Abstract
The cross-talk among cells of the innate immunity can greatly affect both innate and adaptive responses. Here we analyzed the molecular interactions between human natural killer (NK) cells and autologous macrophages. Activated NK cells killed M0 and M2, whereas M1 macrophages were more resistant to lysis because of their higher expression of HLA class I molecules. Following exposure to LPS or bacillus Calmette–Guérin, M0 and M2, but not polarized (endotoxin tolerant) M1 macrophages, induced strong activation of resting NK cells. The expression of CD69 and CD25 activation markers and the acquisition of cytotoxicity against tumor cells and immature dendritic cells required soluble factors being mostly contact independent. On the contrary, IFN-γ production was contact dependent and required the interaction of DNAM-1 and 2B4 (on NK) with their ligands on macrophages as well as IL-18. IL-18 was involved also in the acquisition of CCR7 by NK cells. Interestingly, M0 and M2 cells expressed a membrane-bound form of IL-18, which was released in small amounts after LPS treatment. Our data indicate that, upon interaction with M0 macrophages exposed to microbial products, NK cells may amplify classical type 1 immune responses. In addition, M1-polarizing stimuli can rescue M2 macrophages from their immunomodulatory state and shape their functional behavior toward NK stimulatory capability.
Keywords: cytolityc activity, proinflammatory cytokines, lypopolissacaride, molecular interactions, nectins' family
Natural killer (NK) cells play an important role in immune responses against cancer and pathogen infections by exerting potent cytolytic activity and producing large amounts of T-helper (Th1) cytokines such as IFN-γ. NK cell activation may be induced by IL-12, IL-15, and IL-18 (1) and by the interaction between activating NK receptors and their ligands on target cells. In humans, activating receptors include NKp46, NKp30, and NKp44 (collectively termed natural cytotoxicity receptors, NCR), DNAM-1, and NKG2D (2, 3), whereas NKp80, 2B4, and NTBA are generally considered coreceptors (2, 4). Different cellular ligands have been identified (5). DNAM-1 recognizes PVR and Nectin-2, two Nectin family members that are overexpressed in tumors (6, 7), and NKG2D interacts with MICA/B and ULBP. 2B4 interacts with CD48, NKp80 recognizes the activation-induced C-type lectin (AICL) (8), and NTBA displays homophilic interaction. The NCR cellular ligands are not fully defined, and only recently NKp30 has been shown to interact with B7-H6, a tumor-associated surface molecule that represents a novel member of the B7 family (9).
The function of the activating receptors/coreceptors is under the control of inhibitory receptors that recognize HLA class I molecules on potential targets (2, 10). These receptors include CD94/NKG2A, which recognizes nonclassical HLA-E molecules and the killer Ig-like receptors (KIR, CD158), specific for allotypic determinants shared by groups of classical HLA-A, -B, and -C alleles.
The survival or death of a potential target depends on the type and the number of receptor/ligand interactions occurring at the NK/target cell immune synapse. In an autologous setting, normal cells are spared from NK-mediated killing because of the expression of high (protective) amounts of self-HLA class I molecules and of the lack of (or low) expression of ligands for activating NK receptors. However, tumors become susceptible to killing mediated by autologous NK cells because of a defective (nonprotective) expression of HLA class I molecules and de novo expression or up-regulation of ligands for activating NK receptors (6, 7, 9, 11, 12).
During inflammation, NK cells, after extravasation and recruitment into tissues in response to chemokine gradients, can interact with other immune cell types (13). These interactions may play a critical role not only during early innate immune responses but also in the initiation, amplification, and polarization of adaptive responses. For example, a bidirectional cross-talk occurs between NK and monocyte-derived dendritic cells (DCs) (14). As DCs, macrophages are crucial components of innate immunity. During inflammatory responses, monocytes are recruited into tissues, skewed toward macrophages (M0) by chemokines and growth factors, and polarized toward M1 or M2 functional phenotype (15, 16).
M1 and M2 macrophages represent the two extreme of a spectrum. In vitro, M1 polarization can be induced by IFN-γ alone or in concert with microbial stimuli such as LPS, whereas M2 polarization requires IL-4 or IL-13. M1 and M2 display different cytokine and chemokine gene expression profiles and functional properties (15). M1 are characterized by IL-12high, IL-23high, and IL-10low, whereas M2 macrophages have an IL-12-low, IL-23low, IL-10high, CD206high, and scavenger receptorshigh transcription profiles. In general, M1 macrophages have immunostimulatory Th1-orienting properties, kill intracellular pathogens, and have antitumoral activity. M2 cells have poor Ag-presenting capacity; promote angiogenesis, tissue remodeling, and repair; and suppress Th1 adaptive immunity while supporting TH2 responses necessary for killing and encapsulation of parasites.
