), substantiating the neuronal energy metabolism impairment caused by Aβ oligomers.
Hexokinase activity blocks Aβ-induced generation of reactive oxygen species (ROS)
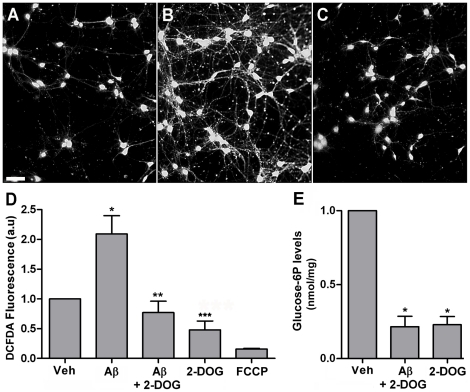
We have previously shown that impaired m-HKI activity results in increased ROS levels in neurons [8]. In order to investigate the possible role of m-HKI in neuronal oxidative stress induced by Aβ, intracellular ROS levels were evaluated in cortical neurons treated with 5 µM Aβ or vehicle for 6 hours, in the absence or presence of 2-deoxyglucose (2DOG), an alternative substrate of hexokinase. Phosphorylation of 2-DOG by hexokinase gives rise to the formation of 2-deoxyglucose 6-phosphate (2-DOG6P), which does not inhibit hexokinase activity in the same range as G6P and consequently preserves HK1 activity [4], [8], [29]. In agreement with previous findings [14], Aβ induced a significant increase in neuronal ROS levels (Fig. 3B, D). Interestingly, addition of 30 mM 2-DOG to the culture medium completely blocked the excessive intracellular ROS production induced by Aβ (Fig. 3C, D). Such results reveal an important antioxidant role of HKI in the prevention of Aβ-induced oxidative stress.
Figure 3. 2-DOG blocks Aβ-induced neuronal ROS formation.
Representative DCF fluorescence images from control (vehicle-treated) cultures (Panel A), cultures exposed to 5 µM Aβ for 6 hours (Panel B) or cultures exposed to 5 µM Aβ+30 mM 2-DOG for 6 hours (Panel C). When present, 2-DOG was added 10 minutes before Aβ. In order to allow direct comparison between ROS levels, identical conditions and exposure times for image acquisition were employed for all experimental conditions. Panel D: Quantitative analysis of DCF fluorescence from five independent experiments (∼ 1,500 cells analyzed per experimental condition in each experiment) using ImageJ software (NIH Windows version). (*) indicates a statistically significant (p<0.005) difference relative to control cultures. (**) and (***) indicates a statistically significant (p<0.005) difference relative to Aβ-treated cultures. Scale bar corresponds to 100 µm. Panel E: 2-DOG decreases neuronal G6P levels in neurons exposed to Aβ. Glucose-6P levels were measured in control cortical neurons or neurons exposed to 5 µM Aβ+30 mM 2-DOG or 30 mM 2-DOG. Values represent means ± SD from three experiments (carried out in duplicate) using independent neuronal cultures. (*) indicates a statistically significant (p<0.001) difference relative to control cultures.
When neurons were treated with 5 µM FCCP (an uncoupler of oxidative phosphorylation that reduces mitochondrial membrane potential) in the presence or absence of Aβ, ROS levels became lower than those observed in control cultures (Fig. 3D), indicating that ROS formation in our experiments originates in mitochondria. We next measured intracellular G6P levels in cortical neurons exposed to Aβ, as G6P is a potent inhibitor of m-HKI at physiological concentrations and causes its release from brain mitochondria [29]. We found that treatment with 2-DOG, both in the absence and in the presence of Aβ greatly decreased neuronal G6P levels (Fig. 3E). Results indicate that the protective effects of 2-DOG may involve a decrease in intracellular G6P levels, thus counteracting the inhibition of HKI by Aβ and leading to decreased mitochondrial ROS production and death via enhanced ADP recycling.
