Abstract
AMPA-type glutamate receptors (AMPARs) mediate the majority of fast excitatory neurotransmission in the mammalian central nervous system. Modulation of AMPAR trafficking supports several forms of synaptic plasticity thought to underlie learning and memory. Protein interacting with C kinase 1 (PICK1) is an AMPAR-binding protein shown to regulate both AMPAR trafficking and synaptic plasticity at many distinct synapses. However, studies examining the requirement for PICK1 in maintaining basal synaptic transmission and regulating synaptic plasticity at hippocampal Schaffer collateral–cornu ammonis 1 (SC–CA1) synapses have produced conflicting results. In addition, the effect of PICK1 manipulation on learning and memory has not been investigated. In the present study we analyzed the effect of genetic deletion of PICK1 on basal synaptic transmission and synaptic plasticity at hippocampal Schaffer collateral–CA1 synapses in adult and juvenile mice. Surprisingly, we find that loss of PICK1 has no significant effect on synaptic plasticity in juvenile mice but impairs some forms of long-term potentiation and multiple distinct forms of long-term depression in adult mice. Moreover, inhibitory avoidance learning is impaired only in adult KO mice. These results suggest that PICK1 is selectively required for hippocampal synaptic plasticity and learning in adult rodents.
Keywords: AMPA receptor, membrane trafficking, endocytosis, receptor recycling, metabotropic glutamate receptor
AMPA-type glutamate receptors (AMPARs) mediate the majority of fast excitatory transmission in the mammalian CNS. Modulation of AMPAR trafficking and expression at synapses has emerged as a key regulator of synaptic plasticity, thought to be a cellular mechanism underlying learning and memory (reviewed in ref. 1). AMPAR trafficking is a highly dynamic process, and the interaction of AMPARs with various AMPAR-binding proteins (AMPAR BPs) plays a critical role in regulating the movement of AMPARs in and out of the synapse (1–3). Of the four AMPAR subunits [AMPA-type glutamate receptors 1–4 (GluA1–4), previously glutamate receptors 1–4 (GluR1–4); see ref. 4], GluA2, GluA3, and GluA4-short have short C-terminal tails containing a type II postsynaptic density 95 (PSD95)/discs large (DlgA)/zonula occludens-1 (Zo-1) (PDZ) ligand. This C-terminal PDZ ligand of GluA2/3 interacts with PDZ domains in multiple AMPAR BPs, including protein interacting with C kinase 1 (PICK1) and glutamate receptor-interacting protein 1 and 2 (GRIP1 and GRIP2, GRIP2 is also called AMPAR binding protein, or ABP) (5–8). C-terminal phosphorylation of GluA2 at serine-880 (S880) differentially regulates interaction with PICK1 and GRIP such that S880 phosphorylation disrupts GluA2 binding to GRIP1/2 but does not affect GluA2 binding to PICK1 (9, 10).
PICK1 is an AMPAR BP (5, 8) originally identified by its interaction with PKC α (11). The PICK1 domain structure includes an N-terminal PDZ domain, a central box-dependent myc-interacting protein-1 (Bin)/amphiphysin/reduced viability to nutrient starvation-homology (Rvs) (BAR) domain, and N- and C-terminal acidic domains (reviewed in ref. 12). Although the precise manner in which GRIP and PICK regulate basal and activity-dependent AMPAR trafficking is still under investigation, GRIP appears to stabilize GluA2-containing AMPARs at the cell surface (13) and/or facilitate recycling of internalized receptors back to the cell surface (14), whereas association with PICK1 facilitates AMPAR endocytosis (15–17), inhibits receptor recycling (14), or retains GluA2-containing receptors at extrasynaptic sites (14, 18, 19). Based on these data, phosphorylation of GluA2 at S880 has been proposed to facilitate long-term depression (LTD) by inducing a switch from GluA2–GRIP to GluA2–PICK interaction (16, 20, 21).
A role for PICK1 in synaptic plasticity has been established most clearly in the cerebellum, where LTD at multiple synapses requires intact PICK1 function (18, 22, 23). PICK1 also plays an essential role in a developmentally regulated form of LTD at hippocampal mossy fiber–cornu ammonis 3 (CA3) synapses (24). At hippocampal Schaffer collateral–cornu ammonis 1 (SC–CA1) synapses numerous studies find a role for PICK1 in regulating LTD (20, 25–27), although the level of impairment upon disruption of PICK1 function varies considerably, and contradictory findings exist (28). In addition, a few studies suggest that PICK1 may regulate activity-dependent insertion of AMPARs (29, 30), and a recent study implicates PICK1 in maintaining long-term potentiation (LTP) at SC–CA1 synapses in the hippocampus (27).
In the present study we investigated the effect of genetic deletion of PICK1 on basal SC–CA1 synaptic transmission and synaptic plasticity in adult and juvenile mice. We also determined whether PICK1 is required for inhibitory avoidance (IA) learning. Surprisingly, we found that loss of PICK1 had no significant effect on synaptic plasticity or IA learning in juvenile mice but resulted in impaired synaptic plasticity and IA learning in adult mice.
Results
Basal Synaptic Transmission Is Unaffected in Adult PICK1-KO Mice.