Macrophages are the most represented leukocytes in cancer tissues and in established progressing tumors generally have M2-like phenotype (17). Cancer cells, by secreting a wide spectrum of soluble factors, attract circulating monocytes and promote their differentiation to macrophages while blocking differentiation to DCs (18). Importantly, the tumor microenvironment twists the polarization of tumor-associated macrophages (TAM) toward an M2-like phenotype that would display strong protumoral activity.
Although NK cells and macrophages are pillars of innate immunity, little information is available on their interplay. The present study was designed to systematically investigate the interaction between different types of macrophages and NK cells and the molecular pathways involved.
Results
M0 and M2 Are Susceptible to Killing by Autologous Cytokine-Conditioned NK Cells.
To analyze whether NK cells could kill unpolarized or polarized macrophages, highly purified NK cells were used as effectors in cytolytic assays against autologous M0, M1, and M2 cells or, for comparison, monocyte-derived immature or mature DCs (iDCs or mDCs; Fig. 1 and Fig. S1).
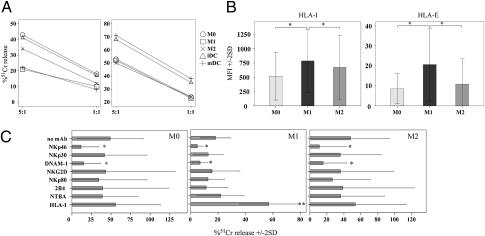
Fig. 1.
Susceptibility of macrophages to lysis mediated by autologous NK cells. (A) Short-term-activated NK cells were analyzed at different E:T ratios for cytolytic activity against autologous M0, M1, M2, iDCs, and mDCs either in the absence (Left) or in the presence (Right) of mAb-mediated masking of HLA class I molecules. Average of 10 independent experiments. (B) Macrophages were analyzed by flow cytometry for the expression of HLA class I molecules. Average of 11 (HLA-I) and four (HLA-E) independent experiments. (C) Short-term-activated NK cells were analyzed for cytolytic activity against autologous M0, M1, and M2 in the presence of mAbs specific for different triggering NK receptors (E:T ratio 5:1). Average of seven independent experiments. SD is indicated. *P < 0.05.
Freshly purified (resting) NK cells did not kill either macrophages or DCs. However, NK cells that had been activated overnight with rIL-12 displayed cytolytic activity against M0 and M2 as well as against iDCs (19, 20). Interestingly, M1, similar to mDCs, were more resistant to lysis than M0 (P = 0.013) and M2 (P = 0.031; Fig. 1A Left). However, upon disruption of inhibitory receptors/HLA class I interactions using anti-HLA-class I mAb, M0, M1, and M2 displayed similar susceptibility to NK-mediated lysis (Fig. 1A Right). These data suggested an important role of HLA class I molecules in protecting M1 macrophages from NK cell-mediated cytotoxicity. Accordingly, the susceptibility to lysis (M0, M2 > M1) inversely correlated with the level of expression of both classical and nonclassical HLA class I molecules (M1 > M2, M0; Fig. 1B and Fig. S1).
To evaluate the contribution of one or another activating NK receptor in killing of macrophages, cytolytic assays were performed in the presence of mAbs specific for major activating NK receptors. As shown in Fig. 1C, NKp46 and DNAM-1 mainly contributed to the lysis of macrophages. Indeed, mAb-mediated disruption of their interaction with the specific ligands on target cells resulted in significant inhibition of lysis. Other activating receptors and coreceptors contributed only marginally or were not involved in killing.
Thus, among the activating receptors, NKp46 played a major role in killing of macrophages (Fig. 1C), whereas killing of DCs was mainly NKp30 dependent (14). On the other hand, DNAM-1 was involved in killing of both macrophages (Fig. 1C) and DCs (19).
LPS-Treated M0 and M2 Enhance the Cytolytic Activity of Resting NK Cells Against K562 and iDCs.
We next analyzed whether macrophages could induce activation of resting NK cells. In these experiments, to avoid interferences of cytokines and stimuli used during differentiation and polarization, M0, M1, and M2 where washed extensively. After washing, cells were cultured with autologous resting NK cells in either the absence or the presence of LPS or bacillus Calmette–Guérin. After coculture, NK cells were recovered (>99% purity) and analyzed for cytolytic activity, cell surface phenotype, and IFN-γ production.
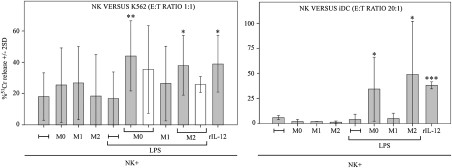
In one set of experiments, NK cells recovered from cocultures were used as effectors in cytolytic assays against K562 (a classical NK-susceptible tumor target) or autologous iDCs, which are resistant to killing by resting NK cells. As shown in Fig. 2, NK cells cocultured with M0, M1, and M2 neither enhanced killing of K562 nor killed iDCs. No significant increases of NK cytotoxicity could be detected in NK cells cultured with M1 in the presence of LPS. On the contrary, NK cells cultured with M0 and M2 in the presence of LPS displayed cytotoxicity against both targets (Fig. 2). It is of note that the cytotoxicity of these NK cells was similar if not greater than that of rIL-12-conditioned NK cells. Similar results were obtained coculturing resting NK cells with bacillus Calmette–Guérin-treated M0 or M2 (Fig. S2).
