Abstract
The subterranean mole rat Spalax is an excellent model for studying adaptation of a mammal toward chronic environmental hypoxia. Neuroglobin (Ngb) and cytoglobin (Cygb) are O2-binding respiratory proteins and thus candidates for being involved in molecular hypoxia adaptations of Spalax. Ngb is expressed primarily in vertebrate nerves, whereas Cygb is found in extracellular matrix-producing cells and in some neurons. The physiological functions of both proteins are not fully understood but discussed with regard to O2 supply, the detoxification of reactive oxygen or nitrogen species, and apoptosis protection. Spalax Ngb and Cygb coding sequences are strongly conserved. However, mRNA and protein levels of Ngb in Spalax brain are 3-fold higher than in Rattus norvegicus under normoxia. Importantly, Spalax expresses Ngb in neurons and additionally in glia, whereas in hypoxia-sensitive rodents Ngb expression is limited to neurons. Hypoxia causes an approximately 2-fold down-regulation of Ngb mRNA in brain of rat and mole rat. A parallel regulatory response was found for myoglobin (Mb) in Spalax and rat muscle, suggesting similar functions of Mb and Ngb. Cygb also revealed an augmented normoxic expression in Spalax vs. rat brain, but not in heart or liver, indicating distinct tissue-specific functions. Hypoxia induced Cygb transcription in heart and liver of both mammals, with the most prominent mRNA up-regulation (12-fold) in Spalax heart. Our data suggest that tissue globins contribute to the remarkable tolerance of Spalax toward environmental hypoxia. This is consistent with the proposed cytoprotective effect of Ngb and Cygb under pathological hypoxic/ischemic conditions in mammals.
Oxygen levels that are inadequate to sustain cellular energy production constitute a life-threatening condition for mammals. Metabolically most active tissues (e.g., nerve cells) are exquisitely sensitive to a reduction of O2 (hypoxia), and humans are severely affected by hypoxic disease conditions like stroke or myocardial ischemia. It is therefore mandatory to investigate the specific adaptations evolved by mammals that live in naturally hypoxic environments where low ambient O2 tensions limit the availability of O2 to the organism (1).
The blind mole rat Spalax spends its entire life in underground burrows that can be extremely hypoxic/hypercapnic (2, 3). The Spalacidae, originating 25–40 million years ago, have evolved physiological strategies enabling their respiratory and cardiovascular systems to cope with hypoxia more efficiently than other mammalian species (2, 4). The four karyotypically distinct allospecies of Spalax in Israel are adapted to different climatic regimes. The strongest differences in ecological conditions are observed between Spalax galili (karyotype 2n = 52), inhabiting the northern cool-humid Upper Galilee Mountains with heavy soil, which often becomes flooded, and Spalax judaei (2n = 60), which reside in the warm-dry south with light-aerated soil. The most efficient hypoxic adaptation has consequently been demonstrated in S. galili, with higher normoxic breathing and heart rate as well as higher hematocrit and Hb levels as compared with S. judaei (2, 5). Another two Spalax species, Spalax golani (2n = 54) and Spalax carmeli (2n = 58), are intermediate in their hypoxia adaptation. Compared with the hypoxia-sensitive rodent Rattus norvegicus, Spalax survives substantially longer at low ambient O2 levels and high CO2 without serious deleterious effects or behavioral changes (6).
Hypoxia tolerance mechanisms identified in Spalax as compared with R. norvegicus include blood properties, anatomical and biochemical changes in respiratory organs (2, 4), and differences in the structure and function of a growing list of gene products (7–10). Transcription patterns of genes related to hypoxic stress differ interspecifically in Spalax (5, 11) and between Spalax and rat, involving key players such as erythropoietin (Epo) and its receptors, and hypoxia-inducible factor-1α (Hif-1α) (12, 13). An important adaptation of Spalax to hypoxic habitats is mediated by an increased blood vessel density, which is triggered by a constitutively higher expression (compared with rat) of vascular endothelial growth factor (Vegf), Hif-1α, and HuR, a posttranscriptional stabilizer of Vegf mRNA (6, 14).
The aerobic metabolism of mammals relies on respiratory proteins that function in the delivery and storage of O2. Hb in erythrocytes transports O2 from the lungs to inner organs (15). Myoglobin (Mb) in cardiac and striated muscles acts as a local O2 store and facilitates intracellular diffusion of O2 (16). Ten years ago, neuroglobin (Ngb) and cytoglobin (Cygb) were discovered as unique members of the mammalian globin family (17). The physiological functions of Ngb and Cygb are still uncertain. In most mammals, Ngb resides in neurons of the central and peripheral nervous systems, as well as in endocrine organs (18, 19). Ngb may have an Mb-like role in supplying O2 to the mitochondrial respiratory chain (18, 20, 21). Alternatively, it may function as a scavenger of reactive oxygen or nitrogen species (ROS/RNS) (22, 23) or protect cells from cytochrome c-induced apoptosis (24, 25). Regardless of its ultimate role, there is conclusive evidence that Ngb localization is tightly linked to active oxidative metabolism and mitochondria (19, 20). The highest Ngb level was found in the neuronal retina, which also has the highest O2-consuming rate in the body (20, 26). Several studies have shown that Ngb is cyto- and neuroprotective (27–29). Recently it was demonstrated that in the hooded seal, a diving mammal that tolerates prolonged hypoxia of the brain (30), Ngb is expressed in astrocytes (31). This may indicate an unusual shift of oxidative metabolism from neurons to glial cells.
Cygb occurs predominantly in the fibroblast cell lineage, as well as in some neurons (32–34). The function of Cygb is even less clear than that of Ngb but has been interpreted in terms of ROS defense or O2 supply to certain enzymes (17, 33, 35).