In juvenile rodents, acute disruption of PICK1 function with a peptide that mimics the phosphorylated C terminus of GluA2 or via expression of a PICK1 mutant that is deficient for lipid binding results in a gradual increase in synaptic responses at hippocampal SC–CA1 synapses in most (20, 26, 31) but not all (28) studies. Conversely, overexpression of PICK1 in hippocampal neurons results in a decrease in surface GluA2 expression (19, 26, 32) consistent with a model in which PICK1 functions to retain GluA2-containing receptors in an intracellular or extrasynaptic pool. However, the role for PICK1 in adult neuronal function has not been investigated. Thus, we examined multiple measures of basal synaptic transmission at SC–CA1 synapses using acute slices from adult (2- to 3-mo-old) mice in which PICK1 expression had been genetically ablated (see ref. 18).
Input–output curves, obtained by plotting the amplitude of the fiber volley vs. the slope of the field excitatory postsynaptic potential (fEPSP) at various stimulation intensities, were not different in adult PICK1-KO mice compared with their WT littermates (WT: 2.97 ± 0.21 ms−1, n = 19; KO: 2.57 ± 0.22 ms−1, n = 19; P > 0.05) (Fig. S1A). In addition to interacting with postsynaptic AMPARs, PICK1 also binds to and regulates the expression of a presynaptic metabotropic glutamate receptor, mGluR7, which modulates neurotransmitter release (33–35). However, analysis of paired-pulse facilitation (PPF) revealed no difference between PICK1-KO and WT mice [WT, n = 19, KO, n = 20; P > 0.05 at all interstimulus intervals (ISI)] (Fig. S1B), suggesting that basal presynaptic release is not altered in adult PICK1-KO mice. To assess synaptic transmission at the level of individual synapses, we recorded miniature excitatory postsynaptic currents (mEPSCs) resulting from action potential-independent spontaneous glutamate release. mEPSC amplitude and frequency were unaffected in adult PICK1-KO mice [WT: amplitude = 12.8 ± 0.40 pA, frequency = 3.34 ± 0.33 Hz, n = 25 cells; KO: amplitude = 12.2 ± 0.44 pA (P > 0.3), frequency = 2.83 ± 0.27 Hz (P > 0.2), n = 22 cells] (Fig. S1C).
AMPARs consist primarily of GluA1/GluA2 heteromers at SC–CA1 synapses (36). The presence of GluA2 in AMPARs not only regulates association with AMPAR BPs, but also renders AMPARs impermeable to Ca2+ and eliminates inward rectification measured by the current–voltage (I–V) relationship of a synapse. To examine the effect of PICK1 deletion on the basal composition of AMPA receptors, we measured the I–V relationship at SC inputs to CA1 pyramidal neurons. The synaptic I–V relationship was not affected in adult PICK1-KO mice (Fig. S1D). Additionally, using outside-out patches from somatic membranes of CA1 pyramidal cells, we found no difference in the amplitude of current induced by 20 μM AMPA at −70 mV or in the I–V relationship between PICK1 WT and KO mice (at −70 mV, WT: 213 ± 28 pA; KO: 172 ± 20 pA; P = 0.12) (Fig. S1E), demonstrating that the composition of extrasynaptic AMPA receptors is not altered in PICK1-KO mice. In summary, basal synaptic transmission at SC–CA1 synapses appears normal in adult PICK1-KO mice.
Multiple Forms of LTD Are Impaired in Adult PICK1-KO Mice.
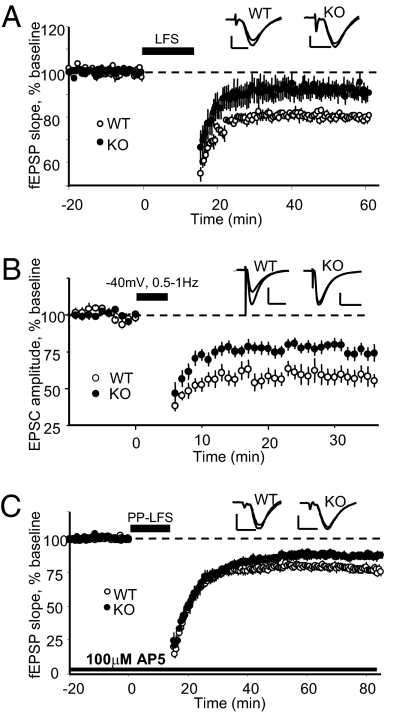
Consistent with a role for PICK1 in retaining intracellular AMPARs after activity-dependent endocytosis (14) or in regulating AMPAR endocytosis (15–17), we found that NMDA receptor (NMDAR)-dependent LTD induced with a standard low-frequency stimulation (LFS) protocol and measured by monitoring extracellular fEPSPs (Fig. 1A) or with whole-cell pairing of −40 mV depolarization with brief LFS (Fig. 1B) was significantly reduced in slices from PICK1-KO mice. In addition to NMDAR-dependent LTD, PICK1 has been implicated in mGluR-dependent LTD in the ventral tegmental area (37), perirhinal cortex (38), and cerebellum (23) of immature rodents. Using paired pulse-LFS (PP-LFS) in the presence of the NMDAR antagonist d,l-2-amino-5-phosphonopentanoic acid (d,l-AP5), we found that NMDAR-independent LTD was reduced in the hippocampus of adult PICK1-KO mice (Fig. 1C). At SC–CA1 synapses this protocol induces LTD that is dependent on group I mGluRs (39–41) and/or muscarinic acetylcholine receptors (mAChRs) (42). These data highlight a central role for PICK1 in multiple forms of hippocampal LTD.
Fig. 1.
