Abstract
Epsin is a ubiquitin-binding endocytic adaptor, which is highly concentrated at clathrin-coated pits and coordinates acquisition of bilayer curvature with coat recruitment and cargo selection. Epsin is encoded by three distinct genes in mammals. Epsin 1 and 2 have broad tissue distribution with high-level expression in the brain. In contrast, epsin 3 was reported to be expressed primarily in immature keratinocytes. Here, we show that epsin 3 is selectively expressed at high levels in the stomach (including the majority of gastric cancers), where it is concentrated in parietal cells. In these cells, epsin 3 is enriched and colocalized with clathrin around apical canaliculi, the sites that control acidification of the stomach lumen via the exo-endocytosis of vesicles containing the H/K ATPase. Deletion of the epsin 3 gene in mice did not result in obvious pathological phenotypes in either the stomach or other organs, possibly because of overlapping functions of the other two epsins. However, levels of EHD1 and EHD2, two membrane tubulating proteins with a role in endocytic recycling, were elevated in epsin 3 knock-out stomachs, pointing to a functional interplay of epsin 3 with EHD proteins in the endocytic pathway of parietal cells. We suggest that epsin 3 cooperates with other bilayer binding proteins with curvature sensing/generating properties in the specialized traffic and membrane remodeling processes typical of gastric parietal cells.
Keywords: adaptor protein, gastric cancer, Hip1R, EH domain, ezrin
Clathrin-mediated endocytosis represents the major pathway that cells use for the selective internalization of plasma membrane proteins and their extracellular ligands. Binding of clathrin to the plasma membrane is mediated by the clathrin adaptors (1, 2). One such adaptor is epsin, which is expressed by three different isoforms in mammals (3–5). Epsin comprises an epsin N-terminal homology (ENTH) domain, which directly interacts with the bilayer and also has membrane-deforming properties, followed by an extended, primarily unfolded tail that contains, in sequence, binding motifs for ubiquitin, the endocytic adaptor AP-2, clathrin, and EH domains (3, 6–10). Through these multiple interactions, epsin is thought to coordinate the recruitment of ubiquitinated cargo proteins with the nucleation and growth of endocytic clathrin-coated pits.
Most studies to date have focused on epsin 1 and 2, which are concentrated in the brain, but have broad tissue distribution (3, 10, 11). Little is known about epsin 3, except that it was reported to be expressed, nearly exclusively, in immature keratinocytes (12). In a survey of several tissues, we have now detected a strikingly selective expression of epsin 3 in the stomach and, more specifically, in its oxyntic region. Such region is enriched in parietal cells (oxyntic cells): that is, the cells specialized for the acidification of the stomach lumen. A unique feature of these cells is a highly developed membrane-recycling machinery at their apical pole that mediates the shuttling of the H/K ATPase between intracellular tubulovesicular structures and the cell surface. Regulation of the pH in the stomach lumen is critically dependent on the exo- and endocytosis of H/K ATPase-containing vesicles. Given the endocytic function of epsin, these findings raised the possibility that high-level expression of epsin 3 in the stomach may reflect its selective enrichment at apical endocytic sites in gastric parietal cells. The massive specialization of these cells for endocytic recycling makes them a powerful model system toward the elucidation of recycling mechanisms in mammalian cells. Thus, we have characterized the precise localization of epsin 3 in the stomach and we have explored the potential significance of this localization.
Results and Discussion
Epsin 3 Is Expressed at High Levels in the Stomach.
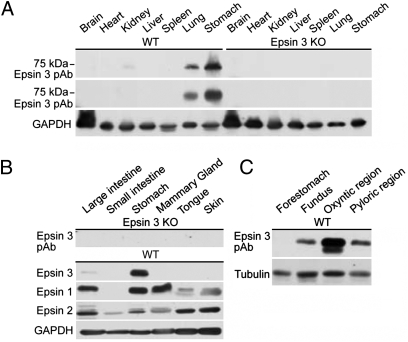
As assayed by Western blotting, the distribution of epsin 3 in adult mouse tissues is highly heterogeneous. By far, the highest expression was detected in the stomach (Fig. 1 A and B). The much broader expression of epsin 1 and 2 (Fig. 1B) (11) confirmed the isoform specificity of the anti-epsin 3 antibodies, which was further validated by the absence of immunoreactivity in the corresponding tissues obtained from an epsin 3 knock-out (KO) mouse (Fig. 1 A and B) (see below). Further analysis of the expression of epsin 3 in different regions of the stomach [forestomach/cardiac region, fundus, oxyntic region, and pyloric region (13)] by Western blotting identified the oxyntic region as the site of highest concentration (Fig. 1C).