Fig. 2.
Analysis of the cytolytic activity of NK cells cocultured with macrophages. After coculture with autologous M0, M1, and M2 (with or without LPS), NK cells were analyzed for cytolytic activity against K562 or autologous iDCs either in the presence of cell-to-cell contacts or in transwell (white bars). Average of six independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
It is of note that the cytolytic activity was reduced but not abrogated in the absence of NK-to-macrophage contact (transwell; M0 contact vs. transwell, P = 0.3; M2 contact vs. transwell, P = 0.071; Fig. 2).
LPS-Treated M0 and M2 Induce Up-Regulation of CD69, CD25, and CCR7 in Resting NK Cells.
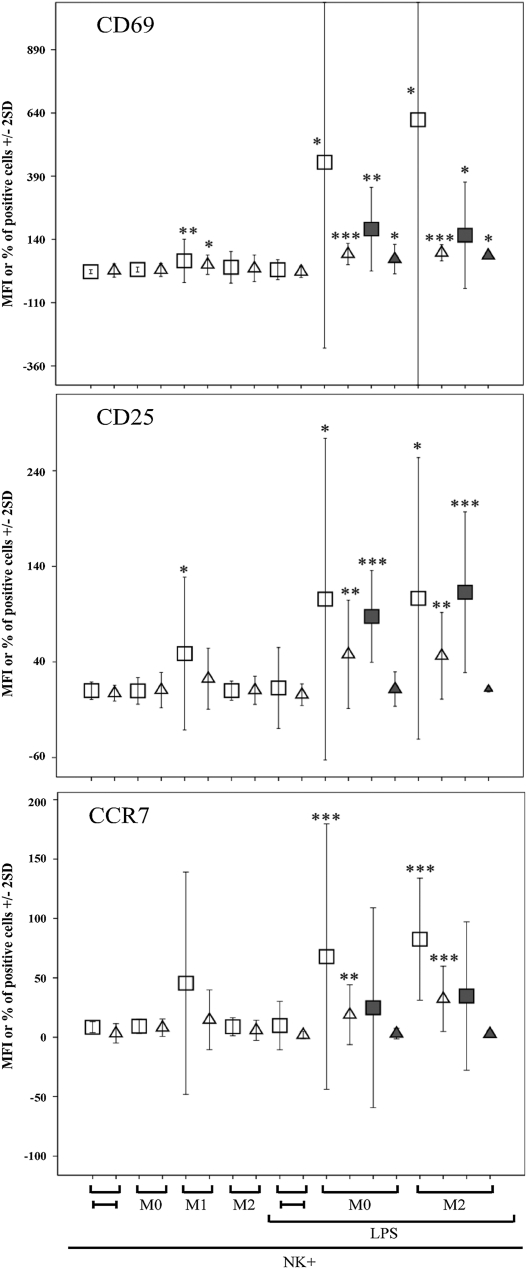
NK cells recovered after coculture with macrophages were assessed for the expression of CD69 and CD25 activation markers. Results are depicted in Fig. 3 and a representative experiment is shown in Fig. S3A. After coculture with M1, NK cells displayed low but significant increases of both CD69 surface density (mean fluorescence intensity, MFI) and percent of CD69+ cells. On the contrary, after coculture with M0 or M2, NK cells did not change CD69 expression. But when NK cells were cultured with either M0 or M2 in the presence of LPS, the surface density and the percent of CD69+ NK cells was strongly up-regulated (Fig. 3). Regarding CD25, the MFI (but not the percent) of positive cells was significantly up-regulated in NK cells cocultured with M1, but not with M0 and M2. However, both the MFI and the percent of CD25+ cells were strongly up-regulated in NK cells cocultured with LPS-treated M0 and M2 (Fig. 3). It is of note that up-regulation of CD69 and CD25 was detected also in the absence of NK-to-macrophages contacts (transwell).
Fig. 3.
Analysis of CD69, CD25, and CCR7 expression in NK cells cocultured with macrophages. After coculture with autologous M0, M1, and M2 (with or without LPS), NK cells were analyzed by flow cytometry for the expression of CD69, CD25, and CCR7. The experiments were performed either in the presence of cell-to-cell contacts or in transwell (gray symbols). Average of 10 independent experiments. Squares and triangles indicate the MFI and the percentage of positive cells, respectively.