Globins are candidates that may enable Spalax to better survive low ambient oxygen conditions. To understand the particular role of Ngb, Cygb, and Mb in hypoxia tolerance, we have studied their sequences, expression patterns, and gene regulation in different Spalax species in comparison with rat. The data provide indirect evidence to the physiological function(s) of Ngb and Cygb in mammals and point to the biomedical significance of these proteins.
Results
Sequence Analysis of Spalax Ngb, Cygb, and Mb.
Ngb and Cygb gene sequences from S. carmeli were reported previously (36). Here we have cloned, sequenced, and compared the coding regions of Ngb and Cygb from all four Spalax species (Fig. S1). As in other mammals (18, 37), the Spalax Ngb cDNA encodes a strongly conserved protein of 151 amino acids. The four Spalax species maximally differ by two amino acids, and the best match to other genera was found with mouse Ngb (94% identity, 96% similarity). Spalax Cygb cDNAs encode a protein of 190 amino acids, as typical for most other mammals. Cygb proteins of S. carmeli and S. galili are identical and show two amino acid differences to S. golani and S. judaei, which has one additional substitution. S. golani Cygb is 95% identical/99% similar to mouse Cygb. Only very few Spalax-specific amino acid replacements were observed for Ngb and Cygb (Fig. S1). Homology modeling showed that these substitutions are on the surface of the Ngb and Cygb proteins (Fig. S2). Except for the minor differences, Spalax Ngb and Cygb include the conserved sequence hallmarks of functional O2 carriers [e.g., the proximal and distal His (F8 and E7) and PheCD1, which are involved in heme and ligand binding].
The coding region of Spalax Mb (462 bp) translates into a protein of 154 amino acids. Its sequence—here obtained from S. carmeli (2n = 58)—matched the published S. judaei Mb protein sequence (38) with 98.1% identity. Again, all functional positions are conserved, and the observed replacements are found in other mammals as well.
Expression Patterns of Ngb and Cygb in Spalax Tissue.
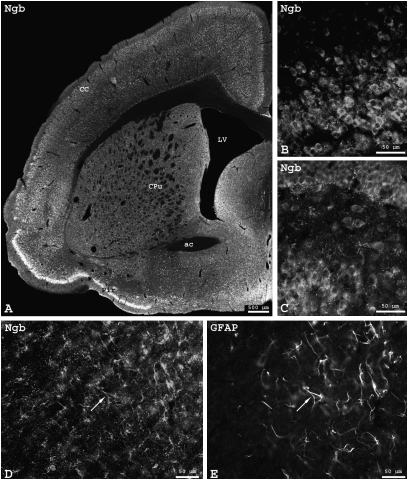
We analyzed the localization of Ngb and Cygb proteins by immunostaining in the brain and other organs of Spalax. We observed a ubiquitous and homogeneous Ngb expression in Spalax brain (Fig. 1A). Stronger signals were obtained from regions with higher neuronal density (such as piriform cortex or hippocampal formation), but fluorescence intensity of single neurons was similar throughout brain sections (Fig. 1 B and C). Neuronal perikarya exhibited Ngb immunofluorescence that was often also seen in cell processes, whereas the cell nuclei remained free of signal (Fig. 1C). The Ngb signals in Spalax neurons were basically identical in regional distribution and intensity to those obtained from mice (19, 39). Immunofluorescence detection of Ngb did not differ between brains prepared from normoxic and hypoxic (5 h, 6% O2) Spalax. Additional Ngb immunoreaction was observed in astrocytes. Fig. 1 D and E, taken from the corpus callosum, show cells that exhibit immunostaining of both Ngb and GFAP.
Fig. 1.
Ngb immunofluorescence in Spalax brain. (A) Frontal section of Spalax brain at the level of the lateral ventricle (LV) and anterior commissure (ac). Ngb staining of neurons was found in the piriform (Pir), insular, sensory, and motor regions of the cerebral cortex (CC) and in the striatum (caudate nucleus-putamen, CPu), as well as medial and basal forebrain regions. Higher magnifications show Ngb-positive neurons from the piriform cortex (B) and dentate gyrus (C). Ngb signal was also seen in astrocytes identified by GFAP staining in the minor forceps of the corpus callosum (D and E; arrows: double-labeled astrocytes). Note cross-sectioned axons positive for Ngb (D) and negative for GFAP (E).
Cygb immunofluorescence was observed in fibroblasts and related, extracellular matrix-producing cell types in various Spalax organs, including heart muscle and liver (Fig. S3). As in mouse (33), Cygb was localized in the cytoplasm of these fibroblast-type cells. In both Spalax and mouse (33), we observed Cygb staining in the nucleus and cytoplasm of distinct neuronal cell populations.
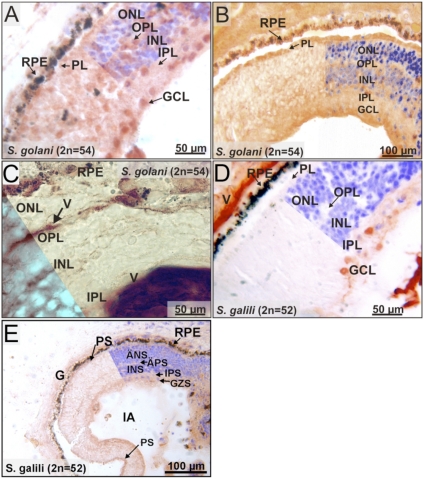
We also investigated the expression patterns of Ngb and Cygb in the Spalax retina. The atrophied eye of the blind Spalax still comprises similar retinal layers as in other rodents. Ngb staining was mainly detected in the ganglion cell layer (GCL) of the Spalax retina (Fig. 2A). Additional weak signals were observed in the inner and outer nuclear layers (INL, ONL). Photoreceptors (PL; inner and outer segments) and plexiform layers (IPL, OPL) were devoid of the intense Ngb staining observed in other rodents (20, 26). To investigate the possible involvement of Ngb in retinal oxidative metabolism, we analyzed the distribution of cytochrome c as a marker for mitochondria. Cytochrome c protein was observed in most layers of the Spalax retina (Fig. 2B), being highest in PL and GCL and lowest in ONL and INL. Ngb and cytochrome c patterns thus do not strictly overlap in Spalax retinal layers. To investigate whether the pattern of Ngb staining in the Spalax retina is determined by a distinct mode of capillary O2 supply, we performed immunostaining applying von Willebrand factor antibodies (Fig. 2C). Results showed that Spalax blood vessels are present in OPL, INL, IPL, and GCs, suggesting O2 supply by deep retinal and superficial capillaries as in the vascularized retina of mouse and rat (20).
