Abstract
The porins OmpF and OmpC are trimeric β-barrel proteins with narrow channels running through each monomer that exclude molecules > 600 Da while mediating the passive diffusion of small nutrients and metabolites across the Gram-negative outer membrane (OM). Here, we elucidate the mechanism by which an entire soluble protein domain (> 6 kDa) is delivered through the lumen of such porins. Following high-affinity binding to the vitamin B12 receptor in Escherichia coli, the bacteriocin ColE9 recruits OmpF or OmpC using an 83-residue intrinsically unstructured translocation domain (IUTD) to deliver a 16-residue TolB-binding epitope (TBE) in the center of the IUTD to the periplasm where it triggers toxin entry. We demonstrate that the IUTD houses two OmpF-binding sites, OBS1 (residues 2–18) and OBS2 (residues 54–63), which flank the TBE and bind with Kds of 2 and 24 μM, respectively, at pH 6.5 and 25 ºC. We show the two OBSs share the same binding site on OmpF and that the colicin must house at least one of them for antibiotic activity. Finally, we report the structure of the OmpF-OBS1 complex that shows the colicin bound within the porin lumen spanning the membrane bilayer. Our study explains how colicins exploit porins to deliver epitope signals to the bacterial periplasm and, more broadly, how the inherent flexibility and narrow cross-sectional area of an IUP domain can endow it with the ability to traverse a biological membrane via the constricted lumen of a β-barrel membrane protein.
Keywords: colicin translocation, intrinsically unstructured protein, protein–protein interaction, transmembrane signalling, isothermal titration calorimetry
Ligand-mediated signalling across a biological membrane typically occurs when the ligand binds a specific membrane receptor causing it to change conformation or oligomeric status (1). Both outcomes communicate to the cell that a receptor-binding event has taken place on the other side of the membrane. The present work describes an alternative transmembrane signalling mechanism in which the signal itself, in the form of an intrinsically unstructured polypeptide epitope, is transferred through a porin in order to meet its cellular target.
Intrinsically unstructured proteins (IUPs) are found in all kingdoms of life (2) but are particularly abundant in eukaryotes, where they are tightly regulated, subject to a wide variety of posttranslational modifications, and key to processes as diverse as cell cycle progression, endocytosis, intracellular signalling, and transcription activation (3–6). Although less abundant in prokaryotes, IUPs are nonetheless important in their lifestyles (2, 7). An example of this is the role IUPs play in the import of bacteriocins in Gram-negative bacteria (8). Bacteriocins are protein antibiotics that translocate into cells following receptor binding at the outer membrane (OM) (9–11). They are particularly abundant in the enterobacteriaciae, where they are the agents of competition between populations in structured environments such as the mammalian colon (12). Bacteriocins typically have a narrow killing spectrum dictated by their associations with particular OM and periplasmic proteins; hence, colicins are specific for Escherichia coli, pyocins target Pseudomonas aeruginosa, and pesticins kill Yersinia pestis. A single bacteriocin molecule is capable of killing a bacterial cell so understanding their modes of entry may uncover novel strategies for the creation of species-specific antibiotics as well as providing insight into mechanisms of intracellular targeting in microbes.
Here we focus on the import mechanism of enzymatic E colicins (ColE2-E9) that are dependent on an IUTD for transport into E. coli. Enzymatic E colicins are cosynthesized with a high-affinity immunity protein that binds and inactivates the nuclease in the producing host (13). The immunity protein is lost during colicin translocation to the cytoplasm (14), with cell death resulting through one of three nuclease activities (8). ColE3, ColE4, and ColE6 are rRNases that inhibit protein synthesis through site-specific cleavage of a single phosphodiester bond within the A-site of 30S ribosomal RNA (15, 16). ColE5 is a tRNase that blocks protein synthesis through the cleavage of the anticodon loops of a subset of tRNAs (17). ColE2 and ColE7-E9 are metal-dependent, nonspecific enzymes that degrade the bacterial genome (18). While the C-terminal nuclease domains of these approximately 60-kDa toxins are unrelated to each other their mechanism of import is the same, dictated by conserved domains involved in receptor binding and membrane translocation. Firstly, a central coiled-coil receptor-binding (R-) domain binds the vitamin B12 receptor BtuB with high affinity (Kd ∼ 1–2 nM), which localizes the colicin to the cell surface (9, 19). From here the colicin recruits a porin via its 83-residue IUTD (19), which is part of a larger 37 kDa translocation (T-) domain.
Colicin delivery and hence cytotoxicity is dependent upon interactions with components of the Tol (group A colicins) or Ton (group B colicins) systems in the bacterial inner membrane and periplasm. The Tol system is a five-protein assembly comprising TolA, TolB, TolQ, TolR, and Pal. These proteins are ubiquitous in Gram-negative bacteria and are required for virulence by some pathogens (20). The Tol assembly is recruited to the septation site during cell division, where it is involved in stabilizing the OM (8). The Ton system composed of TonB, ExbB, and ExbD plays an essential role in the uptake of scarce nutrients across the OM (21). Both Tol and Ton systems are coupled to the proton-motive force of the inner membrane.