Coculture with macrophages also induced the expression of CCR7, a chemokine receptor involved in NK cell migration to lymph nodes (21, 22). In particular, both the MFI and the percent of CCR7+ cells were significantly up-regulated after coculture with LPS-treated M0 and M2 cells. Different from CD69 and CD25, CCR7 expression was not induced under transwell conditions, implying the requirement of cell-to-cell contacts (Fig. 3). Up-regulation of activation markers and CCR7 were detected also in NK cells cocultured with bacillus Calmette–Guérin-treated M0 or M2 cells (Fig. S3B). It is of note that, similar to what was described for NK-DC interactions, CCR7 expression in NK cells was accompanied by down-regulation of GPR56 (23), a surface molecule involved in cellular interactions with extracellular matrix (Fig. S3C).
Our data indicate that M0 and M2 exposed to LPS (or bacillus Calmette–Guérin) induce in resting NK cells an activated/migratory phenotype, which is paralleled by enhancement of their cytolytic activity (Fig. 2).
Role of Macrophage-Derived Cytokines in the Induction of NK Cell Activation.
Resting NK cells upon exposure to rIL-12 and/or rIL-15 increase their cytolytic activity and express activation markers but not CCR7. Moreover, it has been described that IL-12, IL-15, and IL-18 used alone or in combination could be involved in NK cell activation during NK-to-DC or NK-to-macrophages interaction (14, 24, 25). M0 and M2 polarizing macrophages did not release detectable amounts of IL-12 and IL-18 (Fig. S4). Polarization toward M1 with LPS (alone or in concert with IFN-γ) resulted in the release of IL-12 and IL-18, as well as TNF-α and IL-6 by both M0 and M2 cells. M1 polarized cells that (after washing) were maintained in culture for additional 18 h released low amounts of such cytokines and, due to endotoxin tolerance, were no longer responsive to further TLR-mediated stimulation (26, 27). IL-15 was undetectable in the macrophages’ supernatants, whereas IL-15 and its receptor were expressed at the cell membrane in M1 polarized cells and in LPS-treated M0 and M2 macrophages (Fig. S4) (15).
Thus, a correlation existed between the ability of macrophages to release cytokines such as IL-12 and IL-18 (Fig. S4) and their capacity to induce cytotoxicity and activation markers’ expression in resting NK cells (Figs. 2 and 3). However, Ab-mediated neutralization of these cytokines (as well as of IL-15) did not modify significantly both aspects of macrophage-mediated NK cell activation (Fig. S5).
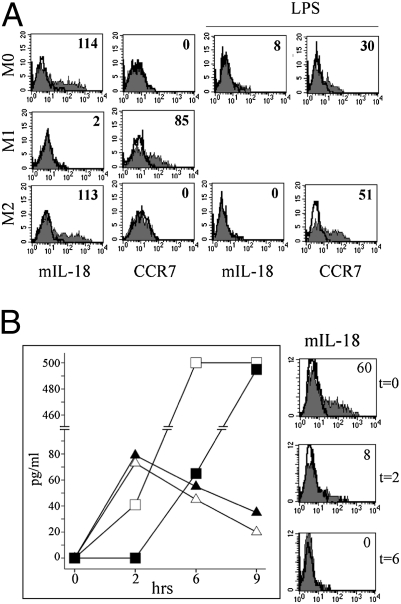
Regarding CCR7, it has been previously shown that it can be induced by IL-18 and receptor uptake from CCR7+ cells (28, 29). In our experiments, CCR7 was detected in M1 but not in M0 and M2. However, polarization toward M1 with LPS resulted in CCR7 expression also by M0 and M2 macrophages (Fig. 4A). Remarkably, a membrane-bound form of IL-18 (mIL-18) could be detected by cytofluorimetric analysis in M0 and M2 cells. Treatment with LPS resulted in disappearance of mIL-18, which was paralleled by the release of soluble IL-18 in the supernatant (Fig. S4) and acquisition of CCR7 (Fig. 4A). Interestingly, treatment with monensin, which blocks intracellular protein transport, did not affect LPS-induced secretion of IL-18 by mIL-18+ macrophages, but strongly affected IL-12 release (Fig. 4B). This finding strongly suggests that IL-18 secretion by macrophages depends on the release of the membrane-bound form of IL-18 rather than on the mobilization from intracellular stores.
Fig. 4.
Analysis of mIL-18 expression and release by macrophages. (A) Macrophages either untreated or LPS treated were analyzed by flow cytometry for mIL-18 and CCR7 expression. (B) M0 were treated at the indicated time intervals with LPS in the absence (open symbols) or in the presence (black symbols) of monensin. Cell culture supernatants were analyzed by ELISA for the presence of IL-18 (triangle) and IL-12 (square). Average of three independent experiments (Left). The analysis was paralleled by the cytofluorimetric analysis of mIL-18 surface expression (Right). The mean MFI is indicated.