Fig. 1.
Epsin 3 is highly expressed in the body region of the stomach. Western blots of total homogenates of multiple mouse organs (A and B) and stomach subregions (C) with anti-epsin 3 specific polyclonal (pAb) and monoclonal (mAb) antibodies, as well as with antibodies directed against epsin 1 and 2. Immunoblotting for GAPDH and tubulin were used as loading controls.
Selective Enrichment of Epsin 3 in Gastric Parietal Cells.
The cellular localization of epsin 3 in the mouse stomach was examined by immunofluorescence. Low-power views of frozen sections (Fig. 2A) showed that epsin 3 was predominantly concentrated in parietal cells of the gastric glands, but no labeling was detected in stomach sections of epsin 3 KO mice (Fig. 2B). Epsin 3 was expressed at only much lower levels in mucosal cells of the stomach surface, and other gastric cells were epsin 3-negative (Fig. 2 A and B). This localization was confirmed by double immunofluorescence for epsin 3 and for H/K ATPase, a specific marker of gastric parietal cells (Fig. 2 C and D) (14).
Fig. 2.
Localization of epsin 3 in the wall of the body of the stomach. Immunofluorescence of thick (10 μm) (A and B) and semithin (0.5 μm) (C–F) frozen sections. (A) Epsin 3 is selectively concentrated in parietal cells (arrows) and also present, but at lower concentration, in the mucous-secreting cells that line the stomach lumen (arrowheads). (B) Epsin 3 immunoreactivity is absent from the stomach of epsin 3 KO mice. (C–F) Immunofluorescence images showing that cells positive for epsin 3 are also positive for H/K ATPase immunoreactivity, a specific marker of parietal cells. Note that not all cells within the gastric gland are positive for epsin 3, as seen by DAPI staining of nuclei. (Scale bar, 10 μm.)
Epsin 3 Is Localized at Clathrin-Coated Pits of Apical Canaliculi of Parietal Cells.
Gastric parietal cells have a unique structure that reflects their specialized function in acid secretion. Their apical luminal surface, which is characterized by deep invaginations (canaliculi), is the site where H/K ATPase pumps protons into the gastric lumen (14). Regulation of this transport is primarily achieved by the incorporation and removal of the H/K ATPase into and from the membrane of the canaliculi by exo-endocytosis. At rest, H/K ATPase is localized primarily in intracellular tubulovesicles. Upon appropriate stimulation, such as in response to histamine, it translocates to the apical membrane leading to a dramatic enlargement of canaliculi and to increased transport of H+ into the gastric lumen. This activated state is reversed by the endocytosis of H/K ATPase-containing apical canalicular membrane to regenerate intracellular tubulovesicles (15).
Because epsin is an endocytic adaptor, we hypothesized that the high levels of epsin 3 in parietal cells reflected a specialized function in the endocytosis of apical membrane. To investigate whether epsin 3 immunoreactivity localized at endocytic sites of canaliculi, semithin sections of the stomach body were stained by immunofluorescence for epsin 3, the H/K ATPase, and ezrin. Ezrin, a component of the membrane-associated cytoskeleton (16), is highly concentrated at canaliculi and outlines their profiles. Before fixation by perfusion, mice were either injected with histamine, to induce massive exocytosis of H/K ATPase-containing membranes, or with the histamine H2-receptor antagonist cimetidine, to counteract endogenous histamine stimulation and thus to observe parietal cells in their resting state.
As expected, the H/K ATPase was localized in the cytoplasm outside the canalicular profiles, as defined by the ezrin signal, in cimetidine-treated samples, but strongly colocalized with ezrin on the dilated canaliculi in histamine-treated samples (Fig. 3A). These changes reflect a redistribution of H/K ATPase from the tubulovesicles to the apical surface. Epsin 3 had a strongly punctate localization that was juxtaposed to the ezrin signal in both conditions, but colocalized with the H/K ATPase only in stimulated samples, revealing a localization next to the apical canaliculi (Fig. 3B). Further double-immunofluorescence experiments showed that all epsin 3 dots colocalized in both cimetidine- and histamine-treated samples with a subset of clathrin immunoreactive dots, indicating a localization of epsin 3 at endocytic clathrin-coated pits (Fig. 3C). However, clathrin immunoreactive dots were not restricted to canaliculi, consistent with the well-established additional presence of clathrin in the Golgi complex and on endosomes (1, 17).
