Abstract
Aging is associated with the functional decline of cells, tissues, and organs. At the same time, age is the single most important prognostic factor in the development of most human cancers, including chronic myelogenous and acute lymphoblastic leukemias initiated by Bcr-Abl oncogenic translocations. Prevailing paradigms attribute the association between aging and cancers to the accumulation of oncogenic mutations over time, because the accrual of oncogenic events is thought to be the rate-limiting step in initiation and progression of cancers. Conversely, aging-associated functional decline caused by both cell-autonomous and non–cell-autonomous mechanisms is likely to reduce the fitness of stem and progenitor cell populations. This reduction in fitness should be conducive for increased selection of oncogenic mutations that can at least partially alleviate fitness defects, thereby promoting the initiation of cancers. We tested this hypothesis using mouse hematopoietic models. Our studies indicate that the dramatic decline in the fitness of aged B-lymphopoiesis coincides with altered receptor-associated kinase signaling. We further show that Bcr-Abl provides a much greater competitive advantage to old B-lymphoid progenitors compared with young progenitors, coinciding with restored kinase signaling pathways, and that this enhanced competitive advantage translates into increased promotion of Bcr-Abl–driven leukemias. Moreover, impairing IL-7–mediated signaling is sufficient to promote selection for Bcr-Abl–expressing B progenitors. These studies support an unappreciated causative link between aging and cancer: increased selection of oncogenic mutations as a result of age-dependent alterations of the fitness landscape.
Keywords: AKT, STAT5, evolution, immunosenescence, chronic myelogenous leukemia
Although hematopoietic stem cells (HSCs) are capable of maintaining hematopoiesis throughout the life of mice and humans, HSCs and progenitor cells show significant age-related functional decline (1). In mice, aging-associated defects in B and T lymphopoiesis are particularly apparent, which can be attributed to both cell-intrinsic and -extrinsic effects of aging (2–6). B-cell development in old mice is severely impaired starting at early B-cell progenitor (EBP) and pro-B cell stages.
Age is the largest risk factor for cancer development in mammals. The incidence of most human cancers rises exponentially with age, dramatically accelerating after midlife (7, 8). Cancer progression represents a process of somatic evolution, whereby a single initially WT cell gives rise to a highly complex tumor containing populations of cells harboring large numbers of genetic and epigenetic alterations. This evolutionary process is driven by two components: diversification of heritable types through acquisition of genetic and epigenetic changes and selection for those cells that harbor mutations increasing cell fitness (wherein “fitness” is a measure of the ability of a cell of a certain genotype to pass this genotype to subsequent cell generations, as governed by competition for similar niches). Predominant paradigms attribute age-related increases in cancer incidence to the accumulation of mutations in oncogenes and tumor suppressor genes, thus providing fuel for tumor evolution (9, 10). The effects of mutations on fitness are highly context dependent, however (11). We have proposed that age-related reductions in the fitness of stem and progenitor pools, attributable both to cell-intrinsic accumulation of genomic damage as well as to alterations in the microenvironment, should increase selection for adaptive oncogenic mutations (12).
We have previously demonstrated that contexts of reduced cellular fitness caused by replicational stress or combined genetic disruption of E2f1 and E2f2 increase the selective advantage conferred by expression of Bcr-Abl in hematopoietic progenitors and that this increased selective advantage translates into increased leukemogenesis (13). The chromosomal translocation generating p210 Bcr-Abl is the major cause of chronic myelogenous leukemia (CML), and translocations generating p210 or p190 Bcr-Abl fusions are present in acute lymphoblastic leukemias (ALLs) (14). The incidence of CML increases exponentially with age (15), and Bcr-Abl+ ALLs account for more than half of ALLs in patients over 50 y of age (16, 17). The Bcr-Abl kinase promotes proliferation, survival, and growth factor independence via a variety of signaling pathways, including signaling dependent on Ras, Rac, Akt, NF-κB, and Jak/STAT (18).
Studies presented here demonstrate that aged B-cell progenitors display impaired receptor signaling and reduced fitness, promoting selection for Bcr-Abl, which is adaptive, at least in part, by restoring kinase signaling. These studies argue that increased selection for certain oncogenic events that alleviate some of the aging-related defects in old progenitor cell pools may contribute to the link between aging and cancer incidence.
Results
Fitness of B-Lymphoid Progenitors Declines with Age.