Fig. 2.
Immunostaining of Spalax retina cryosections. (A) Ngb expression (red-brown signals) is mainly found in the GCL. The PL, ONL, and INL show weak additional signals (RPE, retinal pigment epithelium). (B) Cytochrome c is found in each layer of the retina with the most prominent expression in PL and GCL. (C) Endothelial cells of the blood vessels (V) were stained with a von Willebrand factor antibody. Staining was found in OPL, INL, IPL, and GCs, indicating the presence of deep retinal and superficial capillaries. (D) Cygb protein is present in ganglion cells and their processes and shows weak expression in the IPL. All other retinal layers are unstained. (E) nNOS signal is also detected in GCL and IPL.
In contrast to the presence of Ngb in various retinal layers, positive Cygb staining was exclusively found in the GCL, and with weaker intensity in IPL (Fig. 2D). The Cygb signal colocalized with immunoreactivity toward neuronal NOS (nNOS) (Fig. 2E).
Quantitative Analyses of Gene Expression.
Globin mRNA levels were compared by quantitative RT-PCR (qRT-PCR) and protein levels by Western blotting, between Spalax and rat at normoxia and at different hypoxic conditions. Two positive control genes [Vegf, adrenomedullin (40)] were used to confirm the effects of hypoxia treatment at the mRNA level (Fig. S4).
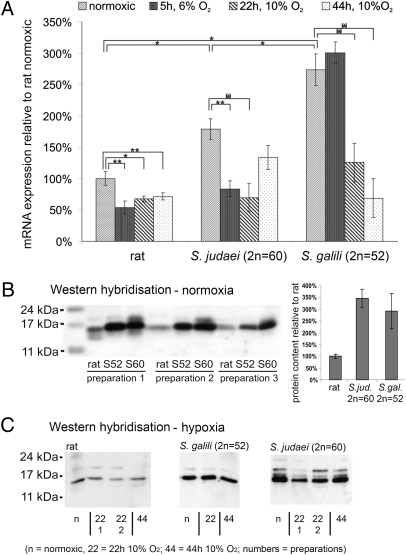
We first compared Ngb mRNA levels in R. norvegicus, S. judaei, and S. galili brain (Fig. 3A). At normoxia, Ngb expression in S. judaei accounted for 180% of the mRNA level in rat. The most hypoxia-resistant species, S. galili, exhibited a relative Ngb level of 280% (P ≤ 0.05). This increased expression of Ngb mRNA under normoxic conditions was confirmed by Western blotting, which revealed up to 3.5-fold more Ngb protein in Spalax than in rat (Fig. 3B).
Fig. 3.
Ngb expression quantification. (A) Ngb mRNA expression in total brain, quantified by qRT-PCR. Under normoxia, Ngb expression is 1.8- and 2.8-fold higher, respectively, in S. judaei and S. galili than in rat. In S. judaei and rat, severe short-time hypoxia (5 h, 6% O2) decreases Ngb mRNA to half of its normoxic value, whereas the amount in S. galili is unchanged. Longer-term moderate hypoxia (22 and 44 h, 10% O2) decreases Ngb expression to 40–75% of the normoxic condition in all three species. Significance levels, indicated by asterisks and horizontal brackets, were obtained by the Student's t test: **P ≤ 0.01, *P ≤ 0.05, (*)P ≤ 0.1. (B) Western blot analysis of Ngb protein expression in rat, S. judaei (2n = 60; S60), and S. galili (2n = 52; S52) normoxic total brain. Three individuals of each species were tested (preparations 1–3). The blot, containing equal amounts of protein per lane, indicates an up to 3.5-fold higher Ngb protein level in the Spalax species as compared with rat. (C) Western blot analysis of Ngb in hypoxic vs. normoxic (n) animals. In rat we observe a slight down-regulation after 22 or 44 h of moderate hypoxic stress (10% O2). In S. galili (S52) and S. judaei (S60), protein levels do not proportionately reflect the decreasing mRNA but show that there is no hypoxic up-regulation of Ngb.
We studied the changes of Ngb expression under short-term severe hypoxia (5 h, 6% O2) and longer-term moderate hypoxia (22 and 44 h, 10% O2). We observed an almost 2-fold decrease of Ngb mRNA in all species (mostly significant at the P < 0.05 level), irrespective of the different hypoxic conditions (Fig. 3A). A notable exception was S. galili after 5 h of 6% O2, which still showed unchanged Ngb mRNA levels. On the protein level, a parallel slight decrease in Ngb was observed after moderate hypoxia in rat, but not so clearly in Spalax (Fig. 3C).
