Abstract
The nonreinforced expression of long-tem memory may lead to two opposite protein synthesis-dependent processes: extinction and reconsolidation. Extinction weakens consolidated memories, whereas reconsolidation allows incorporation of additional information into them. Knowledge about these two processes has accumulated in recent years, but their possible interaction has not been evaluated yet. Here, we report that inhibition of protein synthesis in the CA1 region of the dorsal hippocampus after retrieval of fear extinction impedes subsequent reactivation of the extinction memory trace without affecting its storage or that of the initial fear memory. Our results suggest that extinction memory is susceptible to a retrieval-induced process similar to reconsolidation in the hippocampus.
Keywords: learning, anisomycin, amnesia
Without retrieval, memories would be unusable. Retrieval, however, can weaken long-term memory (LTM). In fact, it is known that reactivation through exposure to training-related stimuli transiently destabilizes the consolidated memory trace, which, to remain behaviorally available, must go through a protein synthesis-dependent process called reconsolidation. Additionally, when retrieval in the absence of appropriate reinforcement is repeated regularly enough, it induces memory extinction, a phenomenon characterized by a decrease in the amplitude and/or frequency of the learned response that also requires protein synthesis in definite areas of the brain.
Reconsolidation and extinction are functionally related; both require acquisition of retrieval-related information connected to previous learning. They are mechanistically different, however; extinction involves additional learning and replaces the expression of the original memory with a newly formed one (1, 2), whereas reconsolidation restabilizes the old memory opened to modification or strengthening by retrieval (3–6).
Given the clinical relevance that reconsolidation blockade and extinction enhancement could have (7, 8), research about the consequences of retrieval on LTM persistence has concentrated on the molecular, neuroanatomical, and electrophysiological requirements of reconsolidation and extinction (9–12) as well as on the elucidation of the behavioral and neurochemical conditions that constrain or facilitate either process (13–15). The potential interaction of extinction and reconsolidation has been comparatively less studied, and when so, the studies produced contradictory results. Thus, it was earlier proposed that extinction can act as a boundary condition preventing fear memories from undergoing reconsolidation (16, 17), but more recent reports indicate that reconsolidation is independent of any influence from fear extinction (18). Indeed, the likelihood of reconsolidation for fear extinction memory has never been evaluated before.
To do that, we analyzed the effect of the intrahippocampal administration of protein synthesis inhibitors on the stability of a consolidated fear extinction memory trace. Our findings strongly support the hypothesis that, as happens for other memory types, fear extinction memory is susceptible to a retrieval-induced process similar to reconsolidation in the hippocampus.
Results
To analyze whether inhibition of protein synthesis after reactivation affects retention of fear extinction, we used a one-trial step-down inhibitory avoidance (IA) task, a form of aversive learning in which stepping down from a platform is paired with a mild foot shock. IA memory is hippocampus-dependent (19) and can be extinguished by allowing the animal to step down to and explore the floor of the training box in the absence of the ensuing foot shock. If this procedure is repeated once a day for 5 consecutive days, it induces clear-cut extinction of the avoidance response that lasts for at least 14 d (Fig. 1A) and requires protein synthesis in dorsal CA1 during a short postretrieval time window to occur (20).
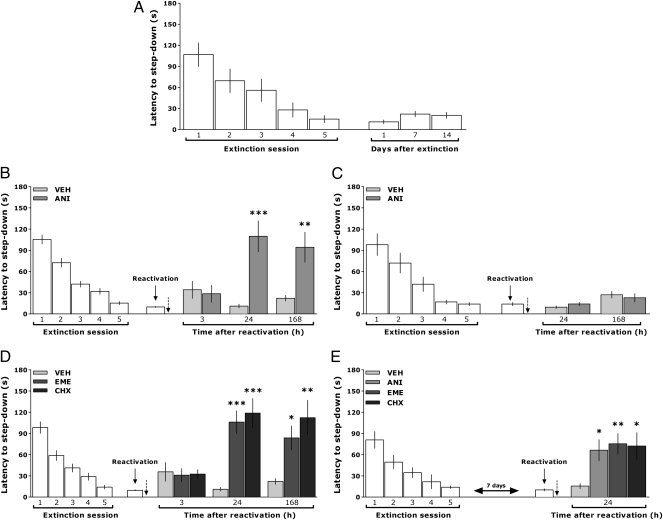
Fig. 1.