Multiple forms of LTD are impaired in adult PICK1-KO mice. (A) LTD induced with LFS (1 Hz, 900 stimuli, 30 °C) is significantly reduced in PICK1-KO mice. WT (n = 8): 80 ± 1% at 55–60 min; KO (n = 9): 92 ± 3% at 55–60 min; P < 0.01. (B) LTD induced by pairing 200–300 pulses at 0.5–1 Hz with −40 mV depolarization (35 °C) is reduced in PICK1-KO mice. WT (n = 10): 59 ± 5% at 31–35 min; KO (n = 11): 76 ± 4% at 31–35 min; P < 0.05. (C) NMDA receptor-independent LTD induced by PP-LFS (50-ms ISI, 30 °C) in the NMDAR antagonist d,l-AP5 (100 μM) is reduced in PICK1-KO mice. WT (n = 9): 79 ± 2% at 75–80 min; KO (n = 8): 89 ± 2% at 75–80 min; P = 0.01. (Inset scale bars: A and C: 0.5 mV, 5 ms; B: 100 pA, 10 ms.)
LTP in Adult PICK1-KO Mice Is Reduced with a Subset of Induction Protocols.
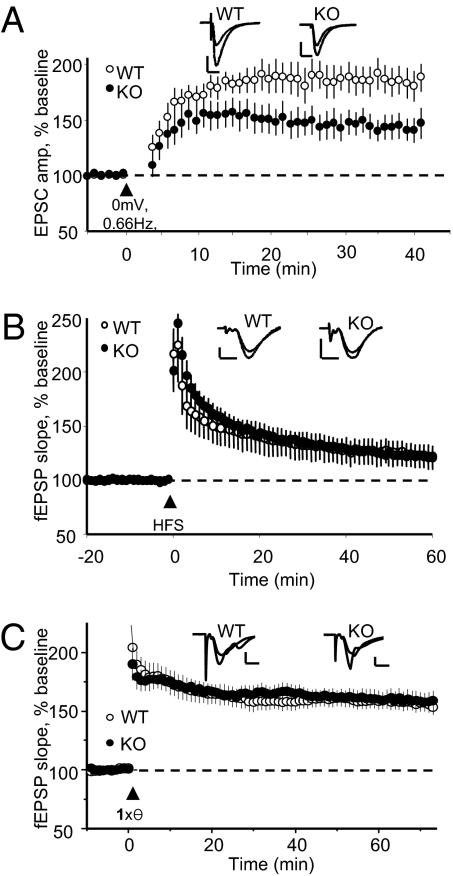
In light of recent findings implicating PICK1 in NMDAR-induced AMPAR insertion and hippocampal LTP in juvenile mice (27, 30), we examined the requirement for PICK1 in LTP at mature hippocampal synapses. LTP induced by pairing postsynaptic depolarization to 0 mV with LFS [0.66 Hz at RT (Fig. 2A) or 2 Hz at 35 °C (Fig. S2)] was significantly reduced in adult PICK1-KO mice. However, high-frequency stimulation (HFS; 2× 100 Hz) (Fig. 2B) or single theta-burst stimulation (TBS) (Fig. 2C) induced LTP that was indistinguishable between WT and PICK1-KO mice. In addition, LTP induced by a single HFS at RT (WT: 125 ± 5%, n = 5; KO: 124 ± 12%, n = 6) or four TBS at 30 °C (WT: 163 ± 6%, n = 8; KO: 166 ± 13%, n = 6) also failed to reveal a deficit in adult PICK1-KO mice. These data suggest that PICK1 is not absolutely required for hippocampal LTP in adult mice, but under some conditions PICK1 is required for normal LTP induction. Given the absolute requirement for PICK1 in LTP observed in juvenile mice (27), we wondered if the induction protocol-specific deficit seen adult PICK1-KO mice in our study reflected a developmental difference in the induction/expression mechanism of LTP in PICK1-KO mice. Thus, we examined basal synaptic transmission as well as LTP and LTD in juvenile PICK1-KO mice.
Fig. 2.
LTP is impaired in an induction protocol-dependent manner in adult PICK1-KO mice. (A) LTP induced by pairing 120 pulses at 0.66 Hz with 0 mV depolarization (RT) is reduced in PICK1-KO mice: WT (n = 10): 185 ± 13%; KO (n = 14): 142 ± 10%; P < 0.05. (B) LTP induced by HFS (2 × 100 Hz, 20-s inter-burst interval, RT) is unaffected in PICK1-KO mice. At 55–60 min after LTP induction, WT (n = 6): 123 ± 9%; KO (n = 5): 123 ± 4%; P > 0.5. (C) LTP induced by a single theta burst (35 °C) is unaffected in PICK1-KO mice. At 56–60 min after induction, WT (n = 7): 159 ± 7%; KO (n = 6): 161 ± 7%; P > 0.5. (Inset scale bars: A: 100 pA, 20 ms; B and C: 0.5 mV, 5 ms.)
Basal Synaptic Transmission Is Modestly Affected in Hippocampal CA1 Pyramidal Neurons of Juvenile PICK1-KO Mice.
Input–output curves (WT: 2.53 ± 0.13 ms−1, n = 47; KO: 2.39 ± 0.12 ms−1, n = 41; P > 0.05) (Fig. S3A), PPF (WT, n = 47; KO, n = 41; P > 0.05 at all ISI) (Fig. S3B), and mEPSC amplitude (WT: 16.6 ± 0.47 pA, n = 35 cells; KO: 15.35 ± 0.35 pA, n = 37 cells; P > 0.1) (Fig. S3C) in juvenile PICK1-KO mice were not different from WT. However, mEPSC frequency was decreased in juvenile PICK1-KO mice (WT: 5.76 ± 0.32 Hz; KO: 3.46 ± 0.23 Hz; P < 0.001) (Fig S3C). Changes in mEPSC frequency may result from changes in presynaptic release probability or from changes in the number of synapses. Given that there is no evidence of altered release probability with evoked responses (Fig. S3B), these data suggest that either the total number of synapses is reduced or the proportion of silent (AMPAR-lacking) synapses is enhanced in juvenile PICK1-KO mice. Alternatively, PICK1 deletion may decrease spontaneous but not evoked neurotransmitter release probability (43, 44).