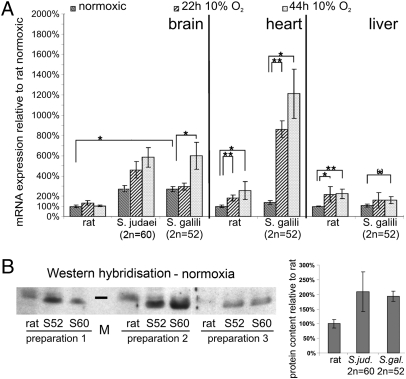
Cygb mRNA levels of rat and Spalax were compared in brain, heart, and liver. The results revealed differences between species, as well as between organs (Fig. 4A). At normoxia, a higher Cygb mRNA level was found in Spalax vs. rat only in the brain (up to 2.5-fold) but not in heart and liver. This result in normoxic brain was confirmed on the protein level (Fig. 4B). Whereas Cygb mRNA expression under hypoxia remained essentially unchanged in rat brain, it increased almost 2-fold in Spalax brain after 44 h of moderate hypoxia (10% O2). In hypoxic heart muscle tissue, both rodents up-regulated Cygb mRNA, although to markedly different extents (rat: 2.5-fold after 44 h; mole rat: up to 12-fold after 44 h). A moderate, approximately 2-fold increase of Cygb mRNA was noted in hypoxic liver in both species.
Fig. 4.
Comparative analyses of Cygb expression. (A) Cygb mRNA expression levels in brain, heart, and liver, determined by qRT-PCR. In brain, constitutive normoxic levels of Cygb mRNA are higher in Spalax (2.5-fold) than in rat. Only Spalax additionally increases brain Cygb mRNA under hypoxia (44 h, 10% O2). In heart and liver, normoxic Cygb mRNA levels are the same in rat and Spalax. In hypoxic heart, Cygb mRNA increases only 2.5-fold in rat but 12-fold in Spalax. In hypoxic liver, both species reveal a moderate 1.5- to 2-fold increase in Cygb mRNA. mRNA levels cannot be compared directly between tissues in the figure, because they are substantially different: normoxic brain and heart express ≈10-fold more Cygb than liver in both species. (B) Western blot analysis of Cygb protein expression in rat, S. judaei (2n = 60; S60), and S. galili (2n = 52; S52). In normoxic brain, Cygb protein expression is approximately 2-fold higher in Spalax than in rat. Three individuals were tested, numbered preparation 1–3. Marker bar (M) indicates a molecular mass of 21 kDa.
We additionally analyzed Mb mRNA expression in neck muscle, used by Spalax for digging, and in heart tissue (Fig. S5). In normoxic neck muscle, S. galili and S. judaei contained 42- and 27-fold more Mb mRNA than rat, respectively. In normoxic heart, both Spalax species had 2.7-fold more Mb mRNA than rat. Short-term severe hypoxia (5 h, 6% O2) did not alter Mb transcription in neck muscle of Spalax, whereas rat Mb mRNA increased 2.7-fold. After longer-term moderate hypoxia, Mb mRNA was found strongly down-regulated at 22 h in rat and S. judaei. The most hypoxia-tolerant species, S. galili, showed the same tendency only after 44 h of hypoxia. Interestingly, Mb mRNA expression in hypoxic heart gave a different picture, increasing slightly, 1.5- to 1.7-fold, in Spalax and rat.
Discussion
In humans a lack of oxygen leads to loss of consciousness within minutes. Acute insults such as cerebral ischemia have a devastating impact on the brain, which is essentially impossible to repair (41). Some mammals, however, can tolerate even prolonged periods of ambient hypoxia; for example, diving mammals show morphological and physiological adaptations that allow them to tolerate periodic hypoxia better than their terrestrial relatives (1, 42). Spalax survives severe chronic hypoxia in its underground burrows and thus is an excellent model system for studying the adaptation of a mammal toward the lack of O2 (2). In fact, genes such as Epo, Hif-1α, or Vegf are instrumental in alleviating hypoxia in Spalax (6, 12–14). Likewise, globin proteins, which enhance O2 supply or decrease hypoxia-caused injuries by other means, may promote viability of Spalax under hypoxic stress. By comparing mRNA and protein expression of Ngb, Cygb, and Mb in two Spalax species with different degrees of hypoxia tolerance and the hypoxia-sensitive rat, we made the following major observations: (i) Ngb mRNA and protein expression in the normoxic brain is substantially higher in Spalax than in rat. (ii) In Spalax, but not in rat, Ngb is expressed in astrocytes in addition to its usual presence in neurons. (iii) Ngb mRNA (and to a lesser extent also the protein) is decreased upon hypoxia in Spalax and rat. A parallel down-regulation after hypoxia is observed for Mb mRNA in Spalax and rat muscle. (iv) Cygb mRNA and protein are also constitutively increased in normoxic brain of Spalax compared with rat and can be further augmented by hypoxia in Spalax. In other tissues, Cygb mRNA is present at comparable levels in normoxic Spalax and rat, and it is up-regulated after hypoxic stress in both taxa.
Increased Globin Levels in Spalax Indicate a Function in Hypoxia Tolerance.
The role of Hb and Mb in O2 supply is well established. Spalax is known to cope with reduced O2 availability in its subterranean burrow systems by means of increased hematocrit and Hb levels and a high O2 affinity of the Hb (43). Likewise, Mb protein levels in Spalax skeletal muscle were reported to be 3-fold higher than in rat (4). This ratio may even be higher in the mole rat neck muscle used for underground digging, as suggested by the 11-fold enhanced Mb mRNA level. The amino acid replacements observed in mole rat Hb and Mb sequences do not seem to be prime mediators of hypoxia adaptation (38, 43). The same is probably true for Ngb and Cygb, which display high sequence conservation in the mole rat. In parallel to Hb and Mb, however, we detected significantly higher Ngb and Cygb mRNA and protein levels in Spalax tissues as compared with rat (Figs. 3, 4), which points to an important role of both globins in hypoxia adaptation. Our findings thus corroborate in vitro and in vivo studies using ectopic globin overexpression, which at least for Ngb conclusively report a survival-enhancing effect in neuronal cells after hypoxic and ischemic insult (27–29). The data also emphasize the importance of gene regulatory changes (vs. sequence changes) as a major adaptive mechanism in Spalax.
