Abstract
MicroRNAs profoundly impact hematopoietic cells by regulating progenitor cell-fate decisions, as well as mature immune effector function. However to date, microRNAs that regulate hematopoietic stem cell (HSC) function have been less well characterized. Here we show that microRNA-125b (miR-125b) is highly expressed in HSCs and its expression decreases in committed progenitors. Overexpression of miR-125b in mouse HSC enhances their function, demonstrated through serial transplantation of highly purified HSC, and enriches for the previously described Slamf1loCD34− lymphoid-balanced and the Slamf1negCD34− lymphoid-biased cell subsets within the multipotent HSC (CD34-KLS) fraction. Mature peripheral blood cells derived from the miR-125b–overexpressing HSC are skewed toward the lymphoid lineage. Consistent with this observation, miR-125b overexpression significantly increases the number of early B-progenitor cells within the spleen and induces the expansion and enrichment of the lymphoid-balanced and lymphoid-biased HSC subset via an antiapoptotic mechanism, reducing the mRNA expression levels of two proapoptotic targets, Bmf and KLF13. The antiapoptotic effect of miR-125b is more pronounced in the lymphoid-biased HSC subset because of their intrinsic higher baseline levels of apoptosis. These effects of miR-125b are associated with the development of lymphoproliferative disease, marked by expansion of CD8+ T lymphocytes. Taken together, these data reveal that miR-125b regulates HSC survival and can promote lymphoid-fate decisions at the level of the HSC by preferentially expanding lymphoid-balanced and lymphoid-biased HSC.
MicroRNAs are a class of evolutionarily conserved small RNAs that induce cleavage or translational repression of target mRNAs by binding to partially complementary seed sequences found on the 3′ UTR regions of target mRNAs (1). MicroRNAs are predicted to profoundly affect gene-expression profiles and regulate the expression of hundreds of mRNAs (1–3).
Several studies have demonstrated that miRNAs may regulate lineage-fate decisions in hematopoietic development. For example, ectopic expression of miR-181 in lineage-negative mouse bone-marrow cells leads to expansion of B cells but a diminution of T cells (4). Myeloid-specific miR-223 has been implicated in granulocytic development, with miR-223 knockout mice exhibiting increased numbers of granulocyte progenitors and mature cells (5). Deficiency of miR-150 leads to expansion of the B1 B-cell compartment, whereas ectopic expression of miR-150 impairs B cell development (6). In addition, miR-150, which is highly expressed in the megakaryocytic lineage, can bias differentiation of megakaryocyte-erythroid progenitors (MEP) toward the megakaryocytic fate at the expense of erythrocytes (7). More recently, studies suggest that miRNAs, such as miR-29a, may regulate hematopoietic stem cell (HSC) self-renewal, as evidenced by the aberrant induction of self-renewal in progenitor populations by miRNAs highly expressed in HSC and human acute myeloid leukemia (AML) (8).
We are interested in identifying genes that regulate HSC function and undertook an effort to identify miRNAs that are differentially expressed in HSC. We found that miR-125b is expressed at highest levels in mouse HSC, and that miR-125b expression decreases progressively as cells differentiate to myeloid and lymphoid committed progenitors, with miR-125b expressed at significantly higher levels in common lymphoid progenitors (CLP) compared with the common myeloid progenitors (CMP). To test the biological function of miR-125b in HSC, we overexpressed miR-125b in highly purified HSC using lentiviral vectors. Overexpression of miR-125b increased HSC engraftment in competitive transplants, and we confirmed that this effect was a result of cell-autonomous effects on HSC and not committed progenitors by recapitulating the phenotype through serial transplantation of highly purified HSC. In addition, miR-125b induced an expansion of the HSC compartment in part by inhibiting expression of at least two antiapoptotic target genes, Bmf (Bcl2 modifying factor) and KLF13 (Krueppel-like factor 13). Both targets were identified as potential miR-125b targets in vivo by evaluating purified stem and progenitor populations. The HSC expansion was associated with a lymphoid differentiation bias in the peripheral blood. In a small fraction of the miR-125b transplanted mice, we observed a lymphoproliferative disease.
Concurrently, O'Connell et al. (9) found that miR-125b is highly expressed in the stem and progenitor-cell compartment of the mouse bone marrow and that 1,000-fold overexpression of miR-125b in the hematopoietic stem and progenitor populations gave rise to a myeloproliferative disease that progressed to AML. Guo et al. (10) also found that miR-125a is highly expressed in the stem- and progenitor-cell compartment of the bone marrow. Overexpression of mir-125a also expanded the HSC compartment via an antiapoptotic mechanism, possibly by targeting the proapoptotic protein, Bak1. Our observations confirm these findings and extend them by demonstrating that miR-125b can induce preferential expansion of the previously described lymphoid-balanced and lymphoid-biased HSC (phenotypic SlamloCD34− and SlamnegCD34− KLS) populations.
Results
Expression Profiling of Hematopoietic Populations.