Fig. 3.
Epsin 3 colocalizes with clathrin at apical canaliculi of parietal cells in both resting (cimetidine) and stimulated (histamine) WT stomachs. Immunofluorescence of semithin sections. (A) H/K ATPase is localized outside the profiles of apical canaliculi (as defined by ezrin immunoreactivity) in a resting cell, but is present at the canaliculi in a stimulated cell. A white asterisk indicates the position of the nucleus. (B) Epsin 3 has a highly punctate localization at the cytoplasmic surface of canaliculi in both conditions. (C) Epsin 3 spots overlap with a subset of clathrin spots (Insets show regions enclosed by rectangles at higher magnification). The green fluorescence observed in some fields (black asterisks) is because of nonspecific fluorescence generated by secondary antibodies. (Scale bars, 10 μm.)
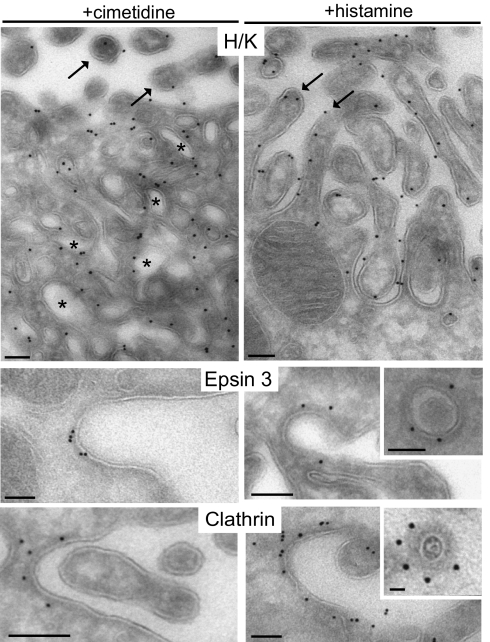
These results were complemented by electron microscopic observation of immunogold-labeled ultrathin frozen sections. H/K ATPase immunoreactivity was primarily localized on the apical microvilli in histamine-treated samples and on internal tubulovesicles in cimetidine-treated samples (Fig. 4). In adjacent sections of the same samples, the great majority of anti-epsin 3 immunogold was localized on pits (40% of the particles) and nearby vesicles (30% of the particles) with a dense coat at the base of apical microvilli in both histamine- and cimetidine-treated stomachs (Fig. 4 and Fig. S1). A similar distribution was observed for anticlathrin immunogold used as a positive control (Fig. 4).
Fig. 4.
Electron microscopy localization of epsin 3 at coated pits and vesicles at the base of apical canaliculi. Immunogold labeling of ultrathin frozen sections. H/K ATPase is localized, as expected, on intracellular tubulovesicles (asterisks) in cimetidine-treated samples and on the surface of microvilli (arrows) in histamine-treated cells. In both conditions, epsin 3 (polyclonal antibodies) and clathrin are found at the surface of invaginations among microvilli and on underlying coated vesicles. (Scale bars, 100 nm.)
Epsin 3 Does Not Play an Essential Role in Gastric Parietal Cells.