Glial Expression of Ngb: A Key Feature of Hypoxia Adaptation?
Inferring adaptive significance by comparing traits of just two distantly related taxa such as Spalax and rat can be dangerous. Therefore it is most important that we observe intriguing parallels between the Spalax–rat data and other animal models, which significantly strengthens our interpretations. Interspecific differences in Ngb expression levels have been reported before in fish: the hypoxia-tolerant goldfish (Carassius auratus) has approximately 5-fold more Ngb protein in the brain than the more hypoxia-sensitive zebrafish (Danio rerio) (44). This quantitative difference can be interpreted in terms of an O2 supply function and/or an ROS detoxification role of Ngb as an adaptive strategy to alleviate hypoxic stress. In Spalax, the interpretation is more complicated: we show that Ngb in the mole rat is localized in neurons and astrocytes, whereas in the brain of hypoxia-sensitive rodents like mouse or rat, Ngb is localized primarily in neurons (19, 39, 45). Thus, both cell types contribute to the elevated Ngb expression level in Spalax brain, and we are currently unable to separately quantify neuronal and glial expression. Interestingly, however, in the brain of the deep-diving hooded seal (Cystophora cristata) Ngb is predominantly present in astrocytes (31). Glial expression of nerve hemoglobins (nHbs) has also been observed in hypoxia-tolerant invertebrates, whereas in related, hypoxia-sensitive species nHbs reside in neurons (46, 47). It is therefore tempting to assume that the glial expression of Ngb in the brains of the hooded seal and Spalax is a common feature of hypoxia tolerance. In the seal, the glial expression of Ngb has been interpreted in terms of a shift of oxidative metabolism from neurons to astrocytes, whereas neurons essentially rely on anaerobic fermentation (31). In fact, seal neurons are more hypoxia tolerant than those of rat (30). It remains to be shown whether this also applies to Spalax neurons.
Adaptation to Chronic Hypoxia: Alleviating the Need for an Acute Hypoxic Up-Regulation of Ngb and Mb in Spalax?
The possible involvement of Ngb and Mb in Spalax hypoxia tolerance led us to study their gene regulatory response after experimental O2 deprivation. In fact, hypoxia causes an increase of Ngb gene expression in zebrafish (mRNA and protein level) (48) and turtle (mRNA) (49). By contrast, no significant changes of Ngb mRNA were found in the brains of mice after prolonged hypoxia (50) or in brains of rats after global ischemia (51), suggesting that Ngb fulfills a constitutive function rather than being an acute stress-response protein. Even more surprising is our observation that experimental hypoxia triggers a significant decrease of Ngb mRNA in rat and Spalax brains, even though protein levels are less affected. At first glance, this result is difficult to reconcile with previously proposed functions of Ngb (17, 21). However, Spalax must adapt to chronic hypoxia in its underground burrows, and thus there may be no evolutionary pressure to evolve pathways, which enable an acute up-regulation of Ngb. Rather, the higher Ngb mRNA/protein content of Spalax brain (and the glial plus neuronal Ngb localization) reflect a constitutive, intrinsic hypoxia tolerance of Spalax tissues.
Interestingly, Mb mRNA is also down-regulated after prolonged hypoxia in Spalax neck muscle, but indicates adaptation to chronic hypoxic conditions by the constitutive normoxic higher Mb mRNA [and protein (4)] expression. This parallel mode of regulation of Ngb and Mb might imply similar functions of the two respiratory proteins (i.e., in O2 supply and ROS/RNS detoxification) (52). The 2.5-fold up-regulation of Mb mRNA under acute strong hypoxia in Rattus neck muscle (and to a lesser extent in heart) in turn indicates that the hypoxia-sensitive species is adapted to deliver an acute stress response, in agreement with a recent study on the molecular pathways of Mb hypoxia regulation in mouse (53).
Evidence for Distinct Tissue-Specific Functions of Ngb and Cygb.
The eye is central to the discussion of Ngb function because in the neuronal retina of sighted rodents, Ngb protein is expressed in substantial amounts in the plexiform layers, ganglion cells, and inner segments of photoreceptors (26). The subcellular colocalization of Ngb with mitochondria and the spatial correlation with retinal vasculature strongly suggest an involvement of Ngb in the intense oxidative metabolism of the retina by supplying O2 and/or by scavenging ROS (20). The subterranean mole rat is blind and possesses only minute regressed eyes covered by skin (54). The Spalax retina, however, still reveals all typical cell layers, although less organized. The outer segments of the photoreceptors are rudimentary and it is considered that the Spalax retina has been restructured to evolve a function in photoperiodic sensing (55). Although we show here that the distribution of blood vessels in Spalax fits a typical “vascular-type” retina, Ngb expression in the Spalax retina is extremely reduced and almost limited to the GCL. An intense Ngb expression in several retinal layers is therefore positively selected to sustain visual processes in sighted mammals. The retained Ngb expression in Spalax ganglion cells may indicate an additional, residual role of this globin in nerve cells, perhaps operating at lower expression levels.
The physiological function of Cygb is currently even less well understood than the role of Ngb (17). The Spalax data, revealing distinct modes of Cygb gene regulation in brain vs. heart and liver, confirm the notion from other rodents that Cygb may have different roles in neurons and in fibroblast-related cell types of diverse organs (33, 34). In brain, the elevated normoxic level of Cygb in Spalax vs. rat suggests involvement in the chronic hypoxia tolerance, as seen for Ngb and Mb. The colocalization of Cygb with nNOS in the GCL of the Spalax retina has been observed before in specific neuronal populations of the mouse brain (47, 56) and may indicate that Cygb and nNOS interact functionally, for example by a delivery of O2 from Cygb to nNOS during the production of NO or by scavenging excess NO.