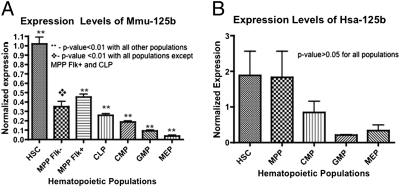
To identify miRNAs that may regulate HSC function, we first analyzed the miRNA expression profiles of multiple hematopoietic populations. We double-sorted HSC and committed progenitor populations from the bone marrow of C57BL/6-Thy 1.1 (BA) mice based on cell-surface markers defined by our laboratory and others (11): HSC (c-kit+Sca-1+Lin−Flk2−CD34−), multipotent progenitor (MPP) flk− cells (c-kit+Sca-1+Lin−Flk2−CD34+), MPP flk+ cells (c-kit+Sca-1+Lin−Flk2+CD34+), CLP cells (Ly6c−Flk2+IL7R+CD27+CD4−B220−CD19−CD11c−), CMP cells (c-kit+Sca-1−Lin−FcGloCD34+), granulocyte-monocyte progenitor (GMP) cells (c-kit+Sca-1−Lin−FcGhiCD34+), and MEP cells (c-kit+Sca1−Lin−FcGloCD34−). MicroRNAs were measured from total RNA isolated from each cell population using a previously described TaqMan real-time PCR strategy (8). Five independently sorted biologic replicates, each representing five pooled mice, were used for these studies. Our analysis revealed that miR-125b expression is consistently and significantly higher in HSC (>twofold, P < 0.05) compared with all other progenitor populations assayed (Fig. 1). In particular, the expression level of miR-125b is significantly higher in the CLP population compared with the CMP population. A similar trend was observed in human HSC and committed progenitor cell populations; however, the differences were not statistically significant (P > 0.05 comparing HSC with all progenitor populations), likely reflecting the genetic heterogeneity of the human cell populations.
Fig. 1.
MicroRNA-125b is highly expressed in HSCs and MPP cells in mice and humans. (A) Normalized expression level of miR-125b was determined by quantitative PCR using miRNA Taqman probes in double-sorted mouse hematopoietic cell populations: HSC (c-kit+Sca1+Lin−CD34−Flk2−), MPP Flk− (c-kit+Sca1+Lin−CD34+Flk2−), MPP Flk+ (c-kit+Sca1+Lin−CD34+Flk2+), CLP (Ly6c−Flk2+IL7R+CD27+CD4−B220−CD19−CD11c−), CMP (c-kit+Sca-1−Lin−FcGloCD34+), GMP (c-kit+Sca-1−Lin−FcGhiCD34+), and MEP(c-kit+Sca1−Lin−FcGloCD34− BM). Expression was normalized against mmu-mir-16 (n = 5). (B) Normalized expression levels of miR-125b were determined by quantitative PCR using miRNA Taqman probes in double-sorted bone marrow human hematopoietic cell populations: HSC (Lin−CD34+CD38−CD90+CD45RA−), MPP (Lin−CD34+CD38−CD90−CD45RA−), CMP (Lin−CD34+CD38+CD123+CD45RA−), GMP (Lin−CD34+CD38+CD123+CD45RA+), and MEP (Lin−CD34+CD38+CD123loCD45RA−). Expression was normalized against sno-R2. (n = 5). The differences were not statistically significant, possibly because of heterogeneity of the human hematopoietic populations. Error bars denote SEM.
Mir-125b exists in two locations in the mouse and human genomes. Mmu-miR-125b1, found on mouse chromosome 9, and hsa-miR-125b1, found on human chromosome 11, exist in a putative polycistronic cluster with miR-let7a2 and miR-100 (Fig. S1). Mmu-miR-125b2 (mouse chromosome 16) and hsa-miR-125b2 (human chromosome 21) exist in a cluster with miR-let7c1 and miR-99a. Interestingly, not all miRNA-125b cluster members have expression levels that covary with miR-125b in early hematopoietic progenitors. Specifically, only miR-99a and miR-100 appear to demonstrate similar profiles to miR-125b, with highest expression levels in HSC and gradually decreasing levels as the progenitors differentiate. Both miR-99a and miR-100 also demonstrate higher expression levels in the early lymphoid progenitors compared with the early myeloid progenitors. These results suggest that these miRNA polycistrons may be regulated by posttranscriptional mechanisms in early hematopoiesis.
Primary Transplants.
To study the role of miR-125b in the hematopoietic system, we ectopically expressed it in HSC using a lentiviral vector. Double-sorted highly enriched HSC (c-kit+Sca-1+Lin−CD34− cells) were transduced with miR-125b or empty control lentiviruses overnight and the transduced cells were transplanted into lethally irradiated congenic recipients with helper marrow cells (Fig. S2A). We measured the level of miR-125b overexpression in transduced (GFP+) unfractionated bone marrow cells of chimeric animals and determined that miR-125b–transduced animals overexpress miR-125b ≈35-fold (n = 4 independent experiments) (Fig. S2B). Primary transplant recipients exhibited similar total chimerism levels in the peripheral blood with a nonstatistically significant relative increase (1.3-fold) in T cells in miR-125b primary chimeras (>10 wk posttransplant). However, when we evaluated the bone marrow of primary chimeric mice >10 wk posttransplant, we found a significant increase (P < 0.05) in HSC chimerism in the miR-125b–overexpressing chimeras (Fig. S2C). This expansion was specific to the highly purified HSC (c-kit+Sca-1+Lin−CD34−) population and was not significant (P > 0.05) at the level of the KLS (c-kit+Sca-1+Lin−) cells, CMP (c-kit+Sca-1−Lin−) cells, or even the MPP (c-kit+Sca-1+Lin−CD34+) population.