LTP Is Intact in Juvenile PICK1-KO Mice.
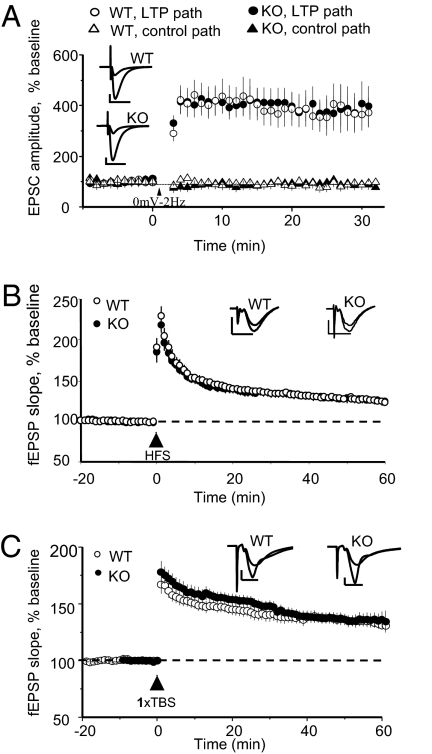
Surprisingly, we found that LTP induced with numerous protocols and under multiple recording conditions was unaffected in juvenile PICK1-KO mice (Fig. 3 and SI Methods). LTP induced with a whole-cell pairing protocol was indistinguishable between WT and PICK1-KO mice (Fig. 3A). Similarly, using extracellular recordings, HFS (Fig. 3B) or TBS (Fig. 3C) induced LTP that was indistinguishable between WT and PICK1-KO mice.
Fig. 3.
LTP is unaffected in juvenile PICK1-KO mice. (A) LTP induced by a whole-cell pairing protocol (200 pulses, 2 Hz, at 0 mV, 35 °C) is unaffected in PICK1-KO mice. At 26–30 min after pairing, WT (n = 5): 383 ± 70%; KO (n = 5): 365 ± 70%; P > 0.5. (B) LTP induced by HFS (2 × 100 Hz, 20-s inter-burst interval, RT) is unaffected in PICK1-KO mice. At 55–60 min after LTP induction, WT (n = 24): 126 ± 3%; KO (n = 23): 123 ± 3%; P > 0.5. (C) LTP induced by a single theta burst (35 °C) is unaffected in PICK1-KO mice. At 56–60 min after TBS, WT (n = 10): 133 ± 4%; KO (n = 8): 136 ± 6%; P > 0.5. (Inset scale bars: A: 100 pA, 20 ms; B and C: 0.5 mV, 5 ms.)
LTD Is Unaffected in Juvenile PICK1-KO Mice.
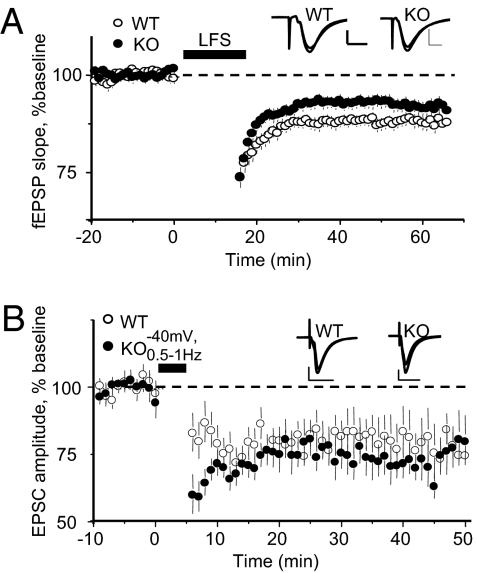
Considering the numerous studies implicating PICK1 in AMPAR endocytosis or in maintaining extrasynaptic pools of GluA2-containing AMPARs, we were surprised to find no significant impairment of LTD in juvenile PICK1-KO mice. LTD induced with a standard LFS protocol and measured by monitoring extracellular fEPSPs (Fig. 4A) and LTD induced with a whole-cell pairing protocol (Fig. 4B) were not significantly affected by loss of PICK1.
Fig. 4.
LTD is modestly affected in juvenile PICK1-KO mice. (A) LTD induced with LFS (35 °C) is not significantly different in PICK1-KO mice. At 61–65 min after beginning LTD induction, WT (n = 18): 88 ± 2%; KO (n = 18): 92 ± 2%; P > 0.1. (B) LTD induced by pairing 200–300 pulses at 0.5–1 Hz with −40 mV depolarization (35 °C) is unaffected in PICK1-KO mice. At 41–45 min after beginning LTD induction, WT (n = 6): 78 ± 9%; KO (n = 9): 70 ± 6%; P > 0.4. (Inset scale bars: A: 0.5 mV, 5 ms; B: 100 pA, 20 ms.)
IA Learning Is Selectively Impaired in Adult PICK1-KO Mice.