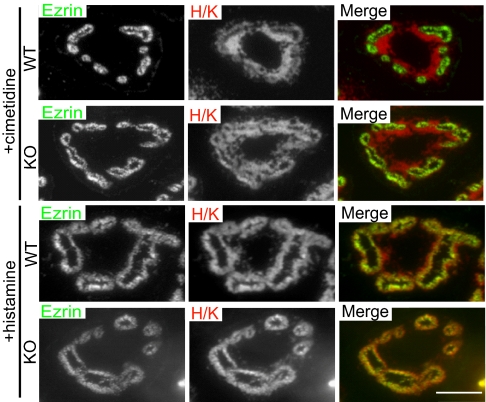
The restricted pattern of epsin 3 expression suggested the possibility that epsin 3 could play a unique and essential role in these cells. To test this hypothesis, an epsin 3 KO mouse was generated (Fig. S2). Epsin 3 KO mice were viable with no obvious developmental, behavioral, or breeding defects. No abnormality was detected by visual inspection of the skin, in spite of the reported expression of epsin 3 in immature keratinocytes (12). The mice stomachs were normal at histological examination (including electron microscopic analysis) and their lumen had a low pH. Furthermore, as in WT stomachs, H/K ATPase immunoreactivity colocalized with ezrin in histamine-treated stomachs, but outside the ezrin-positive canalicular profiles in cimetidine-treated cells, indicating lack of a major defect in the shuttling of membrane from tubulovesicles to the apical canaliculi (Fig. 5). Most likely, epsin 1 and 2, which are also expressed in the stomach (Fig. 1B), have overlapping function with epsin 3. Supporting this possibility, double immunolabeling at the light and electron microscopic levels demonstrated colocalization of epsin 1 and 3 in the stomach (epsin 2-specific antibodies suitable for immunolabeling were not available) (Fig. 6 A and B).
Fig. 5.
The shuttling of H/K ATPase between tubulovesicles and the apical canaliculi is not impaired in epsin 3 KO stomachs. Immunofluorescence of semithin sections. In samples from both WT and epsin 3 KO mice, H/K ATPase is localized outside the ezrin-positive canaliculi in cimetidine-treated samples and relocates to the canaliculi after histamine treatment. (Scale bar, 10 μm.)
Fig. 6.
Colocalization of epsin 1 and 3 in parietal cells. (A) Double immunofluorescence. (Scale bar, 10 μm.) (B) Colocalization of epsin 1 (5-nm gold) and epsin 3 (10-nm gold) on coated vesicles as assessed by immunogold labeling of ultrathin frozen stomach sections. (Scale bar, 100 nm.)
Selective Increase of EHD Proteins in Epsin 3 KO Stomachs.
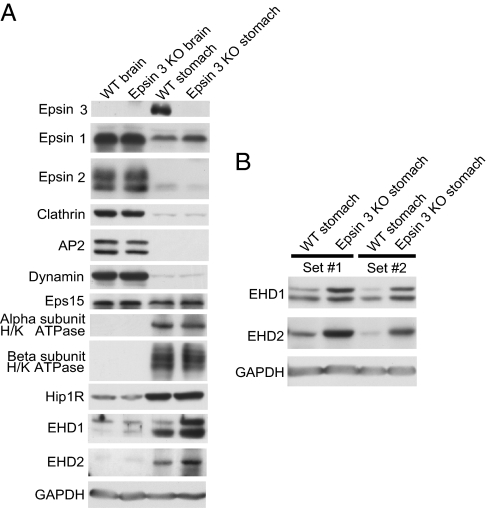
Often, suppression of the expression of a protein results in adaptive changes in the levels of other proteins, thus providing useful insight into its function and about its functional partners. Based on this rationale, the levels of several endocytic proteins was analyzed in WT and epsin 3 KO stomachs (and WT and epsin 3 KO brains as controls) (Fig. 7A). Epsin 1 (but not epsin 2) levels were slightly elevated in the KO stomach, most likely reflecting a compensatory mechanism, but no change in epsin 1 levels was observed in brain, where normal expression of epsin 3 is at the limit of detectability. Clathrin, the clathrin adaptor AP-2, and dynamin were expressed at much lower levels in the stomach than in the brain, but did not undergo any change. No change was observed for the epsin interactor Eps15, or for the α or the β subunit of H/K ATPase, a stomach-specific protein, consistent with the lack of an obvious abnormality in its traffic. Interestingly, expression levels of Hip1R (18), EHD1, and EHD2 (19–21) were higher in the stomach than in the brain. Hip1R, a protein that shares partial domain similarity with epsin (it contains an N-terminal ENTH-like domain) and that links clathrin coats to the actin cytoskeleton, was previously shown to be highly concentrated at apical canaliculi of gastric parietal cells and to be implicated in H/K ATPase endocytosis (18, 22). Furthermore, recent studies in Dictyostelium have suggested a strong functional partnership between epsin and Hip1R at the interface between clathrin-mediated endocytosis and actin (23). EHD2 was one of the proteins identified in a proteomic screen for proteins concentrated in tubulovesicles (24). More importantly, expression level of EHD1 and EHD2 were higher in epsin 3 KO stomach than in WT (Fig. 7 A and B).