Secondary Transplants.
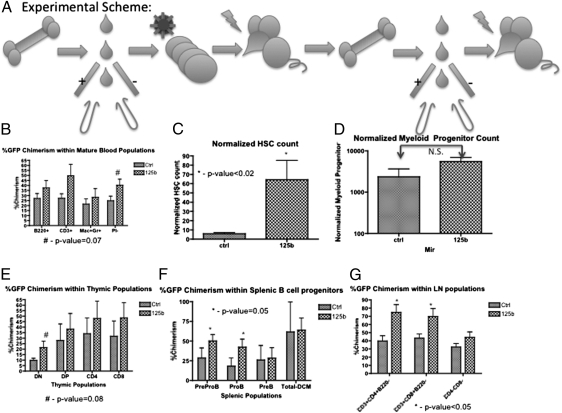
To determine if the miR-125b–induced phenotype observed in primary chimeras was caused by alterations in HSC function, we performed secondary transplants of double-sorted HSC (GFP+c-kit+Sca-1+Lin−CD34−Flk2−) and transplanted equal numbers (150 cells) from control and miR-125b primary chimeric mice into sublethally irradiated secondary recipients. MicroRNA-125b overexpressing HSC reconstituted the lymphoid and myeloid compartments (Mac-1+Gr-1+, CD3+, and B220+Mac-1− cells), and similar to the primary chimeras, secondary miR-125b HSC recipients exhibited significantly higher HSC chimerism (normalized HSC count: 6 vs. 64, P < 0.02) as well as a trend toward increased peripheral blood chimerism (25 vs. 40%, P = 0.07) with a predominance of lymphoid cells. Evaluation of the immature myeloid populations in the bone marrow revealed that the miR-125b HSC secondarily transplanted mice also demonstrated a trend toward higher GFP+ chimerism levels compared with control secondary chimeric mice; however, this difference was not statistically significant. MicroRNA-125b secondary recipients did demonstrate increased chimerism within the early lymphoid progenitors (preproB- and proB-cell populations) in the spleen (P = 0.05), twofold higher chimerism in the DN population of the thymus (P = 0.08), and higher chimerism within the CD4+ and CD8+ T-cell populations in the lymph nodes (P < 0.05) (Fig. 2). Interestingly, a small subset of the miR-125b chimeric mice (one of nine secondary recipients and one of nine tertiary recipients) developed a lymphoproliferative disease characterized by splenomegaly, abnormally large thymi, and an expansion of CD8+ T lymphocytes. The disease was marked by hepatic infiltration of lymphocytes with abnormal histology harboring multiple nuclei. (Fig. S3).
Fig. 2.
Ectopic expression of miR-125b increases engraftment capability of HSC and early lymphoid progenitors. (A) Experimental scheme. Primary transplants were sacrificed and 150 GFP+ HSC were sorted and transplanted into secondary recipients. (B) GFP+ chimerism levels were examined in peripheral blood myeloid, B-, and T-cell populations. (C) Normalized GFP+ HSC counts in bone marrow. (D) Normalized GFP+ myeloid progenitor counts in bone marrow. (E) GFP+ chimerism levels in developing T-progenitor populations in the thymus. (F) GFP+ chimerism levels in developing B-progenitor populations in the spleen. (G) GFP+ chimerism levels in lymphoid cell populations in the lymph nodes. Data from three independent experiments are shown (n = 6). All GFP+ chimerism analysis was conducted by flow cytometry. Error bars denote SEM.
Cell-Cycle Status.
One possible mechanism for the expansion of hematopoietic progenitors in miR-125b–overexpressing cells is an increased rate of cell cycling. To test this possibility, we examined the cell-cycle status of immature hematopoietic cells in miR-125b secondary chimeric mice using Ki-67 as a proliferation marker. Flow cytometry revealed that immature bone marrow c-kit+Lin−CD34− cells contained an equal frequency of Ki67+ cells in control and miR-125b chimeric mice (Fig. S4A). We also evaluated the colony-forming capacity of miR-125b–overexpressing MPPFlk2+ cells (c-kit+Lin−Sca-1+CD34+Flk2+) using in vitro clonal liquid culture assays. MicroRNA-125b overexpression increased the colony forming efficiency of the clone-sorted MPP Flk2+ cells by almost twofold, and single miR-125b–overexpressing MPP Flk2+ cells also generated significantly larger colonies as compared with control cultures (Fig. S4B) (P < 0.01). Taken together, these data indicate that the miR-125b enhances cell survival and that the increased output per cell in vitro is likely not a result of altered cell-cycle status of immature hematopoietic cells, but because of increased cell survival.