How colicin IUTDs translocate to the bacterial periplasm to bind their specific targets remains an unresolved problem in colicin biology. In the case of ColIa, a group B colicin that binds TonB, recent evidence points to the IUTD recruiting another copy of its primary receptor Cir, an iron-siderophore transporter (22). Although the mechanism by which the ColIa IUTD penetrates the periplasm is not known, it is thought the IUTD may mimic a siderophore ligand in order to trigger translocation through the receptor in a TonB-dependent step (22, 23). Group A colicins tend to use porins such as OmpF or OmpC to reach Tol proteins in the periplasm. Lakey and coworkers have argued, based on biochemical and EM studies, that the IUTD of ColN, which uses OmpF both as a receptor and translocator, traverses down the sides of the porin at the interface with the LPS (24). In the case of the ColE3 IUTD, which is essentially identical to that of ColE9 in the present work, Cramer and coworkers have argued for a lumenal translocation route through OmpF based on the occlusion of OmpF voltage-gated channels by the IUTD in planar lipid bilayer experiments and the presence of as yet unassigned electron density in crystals of the IUTD in complex with OmpF (25, 26).
Following OmpF-mediated translocation of the IUTD, enzymatic colicins such as ColE3 and ColE9 bind the periplasmic protein TolB through a 16-residue TBE within the IUTD (residues 32–47) (27). Binding of the colicin TBE to TolB promotes interaction between TolB and the inner membrane protein TolA, which through its association with TolQ and TolR is coupled to the proton-motive force. Colicin-directed contact between TolB and TolA promotes release of the high-affinity immunity protein at the cell surface in a pmf-dependent step that initiates translocation of ColE9 across the OM (14, 28).
The question we address in the present paper is how OmpF is bound by the IUTD and how this establishes the translocation route of the TBE to the periplasm. Using biochemical, biophysical and in vivo experiments we demonstrate that recruitment of OmpF by the ColE9 IUTD occurs through tandem binding sites that flank the TBE. We also report the crystal structure of E. coli OmpF in complex with one of these sites revealing the route of passage of the ColE9 IUTD across the OM. Cumulatively, our results suggest the colicin’s TBE is translocated and displayed in the periplasm by a sequential OmpF-binding mechanism and introduces a new mode of transmembrane signalling in which a peptide signal is posited directly through a membrane-embedded protein pore.
Results
The ColE9 Principal OmpF-Binding Site Spans Residues 2–18 of the IUTD.
Recruitment of OmpF to the BtuB-bound ColE9 complex in vivo has previously been shown to require the colicin IUTD, with the main interaction site localized to the N-terminal half of the disordered domain (19). Preliminary size-exclusion chromatography experiments (see SI Text) indicated that a binary ColE9–OmpF complex could be formed in vitro without prior binding of colicin to BtuB and that binding was mediated entirely by the colicin T-domain (Fig. S1 A and B). Having verified that a binary complex could be formed in vitro we identified the principal OmpF-binding site for ColE9 using fusion proteins in combination with deletion analysis, detecting binding through isothermal titration calorimetry (ITC) (see Fig. S2 and Table S1 for details). We narrowed down the main OmpF-binding site to a 17-residue epitope from the N terminus of the ColE9 IUTD (residues 2–18), for which a peptide encompassing this sequence bound OmpF with a Kd of approximately 2 μM and a stoichiometry of one colicin peptide/OmpF monomer (Fig. 1A). Methionine 1 of the colicin is often missing and so was omitted from the final peptide sequence. We denote this IUTD binding epitope as OmpF-Binding Site 1 (OBS1). The interaction of the OBS1 peptide with OmpF is characterized by a large and favorable enthalpy (ΔH = -19.1 kcal/mol) and an unfavorable entropy change (ΔS = -37.8 cal/mol/K), consistent with the disordered peptide becoming ordered upon complex formation. We also found that LPS had no effect on OBS1 binding (Table S1), suggesting the epitope makes little or no contact with regions of OmpF involved in binding LPS.
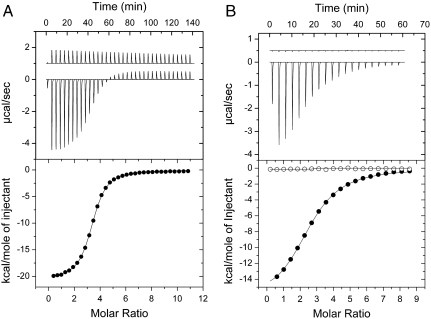
Fig. 1.