Fig. 7.
Western blots showing levels of expression of several endocytic proteins in brain and stomach of WT and epsin 3 KO mice. (A) EHD1 and EHD2 immunoreactive bands are selectively increased in the epsin 3 KO. Note also a slight increase in the epsin 1 band selectively in the KO stomach. (B) The increased levels of the EHD1 and EHD2 immunoreactive bands in the KO stomachs were replicated in mice of two independent litters. GAPDH was used as a loading control.
The increased levels of EHD proteins are of significant interest for several reasons. First, EHD proteins play a role in recycling from the endosome to the plasma membrane (20, 21, 25), and thus may be implicated in membrane shuttling from tubulovesicles to the plasma membrane of canaliculi. Second, purified EHD proteins can deform lipid bilayers both in vitro (19) and in vivo (26, 27) to generate EHD-coated tubules that are in the same size range as tubulovesicles of gastric parietal cells (27, 28). Third, they contain an EH domain homologous to the EH domain of the epsin interactor Eps15 (i.e., a domain predicting a potential interaction with the NPF motifs present in the C-terminal region of the epsins) (25, 29, 30).
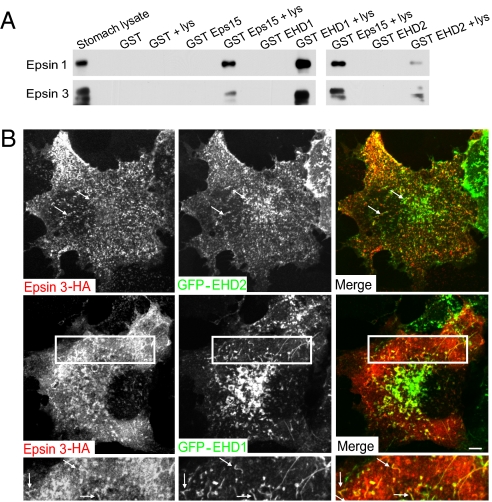
A potential interaction of epsin with EHD1 and EHD2 was directly tested in GST pull-downs from stomach extracts using the EH domains of EHD1 and EHD2 (and of Eps15 as a control) as bait (Fig. 8A). Both epsin 1 and 3 were specifically affinity-purified by the EH domains of all three proteins. Furthermore, EHD1 and EHD2 partially colocalized with HA-tagged epsin 3, both on punctate structures and tubules, when co-overexpressed in COS-7 cells (Fig. 8B). These findings suggest a functional relation between the epsins and the EHD proteins.
Fig. 8.
EHD1 and EHD2 interact with epsin. (A) Anti-epsin 1 and 3 Western blots of material affinity purified by GST or GST fusions of the EH domains of Eps15 and of EHD1 or EHD2. The starting lysate and the pellets obtained by incubating each fusion protein with and without lysate (lys) are shown. (B) Fluorescence images of COS-7 cells cotransfected with HA-tagged epsin 3 and GFP-EHD proteins. The lower panels shows the boxed area of middle panels at higher magnification. Arrows indicate some structures positive for both epsin 3 and EHD proteins. (Scale bars, 10 μm.)
Concluding Remarks.
Collectively, our results demonstrate a selective high-level expression of epsin 3 in gastric parietal cells and suggest a role of epsin in the specialized membrane recycling system of these cells. More specifically, they point to a role of epsin 3 in clathrin-mediated endocytosis at their apical canaliculi. They further suggest a functional link of epsin to the EHD proteins, whose properties makes them likely players in H/K ATPase recycling via tubular membrane intermediates. The intense exo-endocytic traffic in gastric parietal cells is expected to require cyclic conformational changes of membrane proteins. A special property of epsin, among the clathrin adaptors, is to bind ubiquitin. Thus, it will be of interest to determine whether functions of epsin 3 in gastric parietal cells include a role in a quality-control system aimed at removing misfolded and ubiquitinated proteins, similar to what has been recently proposed in another system (31).
Finally, beyond its cell biological significance, selective expression of epsin 3 in specialized, highly differentiated cells of the stomach may have implications for the classification and staging of gastric cancers. In preliminary results, we have found that the majority of gastric cancers express epsin 3 at high levels, but that the expression of this protein is on average lower in metastasis derived from the same tumor (Fig. S3 A and B). Furthermore, on average, epsin 3 mRNA is expressed at lower levels in advanced gastric cancer than in early gastric cancer (Fig. S3C). Thus, analysis of epsin 3 expression in gastric cancer may be of diagnostic value.