Inhibition of hippocampal protein synthesis after reactivation hinders extinction memory. (A) Rats trained in the IA task using a 2-s 0.5-mA foot shock as an unconditioned stimulus were submitted to five extinction sessions 24, 48, 72, 96, and 120 h after training. Retention was evaluated 1, 7, or 14 d later. Note the absence of spontaneous recovery of the avoidance response. (B) Rats trained in the IA task were submitted to five extinction sessions 24, 48, 72, 96, and 120 h posttraining. One day later, extinction memory was reactivated; immediately thereafter, animals were given VEH or ANI in dorsal CA1. Retention was evaluated 3, 24, or 168 h later. (C) Rats were treated as in B except that VEH and ANI were given 6 h after reactivation. (D) Animals were treated as in B except that they received EME or CHX instead of ANI. (E) Rats were treated as in B and C except that reactivation was carried out 7 d after the last extinction training session (n = 10–12 per group). Bars represent the mean (±SEM) of step-down latencies. *P < 0.05; **P < 0.01; ***P < 0.001 using the Student t test or Bonferroni test after ANOVA. The dotted arrows indicate the moment of drug infusion.
Rats trained in the IA task were submitted to the extinction procedure described above (first session, 24 h posttraining), and 24 h after the last extinction session, extinction memory was reactivated. Immediately or 6 h later, the animals received bilateral intra-CA1 infusions of the protein synthesis inhibitor anisomycin (ANI; 160 μg per side) (21–23). Retention was assessed at different postreactivation times. As can be seen in Fig. 1B, when given immediately after reactivation of extinction memory, ANI impaired retention of extinction and, as a consequence, allowed reappearance of the IA response. This effect was observed 24 h and 168 h but not 3 h after reactivation [t(15) = 4.72, P < 0.001 and t(15) = 3.52, P < 0.01 for 24 h and 168 h, respectively]. Conversely, ANI did not affect the retention of extinction at any postinfusion time evaluated when given into dorsal CA1 6 h after reactivation (Fig. 1C), suggesting that the impairment of extinction memory was attributable to a time- and protein synthesis-dependent process and not to an unspecific action of ANI on behavior or hippocampal functionality. Nevertheless, it has been reported that inhibition of protein synthesis with ANI might disrupt other neural functions able to interfere with memory processing (24). Therefore, to discard any ambiguous interpretation of our results, we analyzed the effect of two other widely used protein synthesis inhibitors, emetine (EME) (25) and cycloheximide (CHX) (26), on the persistence of fear extinction. Intra-CA1 infusion of EME (50 μg per side) or CHX (20 μg per side) immediately after reactivation of extinction memory also impaired its retention and allowed recovery of the IA response [F(2,25) = 15.98, P < 0.001; tEME vs. VEH = 4.61, P < 0.001; tCHX vs. VEH = 5.10, P < 0.001 and F(2,24) = 7.70, P < 0.01; tEME vs. VEH = 2.60, P < 0.05; tCHX vs. VEH = 3.82, P < 0.01 for 24 h and 168 h, respectively] (Fig. 1D). It has been suggested that the postretrieval effect of some amnesic agents depends on the age of the mnemonic trace. Thus, young memories would be more prone than older ones to pharmacological disruption following reactivation (27, 28). We found, however, that the amnesic effect of ANI, EME, and CHX on the retention of fear extinction memory also occurred when reactivation was carried out 7 d instead of 1 d after the last extinction training session [F(3,33) = 4.79, P < 0.01; tANI vs. VEH = 2.72; P < 0.05; tEME vs. VEH = 3.21, P < 0.01; tCHX vs. VEH = 3.04, P < 0.05) (Fig. 1E). Importantly, neither ANI, EME, nor CHX affected retention of fear extinction memory when given 1 or 7 d after the end of the extinction procedure but in the absence of relevant behavioral stimuli (Fig. 2).
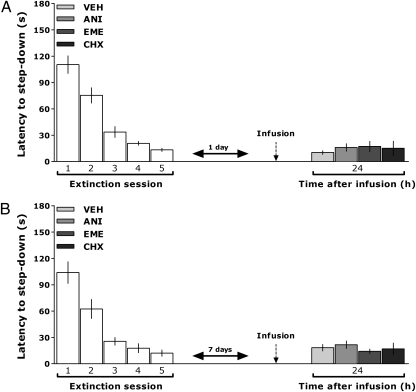
Fig. 2.