Antiapoptotic Effects of miR-125b.
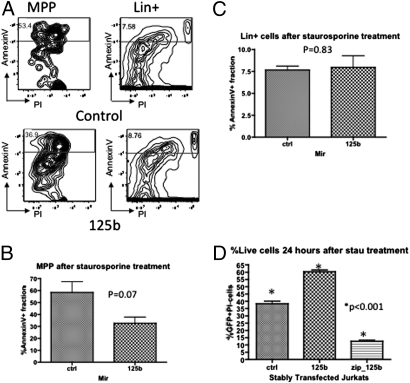
To study the potential antiapoptotic effects of miR-125b in immature hematopoietic cells, we enriched c-kit+ bone-marrow cells from miR-125b primary chimeric mice and challenged the cells with staurosporine, a well-known apoptosis inducer (12, 13). We found that miR-125b–overexpressing MPP cells were less prone to apoptosis compared with control MPP cells, with the annexin V+ fraction in miR-125b–overexpressing cells lower than control (Fig. 3B) (P = 0.07). Interestingly, the Lineage-positive fraction, composed mostly of mature bone marrow cells, did not exhibit differential sensitivity to staurosporine-induced apoptosis (Fig. 3C) (P = 0.83). This finding demonstrates that the antiapoptotic effect of miR-125b is predominantly confined to the immature (Lineage-negative) hematopoietic compartment.
Fig. 3.
Overexpression of microRNA-125b confers a survival advantage to immature hematopoietic cells by inhibiting apoptosis in response to stress. (A) c-Kit+ bone marrow cells were subjected to staurosporine treatment. Representative profile for analyzing annexin V+ fraction is shown for control mice and miR-125b mice. The left plots are representative for the MPP populations and the right plots are representative for the Lin+ populations. The cells scored include propium iodide-positive cells. (B) The annexin V+ fraction within the MPP population of control and miR-125b mice (n = 3). (C) The annexin V+ fraction within the lineage-positive population of control and miR-125b mice. (D) Immature human T-leukemia cell line Jurkat, stably expressing control, miR-125b overexpressing and anti-miR-125b (zip_125b) constructs, were treated with staurosporine and the viability of the cells were examined by propium iodide staining. Representative data from three independent experiments are shown (n = 9). Error bars denote SEM.
To determine if miR-125b can provide antiapoptotic signals in immature lymphoid cells as well, we stably expressed miR-125b in Jurkat cells, an immature human T-cell leukemia cell line. Following staurospine treatment, Jurkat cells overexpressing miR-125b exhibited increased viability, similar to the primary mouse c-kit+ cells. Consistent with this effect, Jurkat cells stably expressing anti-mir-125b (zip125b) demonstrated increased susceptibility to staurosporine-induced apoptosis (Fig. 3D).
MicroRNA-125b Induces Expansion of Slamf1lo and Slamf1neg HSC.
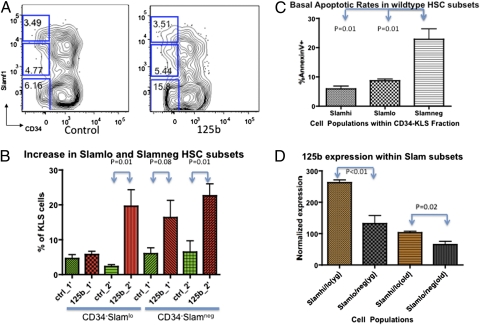
Given miR-125b's ability to expand HSC and lymphoid precursor cells in chimeric animals, we evaluated the cellular basis for the miR-125b–induced lymphoid expansion. Closer examination of miR-125b–overexpressing chimeras revealed a statistically significant higher percentage of Slamf1loCD34− and Slamf1negCD34− cells in the KLS compartment of secondary chimeras (Fig. 4B) (P = 0.01). Within the KLS compartment of the primary chimeras, there was an enrichment of the Slamf1negCD34− population (Fig. 4B) (P = 0.08). Slamf1loCD34− and Slamf1negCD34−cells have been shown to possess a balanced myeloid/lymphoid and lymphoid-biased cell output, respectively, compared with Slamf1hi HSC, which exhibit myeloid-biased differentiation (14, 15). Based on this observation, we hypothesized that the Slamf1loCD34− and Slamf1negCD34− expansion may be a result of the enhanced antiapoptotic effects of miR-125b in this cell population. Analysis of Slamf1hi, Slamf1lo, and Slamf1negCD34− HSC in normal, unperturbed mice demonstrates that the Slamf1negCD34− cell population normally contains higher percentages of annexin V+ cells than Slamf1hiCD34− and Slamf1loCD34− KLS cells (Fig. 4C) (P = 0.01). There is also a general inverse trend of increasing basal apoptosis rates with decreasing Slamf1 expression within the HSC (KLS CD34−) population. In addition, wild-type Slamf1lo/negCD34− HSC express lower levels of miR-125b than Slamf1hi/loCD34− HSC under steady-state physiological settings, and miR-125b expression levels are reduced in both populations with age (Fig. 4D) (P < 0.05). Thus, miR-125b is likely to induce expansion of the Slamlo and Slamneg population by providing them with enhanced survival signals. Over time, the expansion of the Slamf1loCD34− and Slamf1negCD34− population is enhanced in the miR-125b chimeric mice, as shown by a significant increase in the Slamf1loCD34− and Slamf1negCD34− fraction in secondary chimeras compared with primary chimeras (Fig. 4B) (P = 0.01).