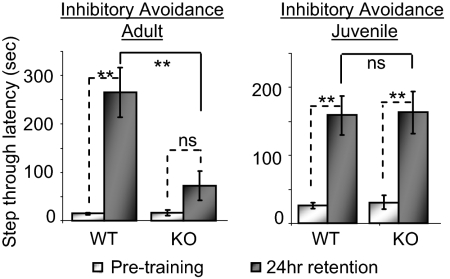
In light of the developmental differences observed in hippocampal synaptic plasticity in PICK1-KO mice, we wanted to determine if a hippocampal-dependent learning task would reflect this age-dependent role for PICK1. We chose to use a step-through IA task because IA learning is dependent on hippocampal function (45–47) and induces AMPAR trafficking and LTP in the hippocampus in vivo (48). Additionally, because this task requires only a single-trial training period, it allows reliable training in a narrow age range in juvenile mice. Memory retention was assessed 24 h after training. Strikingly, adult PICK1-KO mice failed to learn the IA task, but the performance of juvenile PICK1-KO mice was indistinguishable from WT (Fig. 5).
Fig. 5.
Hippocampal-dependent learning/memory is impaired in adult but not in juvenile PICK1-KO mice. Adult (2- to 3-mo-old) or juvenile (~3-wk-old; postnatal day 20–22) mice were trained on a step-through IA task. Latency to cross over to the dark chamber was measured at training and 24 h later. In adult mice, PICK1-KO results in a dramatically reduced latency 24 h after training (WT: n = 10; KO: n = 12). Juvenile PICK1 mice acquire the IA task normally (WT: n = 7; KO: n = 9). **P < 0.01. ns, not significant.
Discussion
In this study we demonstrate a developmentally regulated role for PICK1 in hippocampal synaptic plasticity and learning. We found that the requirement for PICK1 in hippocampal LTP is limited to a subset of LTP induction protocols in adult mice. In contrast, PICK1 appears to be generally required for LTD in the adult hippocampus. Both NMDAR-dependent and mGluR/mAChR-dependent LTD were impaired in adult PICK1-KO mice. Basal transmission was unaffected in adult PICK1-KO mice. Thus, the observed deficits in synaptic plasticity are likely attributable to a direct role for PICK1 in hippocampal synaptic plasticity. To our surprise, when we examined hippocampal LTP and LTD in juvenile mice, we observed no effect of PICK1 deletion. Additionally, IA learning was selectively impaired in adult PICK1-KO mice, supporting an age-dependent role for PICK1 in normal brain function.
Regulation of Basal AMPAR Trafficking and Composition by PICK1.
In neurons of juvenile mice, acute overexpression of PICK1 causes a decrease in surface expression of GluA2 (19, 26, 32), whereas acute disruption of PICK1 function results in an increase in basal synaptic transmission (20, 26, 31, but see ref. 28), consistent with a model in which, under basal conditions, PICK1 functions to sequester and stabilize an intracellular/extrasynaptic pool of GluA2-containing AMPARs (14) or in which PICK1 inhibits recycling of GluA2-containing AMPARs (15–17). In the current study we find no alterations in basal synaptic transmission in adult PICK1-KO mice and only modest changes in juvenile mice. This result may indicate that homeostatic changes are largely able to compensate for the chronic loss of PICK1, resulting in normal steady-state levels of synaptic AMPAR expression.
In juvenile mice, we observe that chronic loss of PICK1 results in a decrease in mEPSC frequency. PICK1 is expressed presynaptically and has been shown to regulate trafficking of presynaptic receptors that could influence neurotransmitter release (33–35, 49–51). However, our finding that PPF, a measure correlated with presynaptic vesicle release, is unchanged in juvenile PICK1-KO mice indicates that the observed decrease in mEPSC frequency is unlikely to result from a general decrease in presynaptic release probability. Interestingly, there is evidence that spontaneous (action potential-independent) and evoked (action potential-dependent) neurotransmitter release use distinct vesicle pools (43, 44). Therefore, PICK1 could selectively affect spontaneous but not evoked neurotransmitter release in juvenile mice. Alternatively, the decrease in mEPSC frequency could reflect a decrease in the number of total or functional (AMPAR-containing) synapses in juvenile PICK1-KO mice.
PICK1 in LTD.
Our results demonstrate that genetic ablation of PICK1 results in significant impairment of several forms of LTD in the mature rodent hippocampus. mGluR/mAChR-dependent and NMDAR-dependent LTD recruit different signaling mechanisms to induce LTD (42, 52–55), but all are expressed via removal of synaptic AMPARs (42, 56–60). The fact that both NMDAR- and mGluR/mAChR-mediated LTD are impaired in adult PICK1-KO mice suggests that PICK1 is an essential mediator of activity-dependent AMPAR trafficking, regardless of the upstream signal transduction pathway recruited.
A recent report showed that intracellular perfusion of a peptide that mimics the S880 phosphorylated GluA2 C-terminal tail, previously shown to inhibit PICK1 binding to endogenous GluA2, decreased mGluR- but not NMDAR-dependent LTD in the perirhinal cortex of immature (postnatal day 7–13) rodents (38). This result suggests that the broad requirement for PICK1 in distinct forms of LTD observed in mature mice in our study may not be generalized across brain regions or developmental age. Indeed, numerous reports find that in juvenile rodents, mGluR LTD at SC–CA1 synapses is expressed presynaptically and does not involve internalization of AMPARs (61–66), making it unlikely that in immature rodents mGluR LTD at SC–CA1 synapses requires PICK1 function. Surprisingly, we find that NMDAR-dependent LTD is not significantly impaired in juvenile PICK1-KO mice. It is not clear what mediates the observed developmental switch in PICK1 dependence of LTD. Induction and expression mechanisms underlying LTD can vary dramatically by developmental age (24, 25, 64, 67, 68); thus juvenile mice may express an additional, PICK1-independent form of LTD. Previous studies investigating the role of PICK1 in LTD at juvenile SC–CA1 synapses generally report some level of impairment when PICK1 function is acutely or chronically blocked, but this impairment ranges from a ~40–60% reduction in the magnitude of LTD (20, 25, 26) to complete block of LTD (27), and one study failed to find that disrupting PICK1–GluA2 interactions had an effect on LTD (28). These differences are not correlated with the method of PICK1 manipulation (acute vs. chronic or inhibition of function vs. removal of PICK1 protein). Taken together, these data suggest that PICK1 is not absolutely required for LTD at juvenile SC–CA1 synapses but that under some conditions PICK1 does contribute significantly to LTD induction. It has been suggested that the level of protein phosphatase 1 at synapses might determine whether PICK1 participates in LTD by affecting basal levels of GluA2 S880 phosphorylation (see below) (25) and different slice preparation, recovery, or recording conditions can result in different basal levels of synaptic signaling molecules (69), which could significantly impact whether PICK1 participates in LTD.