Delineation of the two OmpF-binding sites in the ColE9 IUTD. Figure shows ITC data for ColE9 IUTD peptide sequences binding OmpF in 20 mM potassium phosphate buffer pH 6.5 containing 1 % (w/v) OG at 25 ºC. (A) Titration of 1060 μM OBS1 peptide ( ) into 19 μM OmpF trimer measured on a VP-ITC. Data were corrected for heats of dilution obtained by titration of OBS1 into buffer (control data offset by +1 μcal/ sec in top panel). Fitted parameters for a single site binding model from three independent titrations were: ΔH, -19.1 ± 2.5 kcal/mol; ΔS, -37.8 ± 8.2 cal/mol/K; Kd, 1.8 ± 0.1 μM and N, 3.2 ± 0.1/OmpF trimer. (B) Titration of 2 mM OBS2 peptide (
) into 19 μM OmpF trimer measured on a VP-ITC. Data were corrected for heats of dilution obtained by titration of OBS1 into buffer (control data offset by +1 μcal/ sec in top panel). Fitted parameters for a single site binding model from three independent titrations were: ΔH, -19.1 ± 2.5 kcal/mol; ΔS, -37.8 ± 8.2 cal/mol/K; Kd, 1.8 ± 0.1 μM and N, 3.2 ± 0.1/OmpF trimer. (B) Titration of 2 mM OBS2 peptide (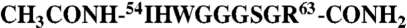 ) into 50 μM OmpF trimer (closed circles), measured on an iTC200. Fitted parameters for a single site binding model from duplicate independent titrations were: ΔH = -16.8 ± 0.2 kcal/mol; ΔS = -35.2 ± 0.5 cal/mol/K; Kd = 23.9 ± 1.6 μM and N = 2.60 ± 0.01/OmpF trimer. Data for the titration of 2 mM OBS2 peptide into 50 μM OmpF/1 mM OBS1 complex are shown as open circles with the raw data offset by +0.5 μcal/ sec in the top panel. We note that for both OBS1 and OBS2 in these experiments three molecules bind per OmpF trimer reflecting independent binding of the peptides to each subunit. Thermodynamic parameters for all T-domain constructs used to identify OBS1 and OBS2 are shown in Table S1.
) into 50 μM OmpF trimer (closed circles), measured on an iTC200. Fitted parameters for a single site binding model from duplicate independent titrations were: ΔH = -16.8 ± 0.2 kcal/mol; ΔS = -35.2 ± 0.5 cal/mol/K; Kd = 23.9 ± 1.6 μM and N = 2.60 ± 0.01/OmpF trimer. Data for the titration of 2 mM OBS2 peptide into 50 μM OmpF/1 mM OBS1 complex are shown as open circles with the raw data offset by +0.5 μcal/ sec in the top panel. We note that for both OBS1 and OBS2 in these experiments three molecules bind per OmpF trimer reflecting independent binding of the peptides to each subunit. Thermodynamic parameters for all T-domain constructs used to identify OBS1 and OBS2 are shown in Table S1.
Deconstructing the Role of OBS1 Residues in OmpF-Binding and Colicin Translocation.
The OBS1 sequence has eleven side-chain-bearing amino acids and six glycines, the latter interspersed throughout the epitope. An N-terminal GST fusion to the ColE9 IUTD completely abolished binding to OmpF in ITC experiments, whereas C-terminal fusions had no effect (Fig. S2). Cramer and colleagues have shown previously that the ability of ColE3 to occlude OmpF voltage-gated channels is dependent on a free N terminus (29). Together these data suggest that the N terminus of an enzymatic colicin IUTD is important for binding OmpF either directly or indirectly. With the exception of Ala13, we next mutated each of the side-chain-bearing amino acids to alanine and assayed their effects in in vivo cell-killing assays (using intact ColE9 mutants; Fig. S3) and in binding OmpF in vitro by ITC (using ColE9 IUTD fusions; Fig. S4). While many of the alanine mutations bound substantially weaker (D5A, R7A, H9A, T11A, and H14A all had ΔΔGs > 1 kcal/mol; Fig. S4D and Table S2) none abolished OmpF binding. Consistent with these residues stabilizing the complex with OmpF, Yamashita et al. have reported that a ColE3 D5A/R7A double mutant abolishes the ability of the colicin to occlude OmpF channels (30).
None of the ColE9 OBS1 alanine mutants had a discernible phenotype in cell-killing assays (Fig. S3), implying the assay is not sensitive to the weakening of OmpF binding. This was borne out by the effects of more drastic mutations. When Gly6 was substituted for proline, for example, all OmpF binding in ITC experiments was lost (Fig. S2) and the mutant exhibited cell-killing kinetics typical of an OBS1 deletion (Fig. S3 and Fig. 2, respectively). In contrast, when Gly6 was substituted for alanine, binding was reduced 50-fold (Fig. S4D), and cell killing was wild type. The Gly-to-Pro effect, presumably due to kinking of the OBS1 polypeptide chain, prompted an investigation of the other glycines. We found that substitution of any of the glycines for proline resulted in ColE9 toxicity equivalent to that of an OBS1 deletion (Fig. S3) showing that proline is not tolerated anywhere along OBS1. Prolines are, however, found within other regions of the ColE9 IUTD that translocate to the periplasm, a point we return to in Discussion. In summary, while multiple OBS1 side chains are undoubtedly involved in stabilizing the complex with OmpF, the most important factors in colicin translocation are the flexibility of the polypeptide backbone and the unconstrained nature of the N terminus.
Fig. 2.
The involvement of OBS1 and OBS2 in ColE9 cytotoxicity in the context of mixed porin, OmpC-specific, and OmpF-specific membrane environments. The figure shows ColE9 induced cell death of 50 ml shake flask cultures grown at 37 ºC upon addition of wild-type (ColE9·Im9, ○), Δ1-30 (▾), I54G H55G W56S R62G (△), or Δ1-30 I54G H55G W56S R62G ColE9·Im9 (▪) to final concentrations of 80 nM (time of colicin addition indicated by arrow), compared to a no colicin control (•). Growth of cultures was monitored at 30 min intervals through measurement of OD600 nm. (A) JM83 (ompF+, ompC+), (B) JW0912 (ompF−, ompC+), and (C) JW2203 (ompF+, ompC−) E. coli cultures.