Fig. 4.
Overexpression of microRNA-125b preferentially expands lymphoid-balanced and lymphoid-biased KLS CD34−Slamf1lo and CD34−Slamf1neg HSC. (A) Representative Slamf1 profiles within the c-Kit+Sca1+Lin− population of control and miR-125b chimeric mice are shown. (B) The fraction of CD34−Slamf1lo and CD34−Slamf1neg cells within the c-Kit+Sca1+Lin− population for control and miR-125b chimeric mice from primary and secondary transplants is shown in the bar graph (n = 6). (C) Wild-type mice exhibit different physiological levels of annexin V+ cells within the Slamf1hi and Slamf1lo and Slamf1neg populations of wild-type mice (n = 6). (D) Normalized expression levels of miR-125b were determined by quantitative PCR using miRNA Taqman probes in double-sorted Slamf1hi/lo and Slamf1lo/neg HSC populations of wild-type young (3.5 mo) and old (15.5 mo) mice (n = 3). Error bars denote SEM.
Identification of miR-125b Targets.
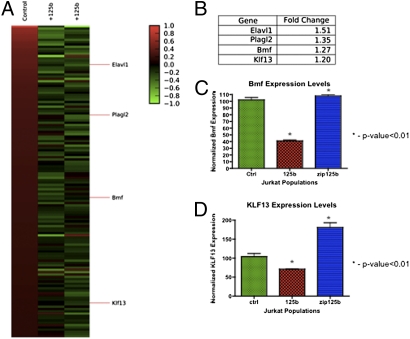
To identify the mRNA targets of miR-125b responsible for its antiapoptotic effects, we sorted GFP+c-kit+ Sca-1+ Lin− (GFP+KLS) cells from miR-125b–overexpressing and control primary chimeras for mRNA microarray analysis. We ranked the transcripts that were most reduced in miR-125b–overexpressing KLS cells and filtered the top 6,000 hits (∼top 15% of total probes represented on the Affymetrix Mouse Genome 430 2.0 Array) (Fig. 5 A and B). Of the 6,000 hits, we identified those mRNAs predicted to be a direct target of miR-125b using TargetScan (v5.1) and found 136 targets (16–18). Of these targets, two interesting genes that emerged, based on their relevance to apoptosis regulation, are Bmf and KLF13. We isolated total mRNA from Jurkat cells stably overexpressing miR-125b and verified by qPCR down-regulation of endogenous Bmf and KLF13 transcript levels by 2.5- and 1.5-fold, respectively (Fig. 5C). Bmf and KLF13 gene expression are respectively restored and slightly increased in Jurkat cells stably expressing the anti-mir-125b (zip125b) (Fig. 5C).
Fig. 5.
Potential mRNA targets of microRNA-125b that mediate the antiapoptotic phenotype in immature hematopoietic cells are Bmf and KLF13. (A) Chimeric mice transplanted with control or miR-125b overexpressing HSC were sacrificed and c-Kit+Sca1+Lin− GFP+ cells were sorted from the bone marrow for microarray analysis. The heat map displays the genes that (i) are down-regulated in miR-125b overexpressing stem and progenitor cells and (ii) are predicted to contain seed sequences for miR-125b. (B) Table corresponding to the heat map listing fold-change of potential gene targets. (C, D) Quantiative PCR analysis of Bmf and KLF13 mRNA levels of Bmf and KLF13 in control vs. miR-125b Jurkat cells. Data were normalized to expression of β-actin (n = 3). Error bars denote SEM.
Discussion
The ability of a single HSC to reconstitute the entire blood system for the lifetime of an organism is made possible by the balance of factors that regulate self-renewal and fate-commitment decisions (19). Recent work has demonstrated that individual HSC may exhibit different lineage capacities, and that such HSC may be prospectively isolated based on differential expression of the Slamf1 cell-surface marker (14, 15). Identifying the mechanisms underlying the differences between myeloid-biased, lymphoid-balanced, and lymphoid-biased HSC populations will be critical to understanding the regulation of cell-fate decisions and may potentially provide key information regarding how to manipulate HSC composition or function for therapeutic purposes.