It is conceivable that the de novo expression of CCR7 in NK cells may require the uptake of both CCR7 and IL-18 by NK cells interacting with macrophages. This hypothesis is supported by the fact that CCR7 expression was detected only in NK cells cocultured with M0 and M2 cells (mIL-18+), which upon treatment with LPS released IL-18 and expressed CCR7. This did not occur when NK cells were cocultured with M0 and M2 (mIL-18+, CCR7−) or M1 polarized cells (mIL-18−, CCR7+) cells, which during polarization with LPS wasted IL-18.
LPS-Treated M0 and M2 Induce IFN-γ Release by Resting NK Cells.
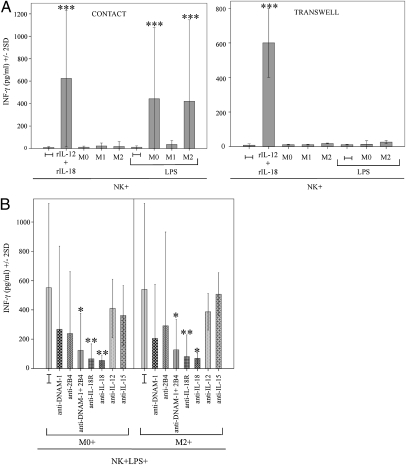
To verify whether macrophages might also promote the production of proinflammatory cytokines in NK cells, coculture supernatants were analyzed for the presence of IFN-γ. As shown in Fig. 5A, M0-, M1-, and M2-polarized cells did not induce IFN-γ production by NK cells. Upon LPS treatment, however, M1 polarizing M0 and M2 promoted the release of high amounts of IFN-γ, which was virtually abrogated in the absence of NK macrophage contacts (i.e., under transwell conditions). Similar results were obtained by coculturing resting NK cells with bacillus Calmette–Guérin-treated M0 and M2 (Fig. S6). To define which molecular interactions were involved in the induction of IFN-γ release, cocultures were performed in the presence of mAbs specific for different NK receptors or cytokines. Ab-mediate blocking of DNAM-1 and 2B4, IL-18, or IL-18R resulted in significant drop of IFN-γ release (Fig. 5B). On the contrary, blocking of other receptors (including NKG2D, NKp46, and NKp44), IL-12, and IL-15 had no substantial effect.
Fig. 5.
Molecular interactions involved in the macrophage-mediated induction of IFN-γ release by NK cells. (A) NK cells were cocultured with M0, M1, and M2 (with or without LPS) either in the presence of cell-to-cell contacts (Left) or in transwell (Right). Supernatants were analyzed by ELISA for the presence of IFN-γ. Average of 13 (contact) and four (transwell) independent experiments. (B) NK cells were cocultured with M0 and M2 in the presence of LPS and the indicated blocking/neutralizing antibodies. Supernatants were analyzed by ELISA for the presence of IFN-γ. Average of five independent experiments.
Our data suggest that the various NK receptors are differently involved in NK macrophages cross-talk. NKp46 participates mainly to the induction of cytotoxicity against macrophages, whereas, in line with a previous report (30), 2B4 would be primarily involved in the induction of IFN-γ secretion. On the other hand, DNAM-1 plays a dual role being involved in the induction of both cytolytic activity and IFN-γ production.
Discussion
The present findings show that interaction between NK cells and unpolarized, polarizing, and polarized macrophages result in different functional outcomes. M0 (unpolarized) and M2 polarized macrophages following capture of microbial products, such as LPS and bacillus Calmette–Guérin, polarize toward M1 and induce strong activation of resting NK cells, resulting in enhancement of cytolytic activity, release of high amounts of IFN-γ, and expression of CCR7, a chemokine receptor crucial for their recruitment into lymph nodes. NK macrophage cross-talk also enhances the DC-editing capability (13) of NK cells, which upon interaction with LPS or bacillus Calmette–Guérin-treated macrophages become capable of killing iDCs. The editing of DCs might be paralleled also by editing of macrophages. Indeed, M0 and M2 exposed to M1-polarizing stimuli induce NK cell activation and, in turn, activated NK cells kill M0- and M2-polarized macrophages, which express low, nonprotective amounts of HLA class I molecules. Conversely, M1-polarized macrophages (HLA class I high) similarly to mDCs, are resistant to NK cells.
Regarding the soluble factors involved in the NK macrophage cross-talk, as also reported (25), Ab-mediated neutralization of IL-15 did not affect NK cell activation (Fig. S5). However, Lapaque et al. (25) showed that the combined Ab-mediated blocking of IL-12 and IL-18 inhibited CD69 expression and cytotoxicity in NK cells cocultured with Salmonella-infected macrophages. Moreover, blocking of IL-12 alone resulted in a marked drop of IFN-γ production. Conversely, another study showed that during plasmodium falciparum infection, the IFN-γ production by NK cells was IL-18 dependent (24).