DOG blocks Aβ-induced neuronal death
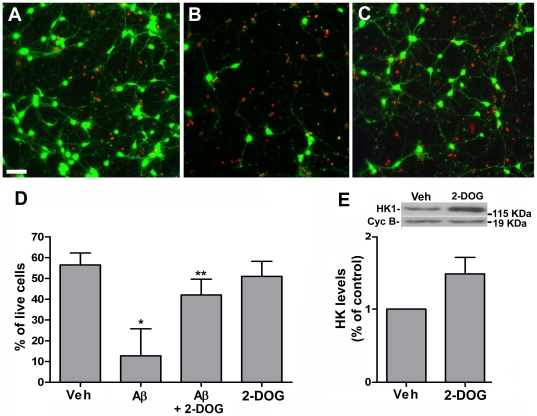
Finally, we investigated whether 2-DOG protects neurons against Aβ-induced neurodegeneration. Cortical neurons were treated for 48 hours with Aβ in the absence or presence of 2-DOG and neuronal viability was determined using the Live/Dead assay. A marked neurodegeneration was observed in cultures exposed to 20 µM Aβ1-42 for 48 hours (Fig. 4). Remarkably, 2-DOG led to significant blockade of Aβ-induced neuronal death (Fig. 4). 2-DOG by itself had no effect on the viability of neurons in culture during 48 hours (Fig. 4D). Finally, we investigated if 2-DOG could interfere with HK1 levels. Increased total levels of HKI were found in cortical neurons treated with 30 mM 2-DOG (Fig. 4E), suggesting that the neuroprotective effect afforded by 2-DOG may also involve up-regulation of HKI levels.
Figure 4. 2-DOG blocks Aβ-induced neurodegeneration.
Neuronal cortical cultures were maintained in the absence or in the presence of Aβ for 48 hours and cell viability was determined using the Live/Dead assay as described in “Materials and Methods”. Live cells are identified by green calcein fluorescence and dead cells are identified by red ethidium bromide fluorescence. Representative images from control (vehicle-treated) cultures (Panel A), cultures exposed to 20 µM Aβ (Panel B) or cultures treated with 20 µM Aβ+30 mM 2-DOG (Panel C). Panel D: Quantitative analysis of neuronal viability was carried out using Image J (described in “Methods”). A total of 3,000–4,000 cells were analyzed in each experimental condition. Bars show means ± SD from four different experiments (carried out in triplicate). (*) and (**) indicates a statistically significant (p<0.005 or p<0.01, respectively) difference relative to control cultures. Scale bar corresponds to 100 µm. Panel E: Effects of 2-DOG on neuronal HKI levels. Western immunoblot analysis of total HKI levels from cultured cortical neurons exposed to 30 mM 2-DOG for 48 hours. Cyclophilin B was used as a loading control. Bars correspond to means ± SD from four independent experiments.
Discussion
In the present work we show that Aβ, the neurotoxin thought to be responsible for neuronal deterioration in Alzheimer's disease (AD), causes the release of hexokinase from mitochondria and a parallel inhibition of enzyme activity. Mitochondrial-bound hexokinase (m-HKI) regulates important cellular processes in neurons, including ATP synthesis, maintenance of glutathione levels and neurite outgrowth [6], [7], [30]. Importantly, we have previously shown that m-HKI participates in a local ADP recycling mechanism that is directly involved in the regulation of mitochondrial oxidative homeostasis, preventing neurons from oxidative damage [8]. Oxidative stress is an important facet of AD [12]–[14]. We have now established that the impact of Aβ on neuronal hexokinase triggers mitochondrial oxidative stress and neuronal death. Importantly, the glucose analogue, 2-DOG, prevented Aβ-induced inactivation of m-HKI, and blocked excessive mitochondrial ROS formation and neuronal death. Aβ-induced cellular redistribution and inactivation of m-HKI may play central roles in oxidative stress and neurodegeneration in AD.
While monomeric Aβ is not neurotoxic, the peptide exhibits a marked toxic gain-of-function upon self-association. Fibrillar forms of Aβ found in amyloid plaques are toxic to neurons [21], [31] and were until recently considered being mainly responsible for neuronal damage in AD. More recently, however, new evidence has emerged demonstrating that Aβ oligomers, aggregates that are soluble and much smaller than fibrils, are potent neurotoxins in AD pathogenesis [17], [18], causing neuronal dysfunction at much lower concentrations than fibrillar aggregates. Aβ oligomers accumulate in the brains of AD patients [32], [33] and have been linked to major AD pathology hallmarks, including neuronal tau hyperphosphorylation [24], oxidative stress [14], [15] and synapse deterioration [25]. Interestingly, oligomers from a non-disease related protein have been shown to mimic Aβ-induced tau hyperphosphorylation and neurodegeneration [26]. Aβ oligomers further inhibit long-term potentiation (LTP) [34], [35]. Our results reveal a novel deleterious neuronal impact of Aβ oligomers, causing m-HKI release from mitochondria (Fig. 2).