Our studies reveal that miR-125b is highly expressed in normal HSC and is down-regulated in committed progenitors. Overexpression of miR-125b in highly purified HSC induces expansion of the HSC compartment in both primary and secondary transplantation assays, demonstrating that the observed phenotypes are the result of differences in HSC function. Investigation of the mechanisms underlying this phenonomen demonstrate that miR-125b–overexpressing HSC do not expand because of increased proliferation but because of decreased apoptosis. Evaluation of both primary HSC and immature T-cell lines expressing miR-125b demonstrate that miR-125b induces an antiapoptotic state and decreases apoptosis in response to in vitro stressors. Similar to other authors (20), we find that normal HSC exhibit low-apoptotic rates; however, baseline apoptotic rates in myeloid-biased and lymphoid-biased HSC differ in wild-type mice. Thus, miR-125b overexpression would be expected to induce larger changes in the number of cells in populations exhibiting higher baseline apoptosis, as even small differences in apoptotic frequency can lead to profound changes in the composition of the HSC pool over time. For example, assuming two HSC populations divide with equal frequency and propensity to self-renew, a 3% decrease in apoptotic rates could potentially translate into a 1.36-fold HSC expansion over 10 cell death/survival cycles, and 1.84-fold expansion over 20 cell death/survival cycles. Consistent with this finding, we found that in the context of ectopic miR-125b expression, the lymphoid-balanced Slamf1lo and the lymphoid-biased Slamf1neg HSC showed a progressive increase in frequency within the KLS population.
Decreased levels of apoptosis in HSC have previously been shown to induce HSC expansion in studies involving H2K-Bcl2 mice. Domen et al. showed that the antiapoptotic property of Bcl2 protects cells from apoptosis inducers and results in a significant (2.4-fold) expansion of stem and progenitor cells as compared with wild-type mice (21). The HSC and committed progenitor populations in H2K-Bcl2 transgenic mice also demonstrated greater repopulating activity in competition with normal HSC in transplantation assays, similar to that observed with miR-125b overexpressing HSC. Interestingly, miR-125b is expressed at higher levels in CLP than in CMP, suggesting that miR-125b plays a significant role in maintaining the size of the lymphoid compartment by limiting apoptosis of lymphoid progenitors. Consistent with this prediction, miR-125b increased pro-B–cell numbers and immature T-cell numbers. These changes did not result in significantly altered mature lymphoid cell chimerism in the peripheral blood, suggesting that physiologic homeostatic control of the peripheral lymphoid compartment is intact.
We also attempted to study the biological effects of miR-125b by knocking-down miR-125b using a lentiviral anti-miR construct; however, we were unable to engraft anti-miR-125b–expressing HSC in congenic transplantation experiments. The transplanted mice were engrafted with GFP− donor cells, demonstrating that only nontransduced HSC engrafted. The lack of engraftment may be a result of miR-125b knockdown effects on HSC homing, direct cytotoxicity, or other technical reasons, as we routinely observed lower lentiviral titers of anti-miR-125b virus.
Given miR-125b's role in HSC, one might predict it may play roles in leukemic transformation or maintenance. Recent studies have identified a novel translocation (22, 23) in B-ALL involving miR-125b and IgH, resulting in overexpression of miR-125b. A specific subset of ALL involving the AML1-TEL translocation (24) was also found to express higher levels of miR-125b that was important for the pathogenesis of the disease. We note with interest that the pro-B–cell population is expanded in miR-125b chimeric mice. The pro-B–cell population undergoes VDJ recombination and thus one might speculate that aberrant recombination between miR-125b and IgH in this cell population can lead to resistance to apoptosis and increased propensity to develop leukemia. In support of miR-125b possible role in AML, one of the two copies of miR-125b is found on human chromosome 21 and is implicated in Down syndrome-related megakaryoblastic leukemias (25). High levels of miR-125b also strongly correlate with the t(2;11)(p21;q23) translocation involved in myelodysplastic syndrome and AML (26). In miR-125b–related hematopoietic malignancies reported thus far, the overexpression of miR-125b in patient samples appears to be in the range of 20- to 500-fold. We observed a lymphoproliferative disease with low penetrance in chimeric mice transplanted with lentivirally transduced HSC overexpressing miR-125b at a level ∼35 times physiological levels. Similarly, O'Connell et al. (9) observed that retroviral transduction of 5-FU cells with miR-125b resulted in the formation of a high penetrance myeloproliferative disorder that develops into AML in chimeric mice transplanted; miR-125b expression levels in these mice were approximately 1,000 times higher than normal physiologic levels. Thus, it is likely that the degree of miR-125b overexpression determines hematologic phenotypes observed in vivo, possibly by affecting HSC survival and lineage bias.
MicroRNA-125b antiapoptotic effects are at least partially mediated by its target genes Bmf and KLF13, both of which have been shown by other groups to be miR-125 targets using luciferase reporter assays (27, 28). Interestingly, the knockout phenotype for KLF13 and Bmf are similar to the miR-125b phenotype, as KLF13−/− thymocytes exhibited decreased apoptosis (29) and Bmf−/− lymphocytes were protected from apoptosis induced by glucocorticoids or histone deacetylase inhibition and developed γ-irradiation–induced thymic lymphomas with shorter latencies (30). The magnitude of the reduction in expression of predicted miR-125b targets identified by mRNA microarrays was not very high (∼1.2- to 3-fold reduction). It appears, however, that these small reductions in mRNA expression are sufficient to tip the balance of apoptotic and antiapoptotic signals. Our studies demonstrate that increased expression of miR-125b also confers an antiapoptotic advantage to committed lymphoid cells, as demonstrated in the Jurkat T-cell line. Hence, it is possible that the increased lymphoid output observed in miR-125b–overexpressing mice may be because of survival advantages induced both at the level of the HSC as well as more mature lymphoid cells.