A Second Porin Binding Site within the IUTD Binds OmpF with Lower Affinity.
Truncation of the ColE9 N terminus up to residue 53 has previously been shown to markedly reduce the ability of the colicin to recruit OmpF at the E. coli cell surface but not abolish it (19). Recruitment of OmpF is only fully lost when more than 53 amino acids are deleted from the IUTD. Moreover, work of Sharma et al. (31) on ColE3 showed that deletions downstream of residue 65 (Δ65-73 ColE3 and Δ72-80 ColE3) retained wild-type colicin activity. Taken together, these experiments suggested the presence of a second OmpF-binding site between residues 53 and 64. Consistent with this hypothesis, ITC measurements on a fusion construct containing ColE9 residues 53–83 at the N terminus revealed weak binding to OmpF (Kd ∼ 130 μM), which was lost when residues 53–64 were deleted (Fig. S2). Delineation of OBS2 was confirmed through ITC experiments using a synthetic peptide of residues 54 to 63 of the ColE9 IUTD binding to OmpF (Fig. 1B). Although OBS2 binds 10-fold weaker to OmpF than OBS1 (Kd = 24 μM) the thermodynamic parameters of complex formation are similar to those of OBS1 (Table S1). Importantly, when OmpF was incubated with an excess of the OBS1 peptide, OBS2 was no longer able to bind OmpF, suggesting the two ColE9 IUTD OmpF-binding sites associate with the same region on the porin (Fig. 1B).
The Presence of at Least One OBS Is Vital for Cytotoxicity.
Enzymatic group A colicins such as ColE3 and ColE9 show no cytotoxic activity against porin deficient cells (32). Hence, we next sought to test the importance of OBS1 and OBS2 in colicin-mediated killing of E. coli JM83 cells using liquid growth assays and a combination of multiple residue mutations and deletion analysis. Interestingly, we found that mutations had subtly different effects to deletions at each of the porin binding sites (Fig. S5). Mutating the majority of the OBS1 side chains in a single construct (ColE9 D5S R7G H9S N10G T11G H14G) yielded less efficient cell killing than its deletion (ColE9 Δ1-30), suggesting mutation of this region affects translocation as well as diminishing binding. Conversely, deletion of residues within OBS2 (ColE9 Δ54-62) had a more profound effect than their combined mutation (ColE9 I54G H55G W56S R62G), highlighting the importance of polypeptide length in this region of the IUTD, in agreement with previous observations (31). Despite a 13-fold difference in OmpF-binding affinities, the effect on colicin-mediated cell killing of losing OBS1 by deletion or OBS2 through mutation is comparable (Fig. 2A). Only upon combining deletion of OBS1 and mutation of OBS2 (ColE9 Δ1-30 I54G H55G W56S R62G) is colicin cytotoxicity against JM83 cells abolished (Fig. 2A). These data show that while neither OmpF-binding site is essential for ColE9 activity, the toxin must house at least one of them to kill E. coli cells.
The OM of JM83 cells contains OmpF and OmpC the proportions of which vary with growth conditions (33), with both porins able to mediate colicin toxicity (32). To assess the impact of OBS1 and OBS2 on OmpF- and OmpC-specific colicin translocation, killing assays were performed using JW0912 (Fig. 2B) and JW2203 (Fig. 2C) E. coli, which are ompF- and ompC-, respectively (34). As observed previously (32), OmpF is more efficient than OmpC in enzymatic colicin translocation, with a shorter delay before the onset of cytotoxicity. The loss of either porin binding site is readily tolerated in the killing of JW2203 cells where translocation occurs via OmpF, with ColE9 Δ1-30 and ColE9 I54G H55G W56S R62G showing near wild-type toxicity. Moreover, a very low residual level of killing is seen with these cells when both OBSs are removed (ColE9 Δ1-30 I54G H55G W56S R62G), indicating that even in the absence of any OmpF-binding sites OmpF can still facilitate translocation of the IUTD across the OM, albeit very poorly. Conversely, the role of each OBS is amplified in JW0912 cells where translocation occurs via OmpC. The loss of either site significantly impairs colicin toxicity, with the combined loss of both sites rendering the colicin inactive. These data highlight that OmpC and OmpF do not behave identically in nuclease colicin-mediated cell killing, their differences likely reflecting subtle differences in the structures of the porins (such as pore size and the sequence and flexibility of external loops) and hence the kinetics of porin recruitment by the colicin IUTD. We conclude that the ColE9 OBSs are required to expedite passage of the IUTD across the OM via a porin in order to present the TBE correctly in the periplasm.
The OBS1 Peptide Spans the Aqueous Channel Running Through OmpF.