We also found that Aβ oligomers cause a marked reduction in neuronal ATP levels (Fig. 2). Impairment of brain energy metabolism is another major facet in AD that has been revealed by in vivo studies of glucose utilization [12], [36]. Brain energy metabolism is also altered in transgenic mouse models of AD that present Aβ deposition and elevated levels of Aβ oligomers [37], [38]. The brain relies almost exclusively on glucose as its source of energy, using approximately 25% of circulating sugar. Normal glucose levels have been shown to protect brain cells from apoptotic events [39], demonstrating that a fine regulation of metabolism is crucial to cellular survival under stress conditions. Glial cells are believed to take up a significant fraction of glucose from the blood and provide neurons with lactate and glucose-derived energy substrates to sustain their activity [40], [41]. Nonetheless, neurons also rely directly on glucose provided via the extracellular space by the cerebral circulation. Conversion of glucose to G6P and oxidative phosphorylation take place both in neurons and glial cells and generate the ATP required to drive many cellular processes, including the excitability and survival of neurons and intracellular signaling pathways related to neuronal plasticity [28]. Our finding that Aβ oligomers decrease neuronal ATP levels indicates that they are linked to the impairment of energy metabolism in neurons. This might, at least in part, explain the deficits in glucose utilization in AD brains, a condition believed to result in synaptic dysfunction, neuronal degeneration and cognitive impairment.
Interestingly, Aβ did not inhibit hexokinase activity measured in an isolated mitochondria preparation (Fig. 1D). Rather, inhibition of m-HKI was observed when cortical neurons in culture were treated with Aβ (Fig. 1B). This suggests that Aβ-induced inactivation of m-HKI only occurs when the enzyme is associated with the mitochondrial membrane in the neuronal cellular context. Further studies will be required to investigate whether Aβ directly interacts with m-HKI in neurons. An elegant study demonstrated that Aβ interacts with alcohol dehydrogenase in the mitochondria of AD patients and transgenic mice [42]. In addition, nicastrin, presenilin, APH-1 and PEN-2 form active gamma-secretase complexes in mitochondria [43], suggesting that Aβ can be physiologically formed in mitochondria.
Another possibility is that Aβ interaction with specific membrane receptors causes deregulation of intracellular signaling cascades that result in m-HKI inhibition. We have shown that Aβ oligomers induce abnormal Ca2+ influx and neuronal oxidative stress through aberrant activation of N-methyl-D-aspartate (NMDA) receptors [14]. Several lines of evidence suggest that Aβ interferes with Wnt/β-catenin signaling [44], [45], causing GSK-3β activation, a kinase abnormally active in AD and involved in tau phosphorylation [46], [24]. GSK-3β phosphorylates VDAC, causing HKII release from mitochondria in HeLa cells [47]. Akt activation is required to maintain hexokinase attachment to mitochondria [30]. Growth factors known to induce Akt activation, such as insulin and insulin growth factor, may increase HK association to VDAC, protecting against Aβ. Insulin in fact has recently been shown to protect synapses against Aβ oligomers [25].
Neuronal oxidative stress is a hallmark of AD pathology. Neurons in culture exposed to Aβ present increased markers of oxidative stress [14], [48], [49]. It is well established that mitochondrial ROS formation triggers early events in apoptosis, including mitochondrial swelling, cytochrome c release and caspase activation [12]. During aging, neurons are subject to increased oxidative stress and impaired energy metabolism, leading to dysfunction of proteins responsible for maintaining proper membrane excitability and subcellular Ca2+ dynamics [28]. Oxidative stress destabilizes Ca2+ homeostasis and renders neurons vulnerable to excitotoxicity and apoptosis [50]. Mitochondria preserve neuronal Ca2+ homeostasis by generating the ATP required to pump Ca2+ out of the cytoplasm and by buffering cytosolic Ca2+ loads needed during normal synaptic activity [51]. Aβ-induced mitochondrial dysfunction and oxidative stress (Fig. 3) may thus be of central importance to disrupt neuronal Ca2+ homeostasis.
Excessive ROS formation due to hyperpolarization of the mitochondrial membrane has also been shown to result from hyperglycemic neuronal injury, a condition in which m-HKI is inhibited by accumulation of G6P [8], [29], [52]. It is possible, therefore, that the decrease in intraneuronal G6P levels in the presence of 2-DOG (Fig. 3E) relieves the inhibition of m-HKI, sustaining a steady-state ADP recycling that sets down the mitochondrial membrane potential to lower levels and, consequently, protects neurons from Aβ-induced oxidative stress. Our finding that 2-DOG completely blocked Aβ-induced ROS formation and neuronal death (Figs. 3 and 4) further indicates that preserving neuronal m-HKI activity by preventing its release from mitochondria plays a key role in neuronal survival. Blocking the impact of Aβ on m-HKI may provide a novel therapeutic approach to prevent oxidative stress and neurodegeneration in AD.