Inhibition of hippocampal protein synthesis in the absence of relevant behavioral stimuli does not affect retention extinction memory. (A) Rats trained in the IA task were submitted to five extinction sessions at 24, 48, 72, 96, and 120 h after training. One day after the last session, the animals received bilateral infusions of VEH, ANI, EME, or CHX in the CA1 region of the dorsal hippocampus. Retention was evaluated 24 h thereafter. (B) Animals were treated exactly as in A except that the drugs were infused 7 d after the last extinction session (n = 10–12 per group). Bars represent the mean (±SEM) of step-down latencies.
So far, two different scenarios have been proposed to explain the amnesia caused by the postreactivation administration of protein synthesis inhibitors and, hence, the reconsolidation phenomenon. One of these hypotheses postulates that the amnesic effect is attributable to a more or less protracted blockade of the retrieval process (29–31), whereas the other states that the amnesia is consequence of storage impairment (32–34).
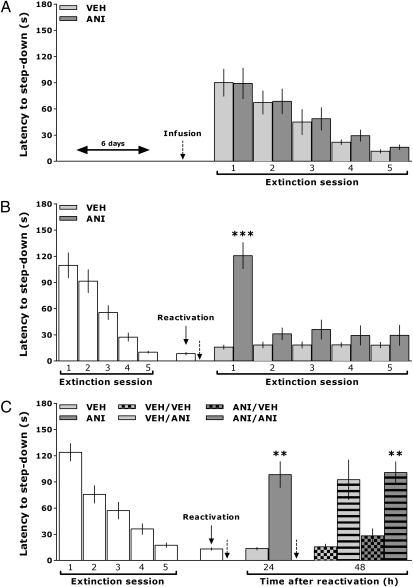
Therefore, to study whether the deleterious effect on extinction memory retention and the resulting recovery of the avoidance response induced by hippocampal protein synthesis inhibition was attributable to erasure or, alternatively, to impaired expression of the fear extinction memory trace, animals trained in the IA task were randomly assigned to one of two experimental groups. Animals in group 1 were not submitted to any relevant behavioral experience and received vehicle (VEH) or ANI into dorsal CA1 6 d after IA training. Animals in group 2 were submitted to one daily extinction session for 5 d. Twenty-four hours after the last extinction training session, extinction memory was reactivated; immediately after that, the animals received VEH or ANI into dorsal CA1. Starting 24 h after these procedures, animals in both groups were submitted to one daily extinction training session for 5 consecutive days. We found that when given 6 d after IA training in the absence of any behaviorally relevant stimuli (group 1), ANI did not affect expression of the IA response or the subsequent acquisition of extinction (Fig. 3A). As expected (Fig. 1), when given after reactivation of extinction memory, ANI induced recovery of the avoidance response, and, importantly, extinction of this recovered response was more rapid than that of the original one [t(22) = 5.49, P < 0.001] (Fig. 3B), indicating that there are savings of extinction memory after ANI treatment. Nevertheless, as can be seen in Fig. 3C, extinction of the recovered IA response was also blocked by ANI given into dorsal CA1 immediately after nonreinforced retrieval [t(24) = 3.40, P < 0.01 for ANI/VEH vs. ANI/ANI] suggesting that, as happens for extinction of the original avoidance memory (20), the occurrence of a protein synthesis-dependent process is also required for extinction of the recovered IA trace.
Fig. 3.
Inhibition of hippocampal protein synthesis after reactivation does not erase extinction memory. (A) IA task-trained rats received bilateral intra-CA1 infusions of VEH or ANI 6 d posttraining and, beginning 1 d later, were submitted to one daily extinction session for 5 d. (B) Rats trained in the IA task were submitted to five extinction sessions at 24, 48, 72, 96, and 120 h posttraining. One day later, extinction memory was reactivated; immediately thereafter, animals received VEH or ANI into dorsal CA1. Beginning 1 d after reactivation, animals were submitted to one daily extinction session for 5 d. (C) IA task-trained rats were submitted to five extinction sessions at 24, 48, 72, 96, and 120 h after training. One day later, extinction memory was reactivated; immediately thereafter, animals received VEH or ANI into dorsal CA1. Twenty-four hours later, animals were submitted to a second reactivation session; immediately thereafter, they received VEH or ANI. Retention was reevaluated 24 h later (n = 10–12 per group). Bars represent the mean (±SEM) of step-down latencies. **P < 0.01; ***P < 0.001 using the Student t test. The dotted arrows indicate the moment of drug infusion.