In heart and liver, interspecific Cygb mRNA levels are similar at normoxia. In these organs, however, Cygb responds to O2 deprivation by stress-induced mRNA up-regulation. This points to a Cygb function that does not require constitutively elevated expression levels but operates in an O2-dependent regulated mode in conserved cellular processes (e.g., collagen maturation) (32, 33).
Conclusions.
Quantitative changes in gene regulation seem to be a major adaptive mechanism in the chronic hypoxia tolerance of the mole rat. Spalax can thus be regarded as a “natural” alternative to transgenic animal models. In globin research, the suggestions of very diverse molecular functions of Ngb and Cygb, mostly obtained in vitro, have to take into consideration observations from natural animal models. For example, hypotheses claiming an involvement of Ngb in complex neuronal signal transduction processes should explain the shift during mammalian evolution into another cell type (glia) in hypoxia model organisms like Spalax and seal. Together, the Spalax data strengthen the argument that Ngb functions in oxidative cellular metabolism, whereas Cygb may have distinct tissue-specific functions.
Materials and Methods
Animals.
Spalax was captured in the field and housed in the Institute of Evolution, Haifa. Sprague-Dawley rats were used. After hypoxic treatment, animals were killed by Ketaset CIII injection (Fort Dodge Animal Health) at 5 mg per kg of body weight. The Ethics Committee of the University of Haifa approved all experiments.
Cloning and Sequencing of Spalax Ngb, Cygb, and Mb cDNAs.
Spalax cDNAs for Ngb, Cygb, and Mb were isolated by RT-PCR from total RNA of brain, liver, and muscle tissues, respectively. PCR primers (SI Materials and Methods) were derived from published globin sequence alignments and the Spalax Mb protein sequence (38). RT-PCR and 5′/3′ RACE products were cloned and sequenced (Starseq). GenBank/European Molecular Biology Laboratory accession numbers are AM419202 (S. judaei Ngb), AM419201 (S. galili Ngb), AM489450 (S. carmeli Ngb), FN821091 (S. golani Ngb), AM419204 (S. judaei Cygb), AM419203 (S. galili Cygb), AM489449 (S. carmeli Cygb), and FN821092 (S. golani Cygb).
Immunostaining and Western Blotting.
For immunohistochemistry and Western hybridization, we used established polyclonal rabbit antisera raised against synthetic peptides of Ngb and Cygb (26, 33; see SI Materials and Methods for experimental details).
Quantitative Real-Time RT-PCR.
mRNA quantities were determined by standard real-time RT-PCR using either Taqman chemistry (Quantitect Probe PCR Kit) or QuantiTect SYBR Green detection (Qiagen). Experimental details (primers, probes, reagent concentrations, PCR conditions, normalization, and reference genes) are given in SI Materials and Methods. We used one to three pooled RNAs, each prepared from at least three animals, for all genes and tissues tested. Data were evaluated by the standard curve method. Graphs show fold changes of expression levels relative to normoxic conditions in the hypoxia/normoxia comparisons, and relative to rat in the interspecies comparisons. Error bars indicate SEM, calculated for biological replicates. Confidence intervals were calculated by Student's t tests at different levels of significance (P < 0.1, P < 0.05, P < 0.01).
Supplementary Material
Acknowledgments
We thank Mrs. Ulrike Maas for technical support to F.G. and T.H. and Scott Permut for language editing. Financial support was provided by the European Union (QLG3-CT-2002-0154, to A.A., T.B., E.N., and T.H.), the Deutsche Forschungsgemeinschaft (DFG Ha2103/3, Bu956/12), the Stiftung für Innovation Rheinland-Pfalz (695, to T.B. and T.H.), and the United States-Israel Binational Science Foundation (2005346, to A.A. and Dr. Mark Band). E.N. was supported by the Ancell-Teicher Research Foundation for Genetics and Molecular Evolution.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015379107/-/DCSupplemental.
References
- 1.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: From the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 2.Nevo E, Ivanitskaya E, Beiles A. Adaptive Radiation of Blind Subterranean Mole Rats. Leiden, The Netherlands: Backhuys Publishers; 2001. [Google Scholar]
- 3.Shams I, Avivi A, Nevo E. Oxygen and carbon dioxide fluctuations in burrows of subterranean blind mole rats indicate tolerance to hypoxic-hypercapnic stresses. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:376–382. doi: 10.1016/j.cbpa.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Widmer HR, Hoppeler H, Nevo E, Taylor CR, Weibel ER. Working underground: respiratory adaptations in the blind mole rat. Proc Natl Acad Sci USA. 1997;94:2062–2067. doi: 10.1073/pnas.94.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arieli R, Nevo E. Hypoxic survival differs between two mole rat species (Spalax ehrenbergi) of humid and arid habitats. Comp Biochem Physiol Comp Physiol. 1991;100:543–545. doi: 10.1016/0300-9629(91)90367-l. [DOI] [PubMed] [Google Scholar]
- 6.Avivi A, Resnick MB, Nevo E, Joel A, Levy AP. Adaptive hypoxic tolerance in the subterranean mole rat Spalax ehrenbergi: The role of vascular endothelial growth factor. FEBS Lett. 1999;452:133–140. doi: 10.1016/s0014-5793(99)00584-0. [DOI] [PubMed] [Google Scholar]