In our experimental setting, optimal macrophage-mediated induction of IFN-γ production by NK cells is dependent on cell-to-cell contacts and requires the interaction of DNAM-1 and 2B4 (on NK) with their ligands (on macrophages) as well as IL-18 released by macrophages. Indeed, in line with Baratin et al. (24), neutralization of IL-18 or blocking of IL-18R virtually abrogated IFN-γ release (Fig. 5). Interestingly, the functional effect of IL-18 on NK cells correlates with the expression, on macrophages, of a membrane-bound form of IL-18 (mIL-18), which is released in small amounts after LPS treatment (Fig. 4B). It is of note that IL-18 belongs to a cytokine family also including IL-1α, which may remain bound to the cell membrane (31). The molecular events leading to the expression of IL-18 at the cell surface and to its release upon stimulation are currently under investigation.
Importantly, IL-18 appears to play a role also in the expression of CCR7 at the NK cell surface. This event would require both the release of IL-18 and the expression of CCR7 by macrophages. This finding suggests the need of cooperation between IL-18 and mechanisms of CCR7 uptake in the up-regulation of CCR7 by NK cells (28, 29). In this context, though resting NK cells expressing CCR7 (or CXCR3 in mice) are sensitized by TLR-stimulated DCs in draining lymph nodes (32–35), inflamed or tumor tissues could be considered as alternative sites of NK cells priming (36–39). Indeed, the majority (>90%) of circulating human NK cells does not express CCR7 while expressing CXCR1 and ChemR (36). These receptors allow their recruitment in inflamed tissues where they interact with monocyte-derived DCs. This results in CXCR1 and ChemR down-regulation (36) and acquisition of CCR7 (29), which allows their response to CCL19 and CCL21, two chemokines that attract NK cells to secondary lymphoid organs such as lymph nodes (21, 22). Our data support the hypothesis that in injured peripheral tissues, the interaction with pathogen-stimulated macrophages might represent a further activation mechanism of resting NK cells leading to CCR7 acquisition. Thus, it is conceivable that NK cells activated in peripheral tissues by M1-polarizing macrophages could participate in Th1 polarization by releasing IFN-γ within lymph nodes (28, 40).
Neutralization of IL-12 did not affect significantly the IFN-γ release by NK cells cocultured with LPS-treated macrophages (Fig. 5). Moreover, although in some experiments the simultaneous neutralization of IL-12 and IL-18 (and IL-15) reduced CD69 expression and cytotoxicity (Fig. S5), overall, the inhibition was not statistically significant. This excludes a major role of these cytokines in the up-regulation of activation markers and cytotoxicity in NK cells cocultured with LPS-treated M0 (and M2) macrophages. The discrepancy between our data and what was observed by Lapaque et al. (25) in Salmonella infection could depend on the type of pathogen or stimulus used to activate macrophages. In this regard, IL-12 seems to play a crucial role in immune responses against Salmonella. Indeed, IL-12Rβ1-deficient patients show a selective susceptibility to Salmonella infection (41).
Thus, IL-18 plays a central role in IFN-γ release and CCR7 expression by NK cells interacting with macrophages activated by LPS or bacillus Calmette–Guérin. However, the identity of the soluble factor(s) playing a pivotal role in up-regulation of NK cell activation markers and cytotoxicity remain to be determined. Interestingly, during Salmonella and plasmodium falciparum infection, macrophage-mediated activation of NK cells required preactivation of NK cells with suboptimal doses of cytokines such as rIL-12, rIL-15, or rIL-2 (24, 25). In our experimental setting, LPS and bacillus Calmette–Guérin-treated macrophages were able to activate resting unconditioned NK cells. The discrepancy could be attributed to the inability of given pathogens or stimuli to induce full M1 polarization of macrophages. For example, in our hands, macrophages treated with LPS alone or in concert with IFN-γ acquired classical M1 phenotype and function, whereas treatment with IFN-γ alone resulted in incomplete polarization of macrophages (Fig. S7). Thus, the “ménage à trois,” e.g., the presence of a third partner (such as DCs or T cells) secreting NK cell-priming cytokines (25) might be necessary or dispensable, depending on the type of pathogen or stimulus acting on macrophages.
Overall, our data suggest that in type 1-oriented immune responses (15, 16), NK cells may play a role in amplifying both innate and adaptive responses, as a result of their interaction not only with maturing DCs but also with M1-polarizing macrophages.
In this context, it is of note that macrophage activation by pathogens through pattern recognition receptors such as TLR4 is regulated by feedback mechanisms that prevent overexuberant inflammation (26, 27). After exposure to LPS, macrophages enter into a transient unresponsive state and are unable to respond to further challenges with endotoxins (endotoxin tolerance). This phenomenon may play a protective role on the host against endotoxin shock. Endotoxin-tolerant macrophages present a gene reprogramming toward an M2-like functional phenotype, which includes increased expression of anti-inflammatory cytokines (IL-10, TGF-β, IL-1Rα) and reduced expression of proinflammatory ones. In our experiments, M1 cells that (after washing) were maintained in culture for additional 18 h and further stimulated with LPS did not express mIL-18, released negligible amounts of cytokines, such as IL-12 and IL-18, and were unable to activate resting NK cells. Lack of NK cell activation could result in drop of IFN-γ production, a further mechanism that prevents excessive inflammatory responses.