The literature supports the notion that miR-125b plays different roles depending on cell context. In the immature hematopoietic system, miR-125b is antiapoptotic and promotes expansion of HSC; its specific effect is determined by context, as different cell populations have different levels of baseline apoptosis during homeostatic hematopoiesis. This context-specific property may explain why miR-125b overexpression is associated with multiple hematologic malignancies. MicroRNA-125b can also confer increased chemoresistance of breast cancer cells by inducing antiapoptotic pathways (31). MicroRNA-125b may also serve a tumor suppressor role in some tumors, as miR-125b has been shown to suppress proliferation of gliomas (32) and ovarian cancer (33). In addition, SNPs in the 3′ UTR of BMPR1B, a direct target of miR-125b, confer a good prognosis in breast cancer by abrogating miR-125b down-regulation of BMPR1B (34). Future studies aimed at understand how miR-125b is regulated in different cellular contexts will be important for elucidating its myriad of biological roles.
Materials and Methods
Mice.
Unless otherwise noted, mouse experiments were performed using young adult (8- to 12-wk-old) C57BL/6-Thy1.1 mice. All mice were maintained in Stanford University's Research Animal Facility in accordance with Stanford University guidelines.
Cell Sorting.
Antibody staining and enrichment procedures for mouse HSC, Flk2− MPP, Flk2+ MPP, CMP, GMP, MEP, and CLP cell sorting and analyses were performed, as previously described (35–38). Hematopoietic cell populations were derived from bone marrow isolated from mouse femurs, tibias, pelvis, and spine.
MicroRNA Profiling.
Total RNA was prepared from sorted mouse and human populations using mirVana RNA prep kits (Ambion) according to the manufacturer's protocol. MicroRNA profiling was done using a set of 283 unique TaqMan mouse miRNA assays and 315 unique Taqman human miRNA assays (Applied Biosystems) (39).
In Vivo Transplants.
HSCs for transplantation assays were double-sorted on the FACSAriaII using a panel of stem-cell surface markers. Lentiviral vectors for inducing stable miR or anti-miR expression were purchased from System Biosciences. The HSC were transduced overnight with lentivirus expressing GFP only, GFP and miR-125b, or GFP and anti-miR-125b in DMEM-F12 with 10% FCS, β-mercaptoethanol, 10 ng/μL SCF, 10 ng/μL Flt3 ligand, 10 ng/μL TPO, 10 ng/μL IL-3, and 10 ng/μL IL-6. The cells were washed and transplantations were performed by retro-orbital injection under isoflurane anesthesia. Recipient mice were lethally irradiated (1,050 rad, delivered in a split dose, 3 h apart) or sublethally irradiated (788 rad, delivered in split dose, 3 h apart) using a Cs irradiator source and given antibiotic containing water for at least 6 wk postirradiation.
In Vitro Culture.
For single-cell cultures, single MPP Flk2− were sorted into 96-well U-bottom plates and cultured in DMEM-F12 with 10% FCS, β-mercaptoethanol, 10 ng/μL SCF, 10 ng/μL Flt3 ligand, 10 ng/μL TPO, 10 ng/μL IL-3, 10 ng/μL IL-6, 10 ng/μL EPO, and 10 ng/μL GM-CSF. Plates were incubated at 37 °C for 1 wk.
Cell-Cycle Analysis.
Bone marrow cells were enriched based on surface c-kit expression and fixed and permeabilized using the FIX & PERM kit (CALTAG GAS-003) according to the manufacturer's instructions. The cells were incubated with anti-Ki67-PE antibody and resuspended in 500 μL PBS containing 5 μg/mL RNaseA and 2 μg/mL DAPI for FACS Analysis.
Apoptosis Assay.
Bone marrow cells were enriched based on surface c-kit expression. The c-kit–enriched bone marrow were incubated in 1 μM staurosporine for 1 h at 37 °C to induce apoptosis. The cells were then stained for hematopoietic cell-surface markers and incubated in annexin V binding solution and annexin V-APC antibody according to manufacturer's instructions (BD Pharmingen; 550474).
Microarray Analysis.
Total RNA was prepared from sorted mouse c-kit+Sca-1+Lin− GFP+ cells using mirVana RNA prep kits (Ambion) according to the manufacturer's protocol for three arrays, one control and two miR-125b–overexpressing arrays. For further details, please see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank M. Inlay, T. Serwold, and D. Rossi for helpful scientific discussions, D. Sam for biostatistical expertise, L. Jerabek and T. Storm for superb laboratory management, A. Mosley for animal supervision, T. Naik for antibody preparations, M. Belen for animal care, and E. Zuo for assistance with microarray experiments. A.G.L.O. is supported by the Singapore Agency for Science Technology and Research. This study was supported in part by a grant from Siebel Stem Cell Institute and the Thomas and Stacey Siebel Foundation (to D.S.), and National Cancer Institute/National Institutes of Health Grants KO8 CA1295470 and 3P30CA008748-44S5 (to C.Y.P.).