- 7.Ashur-Fabian O, et al. Evolution of p53 in hypoxia-stressed Spalax mimics human tumor mutation. Proc Natl Acad Sci USA. 2004;101:12236–12241. doi: 10.1073/pnas.0404998101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravid O, et al. An extracellular region of the erythropoietin receptor of the subterranean blind mole rat Spalax enhances receptor maturation. Proc Natl Acad Sci USA. 2007;104:14360–14365. doi: 10.1073/pnas.0706777104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasser NJ, et al. Alternatively spliced Spalax heparanase inhibits extracellular matrix degradation, tumor growth, and metastasis. Proc Natl Acad Sci USA. 2009;106:2253–2258. doi: 10.1073/pnas.0812846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avivi A, et al. P53 in blind subterranean mole rats—loss-of-function versus gain-of-function activities on newly cloned Spalax target genes. Oncogene. 2007;26:2507–2512. doi: 10.1038/sj.onc.1210045. [DOI] [PubMed] [Google Scholar]
- 11.Avivi A, Brodsky L, Nevo E, Band MR. Differential expression profiling of the blind subterranean mole rat Spalax ehrenbergi superspecies: Bioprospecting for hypoxia tolerance. Physiol Genomics. 2006;27:54–64. doi: 10.1152/physiolgenomics.00001.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shams I, Avivi A, Nevo E. Hypoxic stress tolerance of the blind subterranean mole rat: Expression of erythropoietin and hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA. 2004;101:9698–9703. doi: 10.1073/pnas.0403540101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shams I, Nevo E, Avivi A. Erythropoietin receptor spliced forms differentially expressed in blind subterranean mole rats. FASEB J. 2005;19:1749–1751. doi: 10.1096/fj.05-3975fje. [DOI] [PubMed] [Google Scholar]
- 14.Avivi A, et al. Increased blood vessel density provides the mole rat physiological tolerance to its hypoxic subterranean habitat. FASEB J. 2005;19:1314–1316. doi: 10.1096/fj.04-3414fje. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson RE, Geis I. Hemoglobin: Structure, Function, Evolution, and Pathology. San Francisco: Benjamin/Cummings; 1983. [Google Scholar]
- 16.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 17.Hankeln T, et al. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J Inorg Biochem. 2005;99:110–119. doi: 10.1016/j.jinorgbio.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 19.Hankeln T, et al. The cellular and subcellular localization of neuroglobin and cytoglobin—a clue to their function? IUBMB Life. 2004;56:671–679. doi: 10.1080/15216540500037794. [DOI] [PubMed] [Google Scholar]
- 20.Bentmann A, et al. Divergent distribution in vascular and avascular mammalian retinae links neuroglobin to cellular respiration. J Biol Chem. 2005;280:20660–20665. doi: 10.1074/jbc.M501338200. [DOI] [PubMed] [Google Scholar]
- 21.Burmester T, Hankeln T. What is the function of neuroglobin? J Exp Biol. 2009;212:1423–1428. doi: 10.1242/jeb.000729. [DOI] [PubMed] [Google Scholar]
- 22.Herold S, Fago A, Weber RE, Dewilde S, Moens L. Reactivity studies of the Fe(III) and Fe(II)NO forms of human neuroglobin reveal a potential role against oxidative stress. J Biol Chem. 2004;279:22841–22847. doi: 10.1074/jbc.M313732200. [DOI] [PubMed] [Google Scholar]
- 23.Fordel E, Thijs L, Moens L, Dewilde S. Neuroglobin and cytoglobin expression in mice. Evidence for a correlation with reactive oxygen species scavenging. FEBS J. 2007;274:1312–1317. doi: 10.1111/j.1742-4658.2007.05679.x. [DOI] [PubMed] [Google Scholar]
- 24.Fago A, Mathews AJ, Moens L, Dewilde S, Brittain T. The reaction of neuroglobin with potential redox protein partners cytochrome b5 and cytochrome c. FEBS Lett. 2006;580:4884–4888. doi: 10.1016/j.febslet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Raychaudhuri S, Skommer J, Henty K, Birch N, Brittain T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis. 2010;15:401–411. doi: 10.1007/s10495-009-0436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, et al. How does the eye breathe? Evidence for neuroglobin-mediated oxygen supply in the mammalian retina. J Biol Chem. 2003;278:1932–1935. doi: 10.1074/jbc.M209909200. [DOI] [PubMed] [Google Scholar]
- 27.Sun Y, Jin K, Mao XO, Zhu Y, Greenberg DA. Neuroglobin is up-regulated by and protects neurons from hypoxic-ischemic injury. Proc Natl Acad Sci USA. 2001;98:15306–15311. doi: 10.1073/pnas.251466698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y, et al. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci USA. 2003;100:3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan AA, et al. Neuroglobin-overexpressing transgenic mice are resistant to cerebral and myocardial ischemia. Proc Natl Acad Sci USA. 2006;103:17944–17948. doi: 10.1073/pnas.0607497103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folkow LP, Ramirez JM, Ludvigsen S, Ramirez N, Blix AS. Remarkable neuronal hypoxia tolerance in the deep-diving adult hooded seal (Cystophora cristata) Neurosci Lett. 2008;446:147–150. doi: 10.1016/j.neulet.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Mitz SA, et al. When the brain goes diving: Glial oxidative metabolism may confer hypoxia tolerance to the seal brain. Neuroscience. 2009;163:552–560. doi: 10.1016/j.neuroscience.2009.06.058. [DOI] [PubMed] [Google Scholar]