One mechanism by which PICK1 could regulate hippocampal LTD involves an activity-dependent increase in the fraction of GluA2 associated with PICK1 via phosphorylation of GluA2 at S880 (9, 16, 20, 21, 25). Phosphorylation of GluA2 at this residue disrupts binding with GRIP but not PICK1, shifting the predominant PDZ interaction to PICK1. PICK1 may facilitate LTD by actively participating in AMPAR internalization, as suggested by the presence of a lipid-binding BAR domain in PICK1 (70). Alternatively, release of AMPARs from GRIP may be permissive for other molecules that are actively involved in endocytosis, followed by retention of internalized AMPARs via interaction with PICK1. Numerous studies find that disruption of PICK1 function blocks AMPAR internalization (15–17). However, it is difficult, using static methods for visualizing AMPARs, to distinguish between a direct role for PICK1 in internalization of receptors vs. retention of internalized receptors. Live imaging of AMPAR internalization induced by NMDAR stimulation in neurons cultured from PICK1-KO mice suggests that PICK1 is not necessary for internalization but functions by retaining internalized receptors or inhibiting receptor recycling (14).
PICK1 in LTP.
Although most studies examining the role of PICK1 in activity-induced AMPAR trafficking and synaptic plasticity find a role for PICK1 in LTD, several reports suggest that PICK1 also may participate in AMPAR trafficking required for LTP (27, 29, 30). Consistent with these findings, we observed that hippocampal LTP induced with a pairing protocol is impaired in adult PICK1-KO mice. However, we note that several common LTP induction protocols, including HFS and TBS, failed to reveal a difference between adult PICK1-KO mice and their WT littermates. Thus, our data indicate that in the mature hippocampus PICK1 can regulate LTP but is not essential for LTP induction.
Surprisingly, we find that LTP in juvenile PICK1-KO mice is indistinguishable from that of WT littermates. To the best of our knowledge, the slice preparation, LTP protocol, age of animals, and recording conditions in Fig. 3B are identical to those used by Terashima et al. (27), who observed a loss of LTP in juvenile PICK1-KO. The reason for this discrepancy is not clear, because Terashima and colleagues used the mouse line generated by our laboratory. It is possible that subtle differences in slice preparation or recording conditions facilitate plasticity with different induction or expression requirements. Consistent with this possibility, previous studies from this group demonstrate that LTP at hippocampal synapses is mediated by a transient insertion of GluA2-lacking receptors (71), whereas under the conditions used in Fig. 3B we find that LTP does not require activity through GluA2-lacking receptors (determined by selective antagonism of GluA2-lacking receptors immediately after LTP induction: control: 124 ± 6%, n = 5; 50 μM 1-naphthyl acetyl spermine (NASPM): 122 ± 7%, n = 5; P > 0.5). The requirement for GluA2-lacking receptors in LTP at SC–CA1 synapses is controversial and may depend on the age of animals and type of induction protocol used (71–75).
In acutely prepared cortical slices from juvenile PICK1-KO mice, surface expression of GluA1 is decreased, whereas surface GluA2 is elevated (76). However, basal synaptic transmission is unaffected in this preparation, suggesting that the subunit composition of extrasynaptic surface pools of AMPARs may be aberrant in the absence of PICK1. The GluA1 subunit is thought to be necessary for activity-dependent incorporation of AMPARs during LTP, and recent data support the idea that the immediate source of receptors for LTP may be lateral diffusion from extrasynaptic pools of surface receptors (77, 78). Thus, the redistribution of extrasynaptic receptors observed in PICK1-KO mice could impair the activity-dependent trafficking of GluA1-containing receptors into the synapse during LTP without affecting basal synaptic transmission. Interestingly, juvenile GluA1-KO mice exhibit LTP, but the ability to induce LTP declines as the mice mature (79). Numerous studies report developmental differences in the expression mechanisms of LTP (75, 79–81). If the LTP deficit observed in adult PICK1-KO mice results from depletion of the available pool of extrasynaptic GluA1, LTP in juvenile mice may be mediated by a GluA1-independent form of LTP that exists selectively in young mice (79).
Developmental Regulation of PICK1 Function in Learning and/or Memory.
Our finding that IA learning and/or memory is impaired selectively in adult but not juvenile PICK1-KO mice corroborates our electrophysiological data demonstrating developmental regulation of PICK1 function in synaptic plasticity and suggests that PICK1 function is critical for both hippocampal synaptic plasticity and memory in adult but not in juvenile mice. It should be noted that although IA training induces synaptic plasticity and AMPAR trafficking in the hippocampus, and intact hippocampal function is required for IA learning, IA learning is not exclusively dependent on the hippocampus. These data, however, do support a developmentally regulated requirement for PICK1 in normal brain function.