The previously reported X-ray diffraction data for crystals of the OmpF-ColE3 IUTD complex (26) revealed some electron density within the OmpF lumen, estimated as approximately seven residues of the colicin, although their identity was not established. The present work shows the colicin IUTD has two distinct OmpF-binding sites that flank the TBE and likely share the same binding site on the porin (Fig. 1B). In order to define this common binding site we crystallized OmpF in the presence of the higher affinity OBS1 peptide, the resulting crystals diffracting to 3.0-Å resolution. The monoclinic asymmetric unit contained two OmpF trimers and the electron density maps revealed electron density in the lumen of all six OmpF molecules, indicating the presence of the OBS1 peptide. Fifteen (pdb chain L) or eleven (pdb chains M–Q) residues were built into this electron density (Fig. 3A), and the whole structure refined at 3.0 Å to final R value of 20.8% (Rfree = 26.0%). Although the orientation of the OBS1 peptide cannot be unambiguously assigned, it has been modeled with its N terminus facing the periplasm. This conformation was based on the positions of several bulky side chains (Ser2, Arg7 and Asn10) that are clearly visible in the electron density (Fig. 3 B and C and Fig. S6). The OBS1 peptide adopts a crescent-shaped, extended conformation that spans the aqueous channel running through OmpF, from its extracellular surface through the constriction zone at its center all the way to the periplasm (Fig. 3D). The location of OBS1 within the lumen of OmpF, where it causes no conformational changes to the porin, is consistent with LPS having no effect on its binding because the colicin only makes contact with the aqueous pore. Superposition of our structure with the model of Yamashita et al. for the OmpF-ColE3 IUTD complex (26) shows that residues 7 to 13 of OBS1 occupy the same position as the density observed in this complex (Fig. 3D). Because OBS1 blocks OBS2 binding to OmpF (Fig. 1B) we conclude that OBS2 must also bind in the lumen of the porin.
Fig. 3.
Structure of the OmpF·ColE9 OBS1 peptide complex. (A) OmpF monomer shown in green ribbon with OBS1 peptide (chain L; residues 2-16) modeled into the electron density map (2Fo - Fc shown at 1σ sigma cutoff) within the lumen. (B and C) Hydrogen bond interactions between OBS1 peptide (chain L, red) and OmpF monomer (chain A, green) (dashed lines); electron density map (2Fo - Fc) is shown at 1σ sigma cutoff, length of hydrogen bonds is shown in Å. (D) Superposition of colicin peptide structures from the complex of ColE3 IUTD (26) and the present structure of ColE9 OBS1 bound to OmpF, shown as a molecular surface cutaway to reveal the lumen of the porin. OBS1 peptide is shown in red and fragment from the ColE3 IUTD in yellow. Figures were prepared with the program ccp4mg (48).
Discussion
Sequential OmpF Binding by OBS1 and OBS2 Ensures Anchored Deployment of the Colicin TBE in the Periplasm Following Receptor Binding.
Enzymatic E colicins become localized to the surface of E. coli following high-affinity binding of the long (∼100 Å) coiled-coil R-domain to the vitamin B12 receptor BtuB, present at approximately 100–200 copies per cell in the OM. The unusual orientation of the colicin, which sits at an angle 45° with respect to the membrane (9), projects the N-terminal T-domain above the membrane and away from its initial docking site. From here, the conformational space accessible to the colicin is considerable because the 83-residue IUTD can access a radius of > 300 Å2 in its random two-dimensional search for a porin. It is estimated that OmpF/OmpC are present at approximately 105 copies/cell; consequently, a BtuB-bound colicin will invariably find itself close to a translocator OmpF or OmpC with which its IUTD can make contact. The present work explains how the IUTD binds such porins and suggests a mechanism by which the TBE epitope could be delivered through the porin to the periplasm.
Recruitment of OmpF by the ColE9 IUTD is through two linear epitopes, OBS1 and OBS2, which are separated by 35 amino acids and flank the intervening TBE. ITC data indicate the two OBSs bind to the same site on OmpF, with OBS1 having an approximately 10-fold higher affinity than OBS2 (Table S1). A third of OBS1 residues and a half in OBS2 are glycine, which emphasizes the intrinsically disordered nature of these epitopes. While the two epitopes do not share significant sequence identity they both contain a GGxGRG sequence motif, at the N terminus of OBS1 and C terminus of OBS2, which does not appear anywhere else in the ColE9 IUTD. The OmpF-OBS1 crystal structure indicates that this epitope is located toward the periplasmic surface of the porin. The only other common feature of the two binding sites is their overall basic nature, distinguishing them from the TBE in the center of the IUTD, which is negatively charged and does not bind to OmpF in ITC experiments (Table S1). The positively charged nature of the OBSs is likely to be important for their binding to the OmpF pore, which is mildly cation-selective and implicated previously in the import of cationic antimicrobial peptides into the bacterial periplasm (35). While the low resolution nature of the OmpF·OBS1 peptide crystal structure and the absence of several OBS1 side chains precludes detailed analysis, the side chains of Arg7 and Asn10 of OBS1 are within hydrogen bonding distance of Asp107 and Arg82 of OmpF, respectively (Fig. 3 B and C), in addition to hydrogen bonding between OmpF and the OBS1 backbone. Mutation of Asn10 to Ala had little impact on OmpF binding, whereas the R7A mutation weakened binding 10-fold. Other OBS1 residues not visible in the structure had even greater impact on binding (Asp5, His9, His14) although none was essential.