Discussion
Our results show that inhibition of hippocampal protein synthesis during a restricted postreactivation time window hampers retention of consolidated IA extinction memory lastingly, allowing reappearance of the initial fear memory trace. This phenomenon was observed with three different protein synthesis inhibitors, thus ruling out any possible nonspecific pharmacological byproduct. Moreover, the amnesic effect was time-dependent, concomitant with the reactivation of extinction memory and not attributable to spontaneous recovery (35), because it was not seen in animals that received VEH or were given ANI 6 h after reactivation. Indeed, the training protocol used here induces an extinction memory lasting at least 14 d (Fig. 1). Recovery of the IA response cannot be ascribed to renewal or reinstatement either. The former occurs when the conditioned stimulus is presented outside of the extinction context (36), whereas the latter results from the unexpected delivery of the unconditioned stimulus (37). None of these conditions were present in our experimental design. Hence, the most plausible explanation for our data is that fear extinction weakens after reactivation and, to remain behaviorally available, must undergo a protein synthesis-dependent reconsolidation-like process in the hippocampus.
Currently, the prevailing model about the effect of retrieval on memory persistence states that exposing an animal to a learned situation in the absence of proper reinforcement will reactivate the consolidated trace and initiate one of two opposing and competing processes: consolidation of extinction memory and reconsolidation of the original memory (32, 38; a different standpoint is discussed in 39–41). Which one of these two processes will dominate future behavior is thought to depend on environmental, physiological, and behavioral conditions, the so-called “boundary conditions,” at the moment of reactivation (13, 42). Together with quite recent findings (43), our results suggest that the dynamics of memory processing after reactivation are much more complex than previously thought and indicate that the current model must be amended to include the possibility that consolidated extinction memory is also open to updating and modification by retrieval. This possibility certainly has deep implications at the clinical level and could help us to understand, and avoid, relapse following the apparent success of the extinction-based therapies used for the treatment of anxiety disorders (44, 45).
Whether postreactivation administration of protein synthesis inhibitors disintegrates the neurobiological substrates of the consolidated trace or, instead, affects its future expression, either transiently or lastingly, is still matter of debate (5). We believe that these two seemingly antagonist hypotheses are not mutually exclusive, however. Indeed, one could argue that inhibition of protein synthesis after nonreinforced reactivation could hinder future retention not by deleting the trace or impairing its expression but by affecting an active protein synthesis-dependent process initiated at the moment of retrieval and necessary to access or find the information needed to build up a suitable behavioral representation. In this respect, the existence of extinction savings after the recovery of IA memory induced by ANI and the need of protein synthesis for extinction of the recovered avoidance response indicate that reconsolidation blockade does not fully erase extinction memory but, instead, hinders restabilization of, or access to, information necessary for remembering it (34, 46).
Materials and Methods
Animals, Surgery, and Drug Infusion.
Naive male Wistar rats (3 mo old, 300–350 g) raised in our own facilities or bought at the Fundação Estadual de Produção e Pesquisa em Saúde do Rio Grande do Sul were used. The animals were housed five to a cage and kept with free access to food and water under a 12/12-h light/dark cycle, with light onset at 7:00 AM. The temperature of the animal room was maintained at 22–24 °C. To implant them with indwelling cannulas, rats were deeply anesthetized with thiopental (i.p., 30–50 mg/kg) and 27-gauge cannulas were stereotaxically aimed to the CA1 region of the dorsal hippocampus (anterior, −4.2; lateral, ±3.0; ventral, −1.8) in accordance with coordinates taken from the atlas of Paxinos and Watson (47). Animals were allowed to recover from surgery for 4 d before submitting them to any other procedure. At the time of drug delivery, 30-gauge infusion cannulas were tightly fitted into the guides. Infusions (1 μL per side) were carried out over 60 s with an infusion pump, and the cannulas were left in place for an additional 60 s to minimize backflow. The placement of the cannulas was verified postmortem: 2–4 h after the last behavioral test, 1 μL of a 4% (wt/vol) methylene-blue solution was infused as described above, and the extension of the dye 30 min thereafter was taken as an indication of the presumable diffusion of the VEH or drug previously given to each animal. Only data from animals with correct implants were analyzed. All procedures were conducted in accordance with the National Institutes of Health principles of laboratory animal care. Every effort was made to reduce the number of animals used and to minimize their suffering.