Methods
Electrophysiology.
Whole-cell or extracellular field recordings were obtained from acute hippocampal slices prepared from juvenile (2- to 3-wk-old) or adult (2- to 3-mo-old) PICK1-KO and WT mice.
Behavior.
A standard step-though IA task was used in which mice initially were placed in the light side of a rectangular chamber consisting of a light chamber and a dark chamber separated by a wall with a guillotine-style door. For training, mice were placed in the light side, and the latency to step through to the dark side was measured. This value was taken as the control, pretraining value. After crossing to the dark chamber, mice were given a 0.3-mA, 2-s foot shock. Memory was assessed 24 h later by reintroducing mice to the light side and measuring the latency to step through to the dark chamber. For more details, see SI Methods.
Supplementary Material
Footnotes
Conflict of interest statement: Under a licensing agreement between Millipore Corporation and The Johns Hopkins University, R.L.H. is entitled to a share of royalties received by the University on sales of products described in this article. R.L.H. is a paid consultant to Millipore Corporation. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016103107/-/DCSupplemental.
References
- 1.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- 2.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 4.Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38:635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, et al. GRIP: A synaptic PDZ domain-containing protein that interacts with AMPA receptors. Nature. 1997;386:279–284. doi: 10.1038/386279a0. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava S, et al. Novel anchorage of GluR2/3 to the postsynaptic density by the AMPA receptor-binding protein ABP. Neuron. 1998;21:581–591. doi: 10.1016/s0896-6273(00)80568-1. [DOI] [PubMed] [Google Scholar]
- 8.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 9.Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuda S, Mikawa S, Hirai H. Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J Neurochem. 1999;73:1765–1768. doi: 10.1046/j.1471-4159.1999.731765.x. [DOI] [PubMed] [Google Scholar]
- 11.Staudinger J, Zhou J, Burgess R, Elledge SJ, Olson EN. PICK1: A perinuclear binding protein and substrate for protein kinase C isolated by the yeast two-hybrid system. J Cell Biol. 1995;128:263–271. doi: 10.1083/jcb.128.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Xia J. Structure and function of PICK1. Neurosignals. 2006-2007;15:190–201. doi: 10.1159/000098482. [DOI] [PubMed] [Google Scholar]
- 13.Osten P, et al. Mutagenesis reveals a role for ABP/GRIP binding to GluR2 in synaptic surface accumulation of the AMPA receptor. Neuron. 2000;27:313–325. doi: 10.1016/s0896-6273(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 14.Lin D-T, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanley JG, Henley JM. PICK1 is a calcium-sensor for NMDA-induced AMPA receptor trafficking. EMBO J. 2005;24:3266–3278. doi: 10.1038/sj.emboj.7600801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwakura Y, et al. N-methyl-D-aspartate-induced alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptor down-regulation involves interaction of the carboxyl terminus of GluR2/3 with Pick1. Ligand-binding studies using Sindbis vectors carrying AMPA receptor decoys. J Biol Chem. 2001;276:40025–40032. doi: 10.1074/jbc.M103125200. [DOI] [PubMed] [Google Scholar]
- 17.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10:259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Terashima A, et al. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci. 2004;24:5381–5390. doi: 10.1523/JNEUROSCI.4378-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C-H, Chung HJ, Lee H-K, Huganir RL. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–11730. doi: 10.1073/pnas.211132798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidenman KJ, Steinberg JP, Huganir R, Malinow R. Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells. J Neurosci. 2003;23:9220–9228. doi: 10.1523/JNEUROSCI.23-27-09220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinberg JP, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 24.Ho MTW, et al. Developmental expression of Ca2+-permeable AMPA receptors underlies depolarization-induced long-term depression at mossy fiber CA3 pyramid synapses. J Neurosci. 2007;27:11651–11662. doi: 10.1523/JNEUROSCI.2671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu XD, Huang Q, Yang X, Xia H. Differential regulation of AMPA receptor trafficking by neurabin-targeted synaptic protein phosphatase-1 in synaptic transmission and long-term depression in hippocampus. J Neurosci. 2007;27:4674–4686. doi: 10.1523/JNEUROSCI.5365-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin W, et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26:2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terashima A, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57:872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daw MI, et al. PDZ proteins interacting with C-terminal GluR2/3 are involved in a PKC-dependent regulation of AMPA receptors at hippocampal synapses. Neuron. 2000;28:873–886. doi: 10.1016/s0896-6273(00)00160-4. [DOI] [PubMed] [Google Scholar]
- 29.Maher BJ, Mackinnon RL, 2nd, Bai J, Chapman ER, Kelly PT. Activation of postsynaptic Ca(2+) stores modulates glutamate receptor cycling in hippocampal neurons. J Neurophysiol. 2005;93:178–188. doi: 10.1152/jn.00651.2004. [DOI] [PubMed] [Google Scholar]
- 30.Sossa KG, Court BL, Carroll RC. NMDA receptors mediate calcium-dependent, bidirectional changes in dendritic PICK1 clustering. Mol Cell Neurosci. 2006;31:574–585. doi: 10.1016/j.mcn.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 31.Yao Y, et al. PKM zeta maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez JL, et al. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–5428. doi: 10.1523/JNEUROSCI.21-15-05417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Far O, et al. Interaction of the C-terminal tail region of the metabotropic glutamate receptor 7 with the protein kinase C substrate PICK1. Eur J Neurosci. 2000;12:4215–4221. doi: 10.1046/j.1460-9568.2000.01309.x. [DOI] [PubMed] [Google Scholar]