Our data are consistent with the ColE9 IUTD translocating through the OmpF pore by sequential binding of the two OBSs (Fig. 4). We suggest the following sequence of events after ColE9 binds BtuB. First, OBS1 engages OmpF (or OmpC) through an electrostatically driven interaction. OBS1 is the most likely initial docking site because it carries greater positive charge (+2) than OBS2 (+1) and binds with a higher affinity (Fig. 4A). This would also be consistent with our experiments indicating the importance of the colicin having a free N terminus, allowing the epitope to enter N terminus first and form a weak complex with the OmpF lumen. Second, following unbinding of peptide residues from the walls of the lumen and aided by the inherent flexibility of the IUTD, OBS1 is directed to the periplasm by the overhanging T-domain, kept in place above OmpF by the colicin’s preformed complex with BtuB. Third, the next 30 residues containing the negatively charged TBE and two proline residues (Pro24, Pro45) pass through the porin without interaction (Fig. 4B). Fourth, OBS2 docks in the lumen anchoring the TBE on the underside of the OM (Fig. 4C) allowing the colicin to capture TolB. Binding of the ColE9 TBE promotes contact between TolB and TolA in the inner membrane, which is the initiating, pmf-dependent step for cellular import.
Fig. 4.
Schematic model of the sequential binding mechanism that underpins directed epitope delivery across the OM by the ColE9 IUTD. OmpF is shown in cyan, OBS1 in red, TBE in green, OBS2 in orange, and TolB in purple. The two OBSs are positively charged, whereas the TBE is negatively charged. * denotes positions of prolines in the IUTD. (A) Positively charged OBS1 is guided initially into the lumen of OmpF through an electrostatically driven association after docking of the colicin’s R-domain to its primary receptor, BtuB. (B) Following unbinding of OBS1 residues from the porin lumen (where breaking many of the side-chain-bearing amino acid interactions weakens binding by 10-100-fold; Fig. S4D), diffusion of the epitope is directed through the pore by virtue of the extracellular T-domain, held in place above OmpF by the BtuB-bound colicin. Sequences containing proline and the negatively charged TBE are unable to bind in the pore and so translocate directly to the periplasm. (C) OBS2 passes into the pore and binds, anchoring the IUTD in the OM displaying the TBE sequence in the periplasm. Binding of TolB to the ColE9 TBE initiates entry of the colicin into the cell.
Lumenal Translocation of an IUP—a Novel Mechanism for Transmembrane Signalling.
IUP associations with soluble proteins, nucleic acids, and membranes are well documented (36–38). There are few reports, however, of IUPs recruiting membrane proteins directly (39) and none that define the mechanism by which such binding occurs. As well as Gram-negative bacteria, porins are abundant in eukaryotes where they mediate metabolite diffusion across the mitochondrial OM. They also play a central role in signalling apoptosis to cells. We speculate that the deployment of IUP epitopes through porins in order to engage in transmembrane signalling could therefore be relevant in the biology of eukaryotic porins.
A number of reports have highlighted how the involvement of IUPs in protein–protein interactions brings distinct advantages to biological systems (3, 40–42), such as the capacity to array multiple epitopes in relatively small proteins, the formation of complexes of low affinity but high specificity, binding through “fly casting,” and the moulding of the same IUP sequence to different binding partners. We can now add a further advantage IUPs have over their globular counterparts that centers on their flexibility and lack of structure, characteristics that allow them to traverse a membrane through the narrow channels of a protein pore in order to deliver a signal directly into a cell. The key to this transfer is the intrinsically unstructured nature of an IUP and its narrow cross-sectional area, properties that allow a macromolecule as big as 6,000 Da to pass through a protein pore that has a molecular weight cutoff filter of 600 Da.
Materials and Methods
Protein Purification.
The construction of plasmids for the expression of mutated and truncated ColE9 and fusion proteins and the purification of OmpF from E. coli BE3000 cells and ColE9 constructs from pET21a BL21 (DE3) cultures were all carried out as detailed in SI Text.
Synthetic peptides were purchased from Activotec Ltd. with purities in excess of 90% as determined through HPLC analysis and their predicted molecular weights confirmed by electrospray mass spectrometry.
Protein concentrations were determined through A280 nm measurements using theoretical molar extinction coefficients calculated from amino acid sequence for all colicin constructs and an extinction coefficient of 177742 M-1·cm-1 derived through amino acid analysis (Alta Bioscience) for the OmpF trimer. Concentrations of the OBS1 peptide (NH2)-SGGDGRGHNTGAHSTSG-(CONH2), devoid of aromatic amino acids were determined using the Fluoraldehyde assay (PIERCE) calibrated by amino acid analysis. Concentrations of the OBS2 peptide (CH3CONH)-IHWGGGSGRG-(CONH2) were determined through A280 nm using an extinction coefficient of 5,500 M-1·cm-1.
Isothermal Titration Calorimetry (ITC).
ITC measurements were performed using either a MicroCal VP-ITC or a MicroCal ITC200 thermostated at 25 °C, with all protein samples prepared in 20 mM potassium phosphate buffer pH 6.5 in the presence of 1% (w/v) OG. OmpF was present in the sample cell at a concentration of 18–50 μM with the ligand concentration in the syringe varying from 600 to 2,500 μM depending on the affinity of the binding interaction. Binding isotherms were analyzed using the manufacturer’s software.
In Vivo Cytotoxicity Assay.