Similar to M0, M2, although expressing mIL-18, do not express CCR7; do not release proinflammatory cytokines such as IL-18 and IL-12; and are unable to induce NK cell function. Accordingly, TAM, frequently characterized by a M2-like polarized phenotype and set in a cancer-promoting mode (17), might also be unable to cooperate with NK cells. However, it is noteworthy that M2 are neither terminally differentiated nor “exhausted” cells. Thus, microbial products could revert their functional M2 phenotype toward an immunostimulatory M1-oriented one. Indeed, our present data show that in LPS (or bacillus Calmette–Guérin)-treated M2, the expression of CCR7 and the release of cytokines, including IL-12 and IL-18, was accompanied by a de novo ability to induce, in resting NK cells, expression of activation markers and CCR7, release of IFN-γ, and enhancement of cytotoxicity. This shows that M2 are characterized by a remarkable functional plasticity, because they can be rescued from their immunomodulatory state and shaped toward the capacity to induce NK cell activation. Reprogramming TAM vs. M1 phenotype and function represents an interesting strategy to restore antitumor responses. For example, the use of CpG and anti-IL-10R antibodies promoted innate responses in tumor-bearing mice (42), and bacillus Calmette–Guérin immunotherapy provided substantial clinical benefits in ovarian and bladder cancer patients (43, 44). It is tempting to speculate that restoration of M1 polarization of cancer-propelling TAM might also induce an effective antitumor activity of NK cells. Indeed, reprogramming M2-oriented TAM vs. M1 could also result in strong NK cell activation, which, in turn, would promote type-1 immune responses, NK-mediated editing of DCs and macrophages. Moreover, activated NK cells could exert a potent cytolytic activity not only against cancer cells but also against residual TAM, as suggested by the high susceptibility of M2 macrophages to NK-mediated lysis (Fig. 2).
In conclusion, the interaction between NK cells and M1-polarizing M0 and M2 macrophages emerges as a unique and potentially relevant component of activation, amplification, and regulation of innate and adaptive responses.
Materials and Methods
NK/Macrophage Cocultures.
Cells used in the study are described in SI Materials and Methods.
M0, M1, and M2 cells were washed five times with 10% FCS containing medium and cocultured overnight with autologous resting NK cells with or without 100 ng/mL LPS or bacillus Calmette–Guérin (1:1 bacillus Calmette–Guérin:macrophage ratio). The NK:macrophage ratio used was 1:1. Transwell experiments were performed using 24-well transwell plates (0.4-μm pore size; Corning Costar). In each well, 0.5 × 105 macrophages (with or without LPS or bacillus Calmette–Guérin) and 0.5 × 105 NK cells were placed in the bottom and the upper chamber, respectively.
Flow Cytofluorimetric Analysis, Cytolytic Assays, and Cytokines Release.
For cytofluorimetric analysis (FACSCalibur; Becton Dickinson), cells were stained with PE- or FITC-conjugated mAbs or with unconjugated mAbs followed by PE-conjugated isotype-specific goat anti-mouse second reagent (Southern Biotechnology). Macrophages and DCs were preincubated for 30 min at 4 °C with hIgG (1 mg/mL; Jackson ImmunoResearch Laboratories) before specific mAb staining.
NK cells were tested for cytolytic activity in a 4-h 51Cr-release assay as previously described (7) at the indicated E/T ratios.
ELISA kits used were: IFN-γ, TNF-α, IL-12p40/p70, IL-15, IL-6 (BioSource International, Inc.), and IL-18 (Medical Biological Laboratories).
Ab-mediated blocking/neutralization experiments were performed adding saturating amounts of Abs at the onset of the cell cultures. mAbs of IgM isotype were used, when available, to avoid nonspecific cross-linking of Fc receptors.
Monensin (BD Bioscience) treatment was performed according to the manufacturer's procedures. Briefly, 0.5 × 106 M0 cells were cultured at various time intervals with LPS and monensin (1:5,000 dilution).
Antibodies used in the study and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by investigator grants and Special Project 5x1000 from Associazione Italiana per la Ricerca sul Cancro, Istituto Superiore di Sanità, Ministero del Lavoro, della Salute e delle Politiche Sociali, and Ministero dell'Istruzione, dell'Università e della Ricerca. F.B. and A.D. are recipients of a fellowship awarded by Fondazione Italiana per Ricerca sul Cancro (FIRC).