Footnotes
Competing Interests: WLK is co-founder of Acumen Pharmaceuticals, which has been licensed by Northwestern University to develop ADDL technology for Alzheimer's therapeutics and diagnostics. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by grants from the Human Frontier Science Program (HFSP) and The John Simon Guggenheim Memorial Foundation (FGF), Howard Hughes Medical Institute (STF), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico/Brazil, Fundacao de Amparo à Pesquisa do Estado do Rio de Janeiro/Brazil and Instituto Nacional de Neurociencia Translacional/Brazil (FGF and STF). WLK is supported by grants from the American Health Assistance Foundation, Alzheimer's Association, National Institutes of Health-National Institute on Aging Grants RO1-AG18877 and RO1-AG22547. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defense: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377:347–355. doi: 10.1042/BJ20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang TJ, Verkhratsky A, Fernyhough P. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol Cell Neurosci. 2005;28:42–54. doi: 10.1016/j.mcn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Gimenez-Cassina A, Lim F, Cerrato T, Palomo GM, Diaz-Nido J. Mitochondrial hexokinase II promotes neuronal survival and acts downstream of glycogen synthase kinase-3. J Biol Chem. 2009;284:3001–3011. doi: 10.1074/jbc.M808698200. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JE. Isozmes of mammalian hexokinase: structure, subcelular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 5.Rose IA, Warms JV. Mitochondrial hexokinase. Release, rebinding, and location. J Biol Chem. 1967;242:1635–1645. [PubMed] [Google Scholar]
- 6.Wang Z, Gardiner NJ, Fernyhough P. Blockade of hexokinase activity and binding to mitochondria inhibits neurite outgrowth in cultured adult rat sensory neurons. Neurosci Lett. 2008;434:6–11. doi: 10.1016/j.neulet.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 8.da-Silva WS, Gomez-Puyou A, De Gomez-Puyou MT, Moreno-Sanchez R, De Felice FG, et al. Mitochondrial Bound Hexokinase Activity as a Preventive Antioxidant Defense: steady-state ADP formation as a regulatory mechanism of membrane potential and reactive species generation in mitochondria. J Biol Chem. 2004;279:39846–55. doi: 10.1074/jbc.M403835200. [DOI] [PubMed] [Google Scholar]
- 9.Vyssokikh MY, Brdiczka D. The function of complexes between the outer mitochondrial membrane pore (VDAC) and the adenine nucleotide translocase in regulation of energy metabolism and apoptosis. Acta Biochim Pol. 2003;50:389–404. [PubMed] [Google Scholar]
- 10.Rothman DL, Behar KL, Hyder F. Trends Neurosci. 2004;27:489–495. doi: 10.1016/j.tins.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Serrano F, Klann E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res Rev. 2004;3:431–443. doi: 10.1016/j.arr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, et al. Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis. 2006;9:147–153. doi: 10.3233/jad-2006-9207. [DOI] [PubMed] [Google Scholar]
- 14.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, et al. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 15.Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, et al. Amyloid-β peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in neurons. J Neurochem. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 17.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in Alzheimer's and other amyloid diseases. IUBMB Life. 2007;59:332–345. doi: 10.1080/15216540701283882. [DOI] [PubMed] [Google Scholar]
- 19.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 20.Martins SM, Frosoni DJ, Martinez AM, De Felice FG, Ferreira ST. Formation of soluble oligomers and amyloid fibrils with physical properties of the scrapie isoform of the prion protein from the C-terminal domain of recombinant murine prion protein mPrP-(121-231). J Biol Chem. 2006;281:26121–26128. doi: 10.1074/jbc.M605367200. [DOI] [PubMed] [Google Scholar]
- 21.De Felice FG, Houzel JC, Garcia-Abreu J, Louzada PR, Jr, Afonso RC, et al. Inhibition of Alzheimer's disease beta-amyloid aggregation, neurotoxicity, and in vivo deposition by nitrophenols: implications for Alzheimer's therapy. FASEB J. 2001;15:1297–9. doi: 10.1096/fj.00-0676fje. [DOI] [PubMed] [Google Scholar]
- 22.De Felice FG, Vieira MN, Saraiva LM, Figueroa-Villar JD, Garcia-Abreu J, et al. Targeting the neurotoxic species in Alzheimer's disease: inhibitors of Abeta oligomerization. FASEB J. 2004;12:1366–72. doi: 10.1096/fj.04-1764com. [DOI] [PubMed] [Google Scholar]