IA Learning Task.
Rats were trained in a one-trial step-down IA task during the light phase of the subjective day (between 9:00 and 11:00 AM). The training apparatus was a 50 × 25 × 25-cm Plexiglas box with a 5-cm- high, 8-cm-wide, and 25-cm-long platform on the left end of a series of bronze bars that made up the floor of the box. For training, animals were gently placed on the platform facing the left rear corner of the training box. When they stepped down and placed their four paws on the grid, they received a 2-s 0.5-mA scrambled foot shock and were immediately withdrawn from the training box.
Extinction of IA LTM.
To extinguish the avoidance response, rats were submitted to several nonreinforced IA test sessions 24 h apart. For this purpose, IA-trained animals were put back on the training box platform until they stepped down to the grid. No foot shock was given, and the animals were allowed to explore the training apparatus freely for 30 s after they had stepped down. During this time, the animals stepped up onto the platform and down again several times. To reactivate the extinction memory trace, the animals were put on the training box platform until they stepped down, and right after that, they were removed from the training box. In some experiments (Fig. 3B), animals were submitted to a second extinction protocol after memory reactivation.
Drugs.
ANI, EME, and CHX (Sigma–Aldrich) were dissolved and stored protected from light at −20 °C until use. Right before that, an aliquot was thawed and diluted to a working concentration with 0.1% DMSO in saline (pH 7.2). The doses used were determined based on pilot experiments and previous studies showing the behavioral effects of each compound.
Statistical Analyses.
The Student t test or one-way ANOVA, followed by the Bonferroni test, were used for comparison of two or more than two groups, respectively.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico, and from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
Footnotes
The authors declare no conflict of interest.
References
- 1.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hupbach A, Gomez R, Hardt O, Nadel L. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn Mem. 2007;14:47–53. doi: 10.1101/lm.365707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- 5.Nader K, Hardt O. A single standard for memory: The case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 6.Forcato C, Rodríguez ML, Pedreira ME, Maldonado H. Reconsolidation in humans opens up declarative memory to the entrance of new information. Neurobiol Learn Mem. 2010;93:77–84. doi: 10.1016/j.nlm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Languille S, et al. Extracellular signal-regulated kinase activation is required for consolidation and reconsolidation of memory at an early stage of ontogenesis. Eur J Neurosci. 2009;30:1923–1930. doi: 10.1111/j.1460-9568.2009.06971.x. [DOI] [PubMed] [Google Scholar]
- 12.Herry C, et al. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg M, Kobilo T, Berman DE, Dudai Y. Stability of retrieved memory: Inverse correlation with trace dominance. Science. 2003;301:1102–1104. doi: 10.1126/science.1086881. [DOI] [PubMed] [Google Scholar]
- 14.Yamada D, Zushida K, Wada K, Sekiguchi M. Pharmacological discrimination of extinction and reconsolidation of contextual fear memory by a potentiator of AMPA receptors. Neuropsychopharmacology. 2009;34:2574–2584. doi: 10.1038/npp.2009.86. [DOI] [PubMed] [Google Scholar]
- 15.Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- 16.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 17.Sangha S, Scheibenstock A, Lukowiak K. Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1. J Neurosci. 2003;23:8034–8040. doi: 10.1523/JNEUROSCI.23-22-08034.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duvarci S, Mamou CB, Nader K. Extinction is not a sufficient condition to prevent fear memories from undergoing reconsolidation in the basolateral amygdala. Eur J Neurosci. 2006;24:249–260. doi: 10.1111/j.1460-9568.2006.04907.x. [DOI] [PubMed] [Google Scholar]
- 19.Martel G, Jaffard R, Guillou JL. Identification of hippocampus-dependent and hippocampus independent memory components in step-down inhibitory avoidance tasks. Behav Brain Res. 2010;207:138–143. doi: 10.1016/j.bbr.2009.09.047. [DOI] [PubMed] [Google Scholar]