The ability of ColE9 mutants to translocate into and kill E. coli cells was assessed through liquid growth cell-killing assays. Typically, 50 ml LB-Amp shake flask cultures were inoculated 1∶100 with JM83 pTrc99a overnight cultures (the pTrc99a plasmid included merely for antibiotic resistance). Cultures were grown at 37 °C with shaking, monitoring growth through OD600 nm measurements at 30 min intervals. ColE9 was added 2 h after inoculation to a final concentration of 80 nM, and growth was monitored for a further 3 h. Cell killing mediated specifically though either OmpC or OmpF was investigated using JW0912 and JW2203 cultures, devoid of OmpF and OmpC, respectively.
Crystal Structure Determination.
OmpF in 20 mM MES pH 6.5, 1% (w/v) OG at 90 μM was mixed with excess of OBS1 (1350 μM). Crystallization trials were performed using sitting drop vapor diffusion method. Crystals were obtained from 25% (w/v) PEG 3350, 0.2 M Li2SO4, 0.1 M NaCacodylate 6.5, 0.2% (v/v) BDTM PuraMatrix (BD Bioscience). OmpF·OBS1 complex crystals belonged to space group P21 with unit cell dimensions a = 101.49 Å, b = 101.62 Å, and c = 162.14 Å. A single crystal was transferred into Paratone-N (Hampton Research) and flash-cooled in liquid nitrogen. The single wavelength X-ray diffraction data was collected from a single crystal at 100 K on the Diamond beamline i04 using ADSC Q315 CCD detector. Data were measured with crystal-to-detector distance of 434 mm, using an oscillation range of 0.5°. Two hundred forty seven images were collected to a maximum resolution of 3.0 Å. Recorded data were indexed and scaled using HKL2000 (43). The structure was determined by molecular replacement using the program MolRep (44). The solution contained six molecules in the asymmetric unit. Refinement was carried out using the program REFMAC5 (45) using local NCS and map sharpening options. The structure was visualized and manually modified using program Coot (46). The stereochemistry of the model was evaluated with the program MolProbity (47). Data collection and refinement statistics are shown in Table S2.
Supplementary Material
Acknowledgments.
We are indebted to Garib Murshudov (York Structural Biology Laboratory) for help with refinement of the OmpF·OBS1 complex structure. We are also grateful to Kayleigh Gilmore and Matthew Wiseman (York) for early contributions on T-domain fusions and our collaborators Richard James (Nottingham) and Geoff Moore (Norwich) for helpful discussions. We thank the Kleanthous lab for helpful comments on the manuscript. This work was funded by the Wellcome Trust and the Biotechnology and Biological Sciences Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structural amplitudes have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3O0E).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010780107/-/DCSupplemental.
References
- 1.Groves JT, Kuriyan J. Molecular mechanisms in signal transduction at the membrane. Nat Struct Mol Biol. 2010;17:659–665. doi: 10.1038/nsmb.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol. 2004;337:635–645. doi: 10.1016/j.jmb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 4.Galea CA, et al. Role of intrinsic flexibility in signal transduction mediated by the cell cycle regulator, p27 Kip1. J Mol Biol. 2008;376:827–838. doi: 10.1016/j.jmb.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr Opin Struct Biol. 2008;18:756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Gsponer J, Futschik ME, Teichmann SA, Babu MM. Tight regulation of unstructured proteins: From transcript synthesis to protein degradation. Science. 2008;322:1365–1368. doi: 10.1126/science.1163581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz-Linek U, Hook M, Potts JR. The molecular basis of fibronectin-mediated bacterial adherence to host cells. Mol Microbiol. 2004;52:631–641. doi: 10.1111/j.1365-2958.2004.04027.x. [DOI] [PubMed] [Google Scholar]
- 8.Cascales E, et al. Colicin biology. Microbiol Mol Biol R. 2007;71:158–229. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurisu G, et al. The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat Struct Biol. 2003;10:948–954. doi: 10.1038/nsb997. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan SK, et al. Structure of colicin I receptor bound to the R-domain of colicin Ia: Implications for protein import. EMBO J. 2007;26:2594–2604. doi: 10.1038/sj.emboj.7601693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma O, et al. Structure of the complex of the colicin E2 R-domain and its BtuB receptor. The outer membrane colicin translocon. J Biol Chem. 2007;282:23163–23170. doi: 10.1074/jbc.M703004200. [DOI] [PubMed] [Google Scholar]
- 12.Kirkup BC, Riley MA. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature. 2004;428:412–414. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- 13.Kleanthous C, Walker D. Immunity proteins: Enzyme inhibitors that avoid the active site. Trends Biochem Sci. 2001;26:624–631. doi: 10.1016/s0968-0004(01)01941-7. [DOI] [PubMed] [Google Scholar]
- 14.Vankemmelbeke M, et al. Energy-dependent immunity protein release during tol-dependent nuclease colicin translocation. J Biol Chem. 2009;284:18932–18941. doi: 10.1074/jbc.M806149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boon T. Inactivation of ribosomes in vitro by colicin E 3. Proc Natl Acad Sci USA. 1971;68:2421–2425. doi: 10.1073/pnas.68.10.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng L, et al. Structural basis for ribosomal 16S rRNA cleavage by the cytotoxic domain of colicin E3. Nat Struct Mol Biol. 2010 doi: 10.1038/nsmb.1896. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa T, et al. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science. 1999;283:2097–2100. doi: 10.1126/science.283.5410.2097. [DOI] [PubMed] [Google Scholar]