Materials and Methods
Epsin 3 Gene Targeting.
An epsin 3 conditional KO targeting vector was custom made by Gene Dynamics: all of the nine coding exons (≈8 kb) of the espin 3 gene (EPN3) were bracketed by two loxP sites, and an FRT site-flanked neomycin cassette to allow positive selection of ES cells was inserted after the last coding exon (Fig. S2). The vector was electroporated in Hybrid C57BL/6J-129S1/Sv mouse embryonic stem cells (Yale Cancer Center Animal Genomics Shared Resource); surviving and correctly recombined clones were identified by Southern blotting and injected into blastocysts of C57BL/6J mice. Resulting chimeric mice were mated to a Cre deleter mouse strain (32) to obtain an epsin 3 KO mouse.
Antibodies.
Epsin 3-specific mouse monoclonal and rabbit polyclonal antibodies were raised against the human epsin 3 sequence KQNGTKEPDALDLGILGEAL and the mouse epsin 3 sequence KQNGMKEPEALDLGVLGEAL, respectively (12). Sources of other antibodies are listed in the SI Materials and Methods.
Microscopy.
Mice were starved overnight and injected intraperitoneally with cimetidine (120 mg/kg) or histamine (20 mg/kg) 30 min before perfusion fixation with 4% formaldehyde in sodium phosphate buffer, pH 7.2. Immunofluorescence staining of thick (10 μm) and semithin (0.5 μm) frozen stomach sections and immunogold labeling of ultrathin frozen sections for electron microscopic analysis were carried out by standard procedures (SI Materials and Methods). Animal work was approved by the Yale University Yale Animal Care and Use Committee (YACUC). For the analysis of transfected fluorescent proteins, COS-7 cells were transfected using Lipofectamine 2000, fixed in 4% formaldehyde, and observed using a spinning disk confocal microscope, as described (33). The GFP-EHD1 and GFP-EHD2 plasmids were from our laboratory and a kind gift from H. McMahon (Laboratory of Molecular Biology, Cambridge, United Kingdom), respectively.
Biochemical Analyses.
SDS/PAGE and Western blotting were carried out by standard methods. GST pull-downs from stomach lysates were performed essentially as described (34) using GST fusions of the EH domain containing regions of human Eps15 (3), and of mouse EHD1 (aa 439–535) and mouse EHD2 (aa 444–544) as bait.
Supplementary Material
Acknowledgments
We thank Drs. Michael Caplan, James Goldenring, Tong Wang, Michelle Pirruccello, and Laura Swan for helpful advice and discussion, and Lijuan Liu for technical assistance; and the Molecular Pathology Facility of the Istituto Fondazione Italiana per la Ricerca sul Cancro (FIRC) di Oncologia Molecolare-Istituto Europeo di Oncologia (IFOM-IEO) Campus, Giovanni Mazzarol (IEO), and Caroline Zeiss (Yale Section of Comparative Medicine) for histopathological analyses. This work was supported in part by National Institutes of Health Grants DK45735 and NS36251 (to P.D.C.), the European Community (FP7), the European Research Council, the Ferrari and Monzino Foundations (to P.P.D.F.), Italian Ministry of Research Grant PRIN2008 and Telethon (to O.C.), American Heart Association Grant 0835544N (to H.C.), the Associazione Italiana per la Ricerca sul Cancro (to P.P.D.F. and O.C.), and Cariplo (to P.P.D.F., O.C., and M.V.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016390107/-/DCSupplemental.