Footnotes
Conflict of interest statement: A. Moretta is founder and shareholder of Innate Pharma (Marseille, France). The remaining authors have no conflicting financial interests.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007654108/-/DCSupplemental.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moretta A, Locatelli F, Moretta L. Human NK cells: From HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol Rev. 2008;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: Natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 5.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Pende D, et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112) Blood. 2005;105:2066–2073. doi: 10.1182/blood-2004-09-3548. [DOI] [PubMed] [Google Scholar]
- 7.Castriconi R, et al. NK cells recognize and kill human glioblastoma cells with stem cell-like properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 8.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 9.Brandt CS, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long EO. Negative signaling by inhibitory receptors: The NK cell paradigm. Immunol Rev. 2008;224:70–84. doi: 10.1111/j.1600-065X.2008.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryceson YT, Ljunggren HG. Tumor cell recognition by the NK cell activating receptor NKG2D. Eur J Immunol. 2008;38:2957–2961. doi: 10.1002/eji.200838833. [DOI] [PubMed] [Google Scholar]
- 12.Carlsten M, et al. Primary human tumor cells expressing CD155 impair tumor targeting by down-regulating DNAM-1 on NK cells. J Immunol. 2009;183:4921–4930. doi: 10.4049/jimmunol.0901226. [DOI] [PubMed] [Google Scholar]
- 13.Moretta A, et al. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Ferlazzo G, Münz C. Dendritic cell interactions with NK cells from different tissues. J Clin Immunol. 2009;29:265–273. doi: 10.1007/s10875-009-9283-y. [DOI] [PubMed] [Google Scholar]
- 15.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 16.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 18.Sozzani S, Rusnati M, Riboldi E, Mitola S, Presta M. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28:385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Pende D, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: Relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 20.Moretta A. Natural killer cells and dendritic cells: Rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 21.Campbell JJ, et al. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166:6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 22.Inngjerdingen M, Damaj B, Maghazachi AA. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- 23.Della Chiesa M, et al. GPR56 as a novel marker identifying the CD56dull CD16+ NK cell subset both in blood stream and in inflamed peripheral tissues. Int Immunol. 2010;22:91–100. doi: 10.1093/intimm/dxp116. [DOI] [PubMed] [Google Scholar]
- 24.Baratin M, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci USA. 2005;102:14747–14752. doi: 10.1073/pnas.0507355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapaque N, Walzer T, Méresse S, Vivier E, Trowsdale J. Interactions between human NK cells and macrophages in response to Salmonella infection. J Immunol. 2009;182:4339–4348. doi: 10.4049/jimmunol.0803329. [DOI] [PubMed] [Google Scholar]
- 26.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Porta C, et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci USA. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mailliard RB, et al. IL-18-induced CD83+CCR7+ NK helper cells. J Exp Med. 2005;202:941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marcenaro E, et al. Uptake of CCR7 and acquisition of migratory properties by human KIR+ NK cells interacting with monocyte-derived DC or EBV cell lines: Regulation by KIR/HLA-class I interaction. Blood. 2009;114:4108–4116. doi: 10.1182/blood-2009-05-222265. [DOI] [PubMed] [Google Scholar]
- 30.Nedvetzki S, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–3785. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 31.Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38. doi: 10.1111/j.1600-065X.2008.00624.x. [DOI] [PubMed] [Google Scholar]
- 32.Martín-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 33.Bajénoff M, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferlazzo G, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 36.Parolini S, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- 37.Maghazachi AA. Compartmentalization of human natural killer cells. Mol Immunol. 2005;42:523–529. doi: 10.1016/j.molimm.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 38.Frankel TL, et al. Identification and characterization of a tumor infiltrating CD56(+)/CD16(−) NK cell subset with specificity for pancreatic and prostate cancer cell lines. Cancer Immunol Immunother. 2010;59:1757–1769. doi: 10.1007/s00262-010-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Maux Chansac B, et al. NK cells infiltrating a MHC class I-deficient lung adenocarcinoma display impaired cytotoxic activity toward autologous tumor cells associated with altered NK cell-triggering receptors. J Immunol. 2005;175:5790–5798. doi: 10.4049/jimmunol.175.9.5790. [DOI] [PubMed] [Google Scholar]
- 40.Agaugué S, Marcenaro E, Ferranti B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–1783. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 41.Staretz-Haham O, et al. Interleukin-12 receptor beta1 deficiency presenting as recurrent Salmonella infection. Clin Infect Dis. 2003;37:137–140. doi: 10.1086/375229. [DOI] [PubMed] [Google Scholar]
- 42.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi A, et al. Immunotherapy of ovarian cancer with cell wall skeleton of Mycobacterium bovis Bacillus Calmette–Guérin: Effect of lymphadenectomy. Cancer Sci. 2009;100:1991–1995. doi: 10.1111/j.1349-7006.2009.01271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YJ, et al. Gene signatures for the prediction of response to Bacillus Calmette–Guerin immunotherapy in primary pT1 bladder cancers. Clin Cancer Res. 2010;16:2131–2137. doi: 10.1158/1078-0432.CCR-09-3323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.