- 18.Kleanthous C, et al. Structural and mechanistic basis of immunity toward endonuclease colicins. Nat Struct Biol. 1999;6:243–252. doi: 10.1038/6683. [DOI] [PubMed] [Google Scholar]
- 19.Housden NG, Loftus SR, Moore GR, James R, Kleanthous C. Cell entry mechanism of enzymatic bacterial colicins: Porin recruitment and the thermodynamics of receptor binding. Proc Natl Acad Sci USA. 2005;102:13849–13854. doi: 10.1073/pnas.0503567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubuisson JF, Vianney A, Hugouvieux-Cotte-Pattat N, Lazzaroni JC. Tol-Pal proteins are critical cell envelope components of Erwinia chrysanthemi affecting cell morphology and virulence. Microbiology. 2005;151:3337–3347. doi: 10.1099/mic.0.28237-0. [DOI] [PubMed] [Google Scholar]
- 21.Wiener MC. TonB-dependent outer membrane transport: Going for Baroque? Curr Opin Struct Biol. 2005;15:394–400. doi: 10.1016/j.sbi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Jakes KS, Finkelstein A. The colicin Ia receptor, Cir, is also the translocator for colicin Ia. Mol Microbiol. 2010;75:567–578. doi: 10.1111/j.1365-2958.2009.06966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kleanthous C. Translocator hunt comes full Cir-Col. Mol Microbiol. 2010;75:529–533. doi: 10.1111/j.1365-2958.2009.06967.x. [DOI] [PubMed] [Google Scholar]
- 24.Baboolal TG, et al. Colicin N binds to the periphery of its receptor and translocator, outer membrane protein F. Structure. 2008;16:371–379. doi: 10.1016/j.str.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zakharov SD, et al. Colicin occlusion of OmpF and TolC channels: outer membrane translocons for colicin import. Biophys J. 2004;87:3901–3911. doi: 10.1529/biophysj.104.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashita E, Zhalnina MV, Zakharov SD, Sharma O, Cramer WA. Crystal structures of the OmpF porin: Function in a colicin translocon. EMBO J. 2008;27:2171–2180. doi: 10.1038/emboj.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loftus SR, et al. Competitive recruitment of the periplasmic translocation portal TolB by a natively disordered domain of colicin E9. Proc Natl Acad Sci USA. 2006;103:12353–12358. doi: 10.1073/pnas.0603433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonsor DA, et al. Allosteric beta-propeller signalling in TolB and its manipulation by translocating colicins. EMBO J. 2009;28:2846–2857. doi: 10.1038/emboj.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zakharov SD, Zhalnina MV, Sharma O, Cramer WA. The colicin E3 outer membrane translocon: Immunity protein release allows interaction of the cytotoxic domain with OmpF porin. Biochemistry. 2006;45:10199–10207. doi: 10.1021/bi060694+. [DOI] [PubMed] [Google Scholar]
- 30.Yamashita E, Zhalnina MV, Zakharov SD, Sharma O, Cramer WA. Crystal structures of the OmpF porin: Function in a colicin translocon. EMBO J. 2008;27:2171–2180. doi: 10.1038/emboj.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma O, Cramer WA. Minimum length requirement of the flexible N-terminal translocation subdomain of colicin E3. J Bacteriol. 2007;189:363–368. doi: 10.1128/JB.01344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mock M, Pugsley AP. The BtuB group col plasmids and homology between the colicins they encode. J Bacteriol. 1982;150:1069–1076. doi: 10.1128/jb.150.3.1069-1076.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol R. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006–0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apetrei A, et al. Unimolecular study of the interaction between the outer membrane protein OmpF from E. coli and an analogue of the HP(2-20) antimicrobial peptide. J Bioenerg Biomembr. 2010;42:173–180. doi: 10.1007/s10863-010-9273-z. [DOI] [PubMed] [Google Scholar]
- 36.Dunker AK, et al. The unfoldomics decade: An update on intrinsically disordered proteins. BMC Genomics. 2008;9(Suppl 2):S1. doi: 10.1186/1471-2164-9-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fink AL. Natively unfolded proteins. Curr Opin Struct Biol. 2005;15:35–41. doi: 10.1016/j.sbi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Radivojac P, et al. Intrinsic disorder and functional proteomics. Biophys J. 2007;92:1439–1456. doi: 10.1529/biophysj.106.094045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fristedt R, et al. Intrinsically unstructured phosphoprotein TSP9 regulates light harvesting in Arabidopsis thaliana. Biochemistry. 2009;48:499–509. doi: 10.1021/bi8016334. [DOI] [PubMed] [Google Scholar]
- 40.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 41.Wright PE, Dyson HJ. Linking folding and binding. Curr Opin Struct Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: Introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 43.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 44.Vagin A, Teplyakov A. MOLREP: An automated program for molecular replacement. J Appl Crystallogr. 1997;30:1022–1025. [Google Scholar]
- 45.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 46.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 47.Davis IW, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375, 383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potterton L, et al. Developments in the CCP4 molecular-graphics project. Acta Crystallogr D. 2004;60:2288–2294. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.