- 34.Perroy J, et al. PICK1 is required for the control of synaptic transmission by the metabotropic glutamate receptor 7. EMBO J. 2002;21:2990–2999. doi: 10.1093/emboj/cdf313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suh YH, et al. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 38.Jo J, et al. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca(2+) sensors, NCS-1 and PICK1. Neuron. 2008;60:1095–1111. doi: 10.1016/j.neuron.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faas GC, Adwanikar H, Gereau RWIV, 4th, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: A differential role in acute depression of synaptic transmission and long-term depression. J Neurosci. 2002;22:6885–6890. doi: 10.1523/JNEUROSCI.22-16-06885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 41.Kemp N, Bashir ZI. Induction of LTD in the adult hippocampus by the synaptic activation of AMPA/kainate and metabotropic glutamate receptors. Neuropharmacology. 1999;38:495–504. doi: 10.1016/s0028-3908(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 42.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atasoy D, et al. Spontaneous and evoked glutamate release activates two populations of NMDA receptors with limited overlap. J Neurosci. 2008;28:10151–10166. doi: 10.1523/JNEUROSCI.2432-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung C, Barylko B, Leitz J, Liu X, Kavalali ET. Acute dynamin inhibition dissects synaptic vesicle recycling pathways that drive spontaneous and evoked neurotransmission. J Neurosci. 2010;30:1363–1376. doi: 10.1523/JNEUROSCI.3427-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Izquierdo I, et al. Neurotransmitter receptors involved in post-training memory processing by the amygdala, medial septum, and hippocampus of the rat. Behav Neural Biol. 1992;58:16–26. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- 46.Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat's passive avoidance response: A tetrodotoxin functional inactivation study. Brain Res. 1996;730:32–39. doi: 10.1016/0006-8993(96)00427-1. [DOI] [PubMed] [Google Scholar]
- 47.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 49.Boudin H, et al. Presynaptic clustering of mGluR7a requires the PICK1 PDZ domain binding site. Neuron. 2000;28:485–497. doi: 10.1016/s0896-6273(00)00127-6. [DOI] [PubMed] [Google Scholar]
- 50.Dev KK, et al. PICK1 interacts with and regulates PKC phosphorylation of mGLUR7. J Neurosci. 2000;20:7252–7257. doi: 10.1523/JNEUROSCI.20-19-07252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haglerød C, et al. Protein interacting with C kinase 1 (PICK1) and GluR2 are associated with presynaptic plasma membrane and vesicles in hippocampal excitatory synapses. Neuroscience. 2009;158:242–252. doi: 10.1016/j.neuroscience.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 52.Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 53.Nicholls RE, et al. Transgenic Mice Lacking NMDAR-Dependent LTD Exhibit Deficits in Behavioral Flexibility. 2008;58:104–117. doi: 10.1016/j.neuron.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 54.Park S, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 57.Carroll RC, et al. Dynamin-dependent endocytosis of ionotropic glutamate receptors. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heynen AJ, Quinlan EM, Bae DC, Bear MF. Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron. 2000;28:527–536. doi: 10.1016/s0896-6273(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 59.Snyder EM, et al. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- 60.Xiao M-Y, Zhou Q, Nicoll RA. Metabotropic glutamate receptor activation causes a rapid redistribution of AMPA receptors. Neuropharmacology. 2001;41:664–671. doi: 10.1016/s0028-3908(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 61.Bolshakov VY, Siegelbaum SA. Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science. 1994;264:1148–1152. doi: 10.1126/science.7909958. [DOI] [PubMed] [Google Scholar]
- 62.Feinmark SJ, et al. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J Neurosci. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fitzjohn SM, et al. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001;537:421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 66.Zakharenko SS, Zablow L, Siegelbaum SA. Altered presynaptic vesicle release and cycling during mGluR-dependent LTD. Neuron. 2002;35:1099–1110. doi: 10.1016/s0896-6273(02)00898-x. [DOI] [PubMed] [Google Scholar]
- 67.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918. doi: 10.1523/JNEUROSCI.13-07-02910.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jo J, et al. Experience-dependent modification of mechanisms of long-term depression. Nat Neurosci. 2006;9:170–172. doi: 10.1038/nn1637. [DOI] [PubMed] [Google Scholar]
- 69.Ho OH, Delgado JY, O'Dell TJ. Phosphorylation of proteins involved in activity-dependent forms of synaptic plasticity is altered in hippocampal slices maintained in vitro. J Neurochem. 2004;91:1344–1357. doi: 10.1111/j.1471-4159.2004.02815.x. [DOI] [PubMed] [Google Scholar]
- 70.Dawson JC, Legg JA, Machesky LM. Bar domain proteins: A role in tubulation, scission and actin assembly in clathrin-mediated endocytosis. Trends Cell Biol. 2006;16:493–498. doi: 10.1016/j.tcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 71.Plant K, et al. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- 72.Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray EE, Fink AE, Sariñana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- 74.Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu Y, et al. Age-dependent requirement of AKAP150-anchored PKA and GluR2-lacking AMPA receptors in LTP. EMBO J. 2007;26:4879–4890. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 2010;30:6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi S-H, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 79.Jensen V, et al. A juvenile form of postsynaptic hippocampal long-term potentiation in mice deficient for the AMPA receptor subunit GluR-A. J Physiol. 2003;553:843–856. doi: 10.1113/jphysiol.2003.053637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kirkwood AL, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- 81.Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.