Footnotes
Conflict of interest statement: I.L.W. owns stock in Amgen, Inc., is a Director of Stem Cells, Inc., and is a cofounder of Cellerant, Inc. and Stem Cells, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016218107/-/DCSupplemental.
References
- 1.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baek D, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selbach M, et al. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 4.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 5.Johnnidis JB, et al. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 6.Xiao C, et al. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, et al. MicroRNA-mediated control of cell fate in megakaryocyte-erythrocyte progenitors. Dev Cell. 2008;14:843–853. doi: 10.1016/j.devcel.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YC, et al. MicroRNA-29a induces aberrant self-renewal capacity in hematopoietic progenitors, biased myeloid development, and acute myeloid leukemia. J Exp Med. 2010;207:475–489. doi: 10.1084/jem.20090831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connell RM, et al. MicroRNAs enriched in hematopoietic stem cells differentially regulate long-term hematopoietic output. Proc Natl Acad Sci USA. 2010;107:14235–14240. doi: 10.1073/pnas.1009798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo S, et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc Natl Acad Sci USA. 2010;107:14229–14234. doi: 10.1073/pnas.0913574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MP, Seita J, Weissman IL. Establishment of a normal hematopoietic and leukemia stem cell hierarchy. Cold Spring Harb Symp Quant Biol. 2008;73:439–449. doi: 10.1101/sqb.2008.73.031. [DOI] [PubMed] [Google Scholar]
- 12.Tafani M, et al. Regulation of intracellular pH mediates Bax activation in HeLa cells treated with staurosporine or tumor necrosis factor-alpha. J Biol Chem. 2002;277:49569–49576. doi: 10.1074/jbc.M208915200. [DOI] [PubMed] [Google Scholar]
- 13.Tafani M, Minchenko DA, Serroni A, Farber JL. Induction of the mitochondrial permeability transition mediates the killing of HeLa cells by staurosporine. Cancer Res. 2001;61:2459–2466. [PubMed] [Google Scholar]
- 14.Beerman I, et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci USA. 2010;107:5465–5470. doi: 10.1073/pnas.1000834107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 17.Grimson A, et al. MicroRNA targeting specificity in mammals: Determinants beyond seed pairing. Mol Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 20.Opferman JT, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 21.Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191:253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tassano E, et al. MicroRNA-125b-1 and BLID upregulation resulting from a novel IGH translocation in childhood B-Cell precursor acute lymphoblastic leukemia. Genes Chromosomes Cancer. 2010;49:682–687. doi: 10.1002/gcc.20776. [DOI] [PubMed] [Google Scholar]
- 23.Chapiro E, et al. A new recurrent translocation t(11;14)(q24;q32) involving IGH@ and miR-125b-1 in B-cell progenitor acute lymphoblastic leukemia. Leukemia. 2010;24:1362–1364. doi: 10.1038/leu.2010.93. [DOI] [PubMed] [Google Scholar]
- 24.Gefen N, et al. Hsa-mir-125b-2 is highly expressed in childhood ETV6/RUNX1 (TEL/AML1) leukemias and confers survival advantage to growth inhibitory signals independent of p53. Leukemia. 2010;24:89–96. doi: 10.1038/leu.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klusmann JH, et al. miR-125b-2 is a potential oncomiR on human chromosome 21 in megakaryoblastic leukemia. Genes Dev. 2010;24:478–490. doi: 10.1101/gad.1856210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bousquet M, et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J Exp Med. 2008;205:2499–2506. doi: 10.1084/jem.20080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia HF, et al. MiR-125b expression affects the proliferation and apoptosis of human glioma cells by targeting Bmf. Cell Physiol Biochem. 2009;23:347–358. doi: 10.1159/000218181. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, et al. MicroRNA-125a contributes to elevated inflammatory chemokine RANTES via targeting KLF13 in systemic lupus erythematosus. Arthritis Rheum. 2010;62:3425–3435. doi: 10.1002/art.27632. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, et al. Kruppel-like transcription factor 13 regulates T lymphocyte survival in vivo. J Immunol. 2007;178:5496–5504. doi: 10.4049/jimmunol.178.9.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Labi V, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou M, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi L, et al. MiR-125b is critical for the suppression of human U251 glioma stem cell proliferation. Brain Res. 2010;1312:120–126. doi: 10.1016/j.brainres.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 33.Guan Y, Yao H, Zheng Z, Qiu G, Sun K. MiR-125b targets BCL3 and suppresses ovarian cancer proliferation. Int J Cancer. 2010 doi: 10.1002/ijc.25575. in press. [DOI] [PubMed] [Google Scholar]
- 34.Saetrom P, et al. A risk variant in an miR-125b binding site in BMPR1B is associated with breast cancer pathogenesis. Cancer Res. 2009;69:7459–7465. doi: 10.1158/0008-5472.CAN-09-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 36.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci USA. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 38.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Chen C, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.