- 23.Paula-Lima AC, De Felice FG, Brito-Moreira J, Ferreira ST. Activation of GABA(A) receptors by taurine and muscimol blocks the neurotoxicity of beta-amyloid in rat hippocampal and cortical neurons. Neuropharmacology. 2005;49:1140–1148. doi: 10.1016/j.neuropharm.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 24.De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, et al. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A-beta oligomers. Neurobiol Aging. 2008;29:1334–47. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, et al. Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–6. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira MN, Forny-Germano L, Saraiva LM, Sebollela A, Martinez AM, et al. Soluble oligomers from a non-disease related protein mimic Abeta-induced tau hyperphosphorylation and neurodegeneration. J Neurochem. 2007;103:736–748. doi: 10.1111/j.1471-4159.2007.04809.x. [DOI] [PubMed] [Google Scholar]
- 27.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 28.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skaff DA, Kim CS, Tsai HJ, Honzatko RB, Fromm HJ. Glucose 6-phosphate release of wild-type and mutant human brain hexokinases from mitochondria. J Biol Chem. 2005;280:38403–38409. doi: 10.1074/jbc.M506943200. [DOI] [PubMed] [Google Scholar]
- 30.Majewski N, Nogueira V, Bhaskar P, Coy PE, Skeen JE, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–830. doi: 10.1016/j.molcel.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 31.Lorenzo A, Yankner BA. Beta-amyloid neurotoxicity requires fibril formation and is inhibited by congo red. Proc Natl Acad Sci USA. 1994;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, et al. Alzheimer's disease-affected brain: presence of oligomeric Ab ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccini A, Russo C, Gliozzi A, Relini A, Vitali A, et al. Beta-amyloid is different in normal aging and in Alzheimer disease. J Biol Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- 34.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;6880:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 36.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigl M, Apelt J, Eschrich K, Schliebs R. Cortical glucose metabolism is altered in aged transgenic Tg2576 mice that demonstrate Alzheimer plaque pathology. J Neural Transm. 2003;110:77–94. doi: 10.1007/s00702-002-0772-x. [DOI] [PubMed] [Google Scholar]
- 38.Sadowski M, Pankiewicz J, Scholtzova H, Ji Y, Quartermain D, et al. Amyloid-beta deposition is associated with decreased hippocampal glucose metabolism and spatial memory impairment in APP/PS1 mice. J Neuropathol Exp Neurol. 2004;63:418–428. doi: 10.1093/jnen/63.5.418. [DOI] [PubMed] [Google Scholar]
- 39.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mo Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pellerin L. How astrocytes feed hungry neurons. Mol Neurobiol. 2005;32:59–72. doi: 10.1385/MN:32:1:059. [DOI] [PubMed] [Google Scholar]
- 41.Jolivet R, Magistretti PJ, Weber B. Deciphering neuron-glia compartmentalization in cortical energy metabolism. Front Neuroenergetics. 2009;1:4. doi: 10.3389/neuro.14.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer's disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 43.Andersson CX, Fernandez-Rodriguez J, Laos S, Baeckstrom D, Haass C, Hansson GC. Shedding and gamma-Secretase mediated intramembrane proteolysis of the mucin-type molecule CD43. Biochem J. 2004;387:377–384. doi: 10.1042/BJ20041387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inestrosa NC, Toledo EM. The role of Wnt signaling in neuronal dysfunction in Alzheimer's Disease. Mol Neurodegener. 2008;24:3–9. doi: 10.1186/1750-1326-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magdesian MH, Carvalho MM, Mendes FA, Saraiva LM, Juliano MA, et al. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem. 2008;283:9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol Chem Neuropathol. 1996;29:253–261. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- 47.Pastorino GJ, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3β disrupts the binding of hkII to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65:10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]
- 48.Mattson MP, Cheng B, Davis D, Bryant K, Lieberburg I, Rydel RE. Beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moreira PI, Smith MA, Zhu X, Nunomura A, Castellani RJ, Perry G. Oxidative stress and neurodegeneration. Ann N Y Acad Sci. 2005;1043:545–552. doi: 10.1196/annals.1333.062. [DOI] [PubMed] [Google Scholar]
- 51.Ly CV, Verstreken P. Mitochondria at the synapse. Neuroscientist. 2006;12:291–299. doi: 10.1177/1073858406287661. [DOI] [PubMed] [Google Scholar]
- 52.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]