- 20.Cammarota M, Bevilaqua LR, Kerr D, Medina JH, Izquierdo I. Inhibition of mRNA and protein synthesis in the CA1 region of the dorsal hippocampus blocks reinstallment of an extinguished conditioned fear response. J Neurosci. 2003;23:737–741. doi: 10.1523/JNEUROSCI.23-03-00737.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBPbeta. Nat Neurosci. 2001;4:813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- 22.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris RG, et al. Memory reconsolidation: Sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron. 2006;50:479–489. doi: 10.1016/j.neuron.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Canal CE, Chang Q, Gold PE. Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci USA. 2007;104:12500–12505. doi: 10.1073/pnas.0705195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stollhoff N, Menzel R, Eisenhardt D. Spontaneous recovery from extinction depends on the reconsolidation of the acquisition memory in an appetitive learning paradigm in the honeybee (Apis mellifera) J Neurosci. 2005;25:4485–4492. doi: 10.1523/JNEUROSCI.0117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase- mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur J Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 27.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 28.Boccia MM, Blake MG, Acosta GB, Baratti CM. Post-retrieval effects of icv infusions of hemicholinium in mice are dependent on the age of the original memory. Learn Mem. 2006;13:376–381. doi: 10.1101/lm.150306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Litvin OO, Anokhin KV. Mechanisms of memory reorganization during retrieval of acquired behavioral experience in chicks: The effects of protein synthesis inhibition in the brain. Neurosci Behav Physiol. 2000;30:671–678. doi: 10.1023/a:1026698700139. [DOI] [PubMed] [Google Scholar]
- 30.Anokhin KV, Tiunova AA, Rose SP. Reminder effects—Reconsolidation or retrieval deficit? Pharmacological dissection with protein synthesis inhibitors following reminder for a passive-avoidance task in young chicks. Eur J Neurosci. 2002;15:1759–1765. doi: 10.1046/j.1460-9568.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- 31.Lattal KM, Abel T. Behavioral impairments caused by injections of the protein synthesis inhibitor anisomycin after contextual retrieval reverse with time. Proc Natl Acad Sci USA. 2004;101:4667–4672. doi: 10.1073/pnas.0306546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 33.Dudai Y, Eisenberg M. Rites of passage of the engram: Reconsolidation and the lingering consolidation hypothesis. Neuron. 2004;44:93–100. doi: 10.1016/j.neuron.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Alberini CM. Mechanisms of memory stabilization: Are consolidation and reconsolidation similar or distinct processes? Trends Neurosci. 2005;28:51–56. doi: 10.1016/j.tins.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Rescorla RA. Spontaneous recovery. Learn Mem. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- 36.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 37.McAllister WR, McAllister DE. Recovery of conditioned fear by a single postextinction shock: Effect of similarity of shock contexts and of time following extinction. Learn Behav. 2006;34:44–49. doi: 10.3758/bf03192870. [DOI] [PubMed] [Google Scholar]
- 38.Dudai Y. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol. 2004;55:51–86. doi: 10.1146/annurev.psych.55.090902.142050. [DOI] [PubMed] [Google Scholar]
- 39.Amaral OB, Osan R, Roesler R, Tort AB. A synaptic reinforcement-based model for transient amnesia following disruptions of memory consolidation and reconsolidation. Hippocampus. 2008;18:584–601. doi: 10.1002/hipo.20420. [DOI] [PubMed] [Google Scholar]
- 40.Lattal KM, Stafford JM. What does it take to demonstrate memory erasure? Theoretical comment on Norrholm et al. (2008) Behav Neurosci. 2008;122:1186–1190. doi: 10.1037/a0012993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stafford JM, Lattal KM. Direct comparisons of the size and persistence of anisomycin-induced consolidation and reconsolidation deficits. Learn Mem. 2009;16:494–503. doi: 10.1101/lm.1452209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Delatorre P, Rodríguez-Ortiz CJ, Balderas I, Bermúdez-Rattoni F. Differential participation of temporal structures in the consolidation and reconsolidation of taste aversion extinction. Eur J Neurosci. 2010;32:1018–1023. doi: 10.1111/j.1460-9568.2010.07365.x. [DOI] [PubMed] [Google Scholar]
- 44.Rachman S, Whittal M. The effect of an aversive event on the return of fear. Behav Res Ther. 1989;27:513–520. doi: 10.1016/0005-7967(89)90085-5. [DOI] [PubMed] [Google Scholar]
- 45.Foa EB. Psychosocial treatment of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl 5):43–48. discussion 49–51. [PubMed] [Google Scholar]
- 46.Miller CA, Sweatt JD. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem. 2006;13:498–505. doi: 10.1101/lm.304606. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. Compact 3rd Ed Vol 1, pp 33–37. [Google Scholar]