- 32.Nakatani K, et al. Cytoglobin/STAP, its unique localization in splanchnic fibroblast-like cells and function in organ fibrogenesis. Lab Invest. 2004;84:91–101. doi: 10.1038/labinvest.3700013. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M, et al. Cytoglobin is a respiratory protein in connective tissue and neurons, which is up-regulated by hypoxia. J Biol Chem. 2004;279:8063–8069. doi: 10.1074/jbc.M310540200. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt M, Laufs T, Reuss S, Hankeln T, Burmester T. Divergent distribution of cytoglobin and neuroglobin in the murine eye. Neurosci Lett. 2005;374:207–211. doi: 10.1016/j.neulet.2004.10.071. [DOI] [PubMed] [Google Scholar]
- 35.Burmester T, Gerlach F, Hankeln T. Regulation and role of neuroglobin and cytoglobin under hypoxia. Adv Exp Med Biol. 2007;618:169–180. doi: 10.1007/978-0-387-75434-5_13. [DOI] [PubMed] [Google Scholar]
- 36.Gerlach F, et al. Genomic organization and molecular evolution of the genes for neuroglobin and cytoglobin in the hypoxiatolerant Israeli mole rat, Spalax carmeli. Isr J Ecol Evol. 2007;52:389–403. [Google Scholar]
- 37.Burmester T, et al. Neuroglobin and cytoglobin: Genes, proteins and evolution. IUBMB Life. 2004;56:703–707. doi: 10.1080/15216540500037257. [DOI] [PubMed] [Google Scholar]
- 38.Gurnett AM, et al. The myoglobin of rodents: Lagostomus maximus (viscacha) and Spalax ehrenbergi (mole rat) J Protein Chem. 1984;3:445–454. [Google Scholar]
- 39.Wystub S, et al. Localization of neuroglobin protein in the mouse brain. Neurosci Lett. 2003;346:114–116. doi: 10.1016/s0304-3940(03)00563-9. [DOI] [PubMed] [Google Scholar]
- 40.Bernaudin M, Tang Y, Reilly M, Petit E, Sharp FR. Brain genomic response following hypoxia and re-oxygenation in the neonatal rat. Identification of genes that might contribute to hypoxia-induced ischemic tolerance. J Biol Chem. 2002;277:39728–39738. doi: 10.1074/jbc.M204619200. [DOI] [PubMed] [Google Scholar]
- 41.Weil ZM, Norman GJ, DeVries AC, Nelson RJ. The injured nervous system: A Darwinian perspective. Prog Neurobiol. 2008;86:48–59. doi: 10.1016/j.pneurobio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Butler PJ. Metabolic regulation in diving birds and mammals. Respir Physiol Neurobiol. 2004;141:297–315. doi: 10.1016/j.resp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Kleinschmidt T, Nevo E, Goodman M, Braunitzer G. Mole rat hemoglobin: Primary structure and evolutionary aspects in a second karyotype of Spalax ehrenbergi, Rodentia, (2n = 52) Biol Chem Hoppe Seyler. 1985;366:679–685. doi: 10.1515/bchm3.1985.366.2.679. [DOI] [PubMed] [Google Scholar]
- 44.Roesner A, Mitz SA, Hankeln T, Burmester T. Globins and hypoxia adaptation in the goldfish, Carassius auratus. FEBS J. 2008;275:3633–3643. doi: 10.1111/j.1742-4658.2008.06508.x. [DOI] [PubMed] [Google Scholar]
- 45.Laufs TL, et al. Neuron-specific expression of neuroglobin in mammals. Neurosci Lett. 2004;362:83–86. doi: 10.1016/j.neulet.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 46.Kraus DW, Colacino JM. Extended oxygen delivery from the nerve hemoglobin of Tellina alternata (Bivalvia) Science. 1986;232:90–92. doi: 10.1126/science.232.4746.90. [DOI] [PubMed] [Google Scholar]
- 47.Burmester T, Hankeln T. Neuroglobin and other nerve globins. In: Bolognesi M, di Prisco G, Verde C, editors. Protein Reviews: Dioxygen Binding and Sensing Proteins. Vol. 9. Milan: Springer; 2008. pp. 211–222. [Google Scholar]
- 48.Roesner A, Hankeln T, Burmester T. Hypoxia induces a complex response of globin expression in zebrafish (Danio rerio) J Exp Biol. 2006;209:2129–2137. doi: 10.1242/jeb.02243. [DOI] [PubMed] [Google Scholar]
- 49.Nayak G, Prentice HM, Milton SL. Role of neuroglobin in regulating reactive oxygen species in the brain of the anoxia-tolerant turtle Trachemys scripta. J Neurochem. 2009;110:603–612. doi: 10.1111/j.1471-4159.2009.06157.x. [DOI] [PubMed] [Google Scholar]
- 50.Mammen PP, et al. Neuroglobin, a novel member of the globin family, is expressed in focal regions of the brain. J Histochem Cytochem. 2002;50:1591–1598. doi: 10.1177/002215540205001203. [DOI] [PubMed] [Google Scholar]
- 51.Büttner F, et al. Genomic response of the rat brain to global ischemia and reperfusion. Brain Res. 2009;1252:1–14. doi: 10.1016/j.brainres.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 52.Wittenberg JB. On optima: The case of myoglobin-facilitated oxygen diffusion. Gene. 2007;398:156–161. doi: 10.1016/j.gene.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 53.Kanatous SB, et al. Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am J Physiol Cell Physiol. 2009;296:C393–C402. doi: 10.1152/ajpcell.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanyal S, Jansen HG, de Grip WJ, Nevo E, de Jong WW. The eye of the blind mole rat, Spalax ehrenbergi. Rudiment with hidden function? Invest Ophthalmol Vis Sci. 1990;31:1398–1404. [PubMed] [Google Scholar]
- 55.Cernuda-Cernuda R, DeGrip WJ, Cooper HM, Nevo E, García-Fernández JM. The retina of Spalax ehrenbergi: Novel histologic features supportive of a modified photosensory role. Invest Ophthalmol Vis Sci. 2002;43:2374–2383. [PubMed] [Google Scholar]
- 56.Hundahl CA, et al. Anatomical characterization of cytoglobin and neuroglobin mRNA and protein expression in the mouse brain. Brain Res. 2010;1331:58–73. doi: 10.1016/j.brainres.2010.03.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.