References
- 1.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 2.Pearse BM, Robinson MS. Clathrin, adaptors, and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, et al. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 4.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wendland B. Epsins: Adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3:971–977. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 6.De Camilli P, et al. The ENTH domain. FEBS Lett. 2002;513(1):11–18. doi: 10.1016/s0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- 7.Ford MG, et al. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–366. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 8.Itoh T, et al. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 9.Polo S, et al. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 10.Rosenthal JA, et al. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci USA. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276:29257–29267. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 13.Karam SM, Tomasetto C, Rio MC. Trefoil factor 1 is required for the commitment programme of mouse oxyntic epithelial progenitors. Gut. 2004;53:1408–1415. doi: 10.1136/gut.2003.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forte JG, Zhu L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu Rev Physiol. 2010;72:273–296. doi: 10.1146/annurev-physiol-021909-135744. [DOI] [PubMed] [Google Scholar]
- 15.Sawaguchi A, Aoyama F, Ohashi M, Ide S, Suganuma T. Ultrastructural transformation of gastric parietal cells reverting from the active to the resting state of acid secretion revealed in isolated rat gastric mucosa model processed by high-pressure freezing. J Electron Microsc (Tokyo) 2006;55(2):97–105. doi: 10.1093/jmicro/dfl004. [DOI] [PubMed] [Google Scholar]
- 16.Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 1991;10:2363–2373. doi: 10.1002/j.1460-2075.1991.tb07775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14(4):167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Engqvist-Goldstein AE, et al. The actin-binding protein Hip1R associates with clathrin during early stages of endocytosis and promotes clathrin assembly in vitro. J Cell Biol. 2001;154:1209–1223. doi: 10.1083/jcb.200106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daumke O, et al. Architectural and mechanistic insights into an EHD ATPase involved in membrane remodelling. Nature. 2007;449:923–927. doi: 10.1038/nature06173. [DOI] [PubMed] [Google Scholar]
- 20.Grant B, et al. Evidence that RME-1, a conserved C. elegans EH-domain protein, functions in endocytic recycling. Nat Cell Biol. 2001;3:573–579. doi: 10.1038/35078549. [DOI] [PubMed] [Google Scholar]
- 21.Lin SX, Grant B, Hirsh D, Maxfield FR. Rme-1 regulates the distribution and function of the endocytic recycling compartment in mammalian cells. Nat Cell Biol. 2001;3:567–572. doi: 10.1038/35078543. [DOI] [PubMed] [Google Scholar]
- 22.Jain RN, et al. Hip1r is expressed in gastric parietal cells and is required for tubulovesicle formation and cell survival in mice. J Clin Invest. 2008;118:2459–2470. doi: 10.1172/JCI33569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brady RJ, Damer CK, Heuser JE, O'Halloran TJ. Regulation of Hip1r by epsin controls the temporal and spatial coupling of actin filaments to clathrin-coated pits. J Cell Sci. 2010;123:3652–3661. doi: 10.1242/jcs.066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lapierre LA, et al. Characterization of immunoisolated human gastric parietal cells tubulovesicles: Identification of regulators of apical recycling. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1249–G1262. doi: 10.1152/ajpgi.00505.2006. [DOI] [PubMed] [Google Scholar]
- 25.Grant BD, Caplan S. Mechanisms of EHD/RME-1 protein function in endocytic transport. Traffic. 2008;9:2043–2052. doi: 10.1111/j.1600-0854.2008.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caplan S, et al. A tubular EHD1-containing compartment involved in the recycling of major histocompatibility complex class I molecules to the plasma membrane. EMBO J. 2002;21:2557–2567. doi: 10.1093/emboj/21.11.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shao Y, et al. Pincher, a pinocytic chaperone for nerve growth factor/TrkA signaling endosomes. J Cell Biol. 2002;157:679–691. doi: 10.1083/jcb.200201063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito S, Schofield GC. Studies on the depletion and accumulation of microvilli and changes in the tubulovesicular compartment of mouse parietal cells in relation to gastric acid secretion. J Cell Biol. 1974;63:364–382. doi: 10.1083/jcb.63.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naslavsky N, Caplan S. C-terminal EH-domain-containing proteins: Consensus for a role in endocytic trafficking, EH? J Cell Sci. 2005;118:4093–4101. doi: 10.1242/jcs.02595. [DOI] [PubMed] [Google Scholar]
- 30.Salcini AE, Chen H, Iannolo G, De Camilli P, Di Fiore PP. Epidermal growth factor pathway substrate 15, Eps15. Int J Biochem Cell Biol. 1999;31:805–809. doi: 10.1016/s1357-2725(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 31.Okiyoneda T, et al. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewandoski M, Martin GR. Cre-mediated chromosome loss in mice. Nat Genet. 1997;17:223–225. doi: 10.1038/ng1097-223. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson SM, et al. Coordinated actions of actin and BAR proteins upstream of dynamin at endocytic clathrin-coated pits. Dev Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erdmann KS, et al. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.