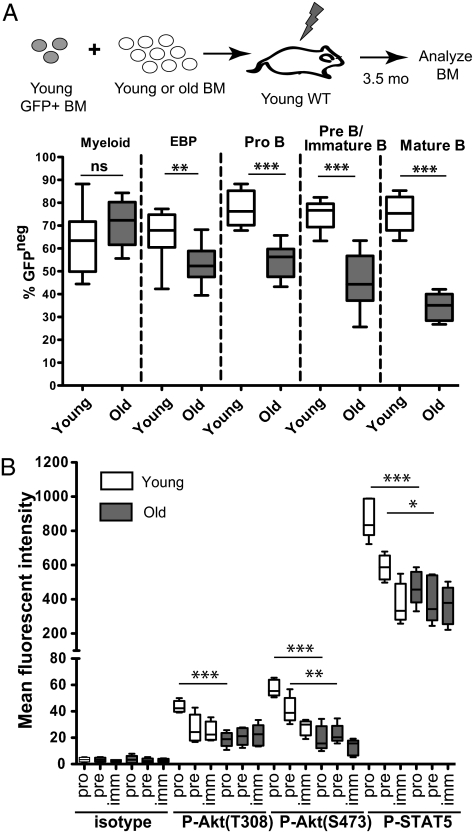
To compare the relative abilities of young and old bone marrow (BM) to contribute to B-progenitor stages in a competitive context, BM from young (∼2 mo of age) or old (22–24 mo of age) BALB/c mice was mixed at 85:15 ratios with BM from GFP transgenic BALB/c mice (19) and then transplanted into lethally irradiated young BALB/c recipients. At 3.5 mo after bone marrow transplantation (BMT), BM was harvested and stained for markers of B progenitors and myeloid cells. As expected (4), contributions to myelopoiesis were not affected by the age of the donor BM (Fig. 1A). In contrast, aged BM exhibited progressively diminished contributions to B-progenitor pools, starting at the EBP stage, likely reflecting reduced fitness at these stages. In addition, by limiting dilution assays, we observed reductions in the frequency of functional HSCs in old mice and sustained defects in contributions per HSC to the B cell but not to the myeloid lineage (Fig. S1).
Fig. 1.
Declining B-progenitor fitness with age coincides with reduced kinase signaling. (A) Total BM from a 2-mo-old GFP transgenic mouse was mixed with BM from either a young (1.5–2 mo of age) or old (24 mo of age) BALB/c mouse at a 15:85 ratio. The mixtures were transplanted into lethally irradiated young recipients. At 3.5 mo posttransplantation, GFP expression was analyzed in various BM progenitor populations as determined via surface staining: myeloid lineage cells (Mac+B220neg), EBPs (LinnegSca1loIL-7Rα+), pro-B cells (B220medCD93+CD43+Mac1neg), pre-B cells/immature B cells (B220medCD93+CD43negMac1neg), and mature B cells (B220highCD93negCD43negMac1neg). The percentage of GFPneg cells derived from the young or old donors is graphed, with the mean, SE, minimum, and maximum values shown. (B) BM was isolated from young (Y; white bars) and old (O; gray bars) mice and analyzed for P-Akt (T308 or S473) and P-STAT5, together with surface staining for B220, CD43, and CD93, by flow cytometry. The detection of the indicated phosphorylated effector protein, as determined by the mean fluorescence intensity, within the indicated B-progenitor populations (pro-B, pre-B, and mature/“mat” B cells, as defined in A, but without Mac1 analysis) is graphed as in A. Data for A and B are derived from at least five mice per group. An unpaired Student's exact t test was used for statistical analyses. ns, not significant (indicates P > 0.05); *P value between 0.05 and 0.01; **P value between 0.01 and 0.001; ***P < 0.001.
B-cell development is critically dependent on signaling from lineage-specific receptors, such as the IL-7 receptor (IL-7R) and the pre-B cell receptor (BCR). Aged B progenitors have been shown to be poorly responsive to IL-7 in vitro (5, 20) and to exhibit reduced expression of components of the pre-BCR (21, 22). Akt and STAT5 are key effectors of IL-7R signaling (23). Indeed, the levels of phosphorylated/activated Akt and STAT5 were two- to threefold lower in old BM B-cell progenitors relative to young B-cell progenitors (Fig. 1B and Fig. S2) but were unaffected by age in myeloid cells (Fig. S2), consistent with skewing of hematopoiesis toward myelopoiesis in old mice (Fig. S3B). The defect in Akt and STAT5 activation was most apparent at the pro-B cell stage (Fig. 1B), coinciding with reduced frequencies of pre-B and pro-B cells in old mice (2–5) (Figs. S3 A and C). In contrast, phosphorylation of Erk and STAT3 was similar in young and old progenitors (Fig. S2C). Of note, the percentage of cells in cycle was similar for old and young B progenitors, probably because of homeostatic pressure; thus, age-dependent signaling perturbations cannot be attributed to altered cell cycling (Fig. S3D).
Bcr-Abl Is Adaptive in Old B Progenitors.
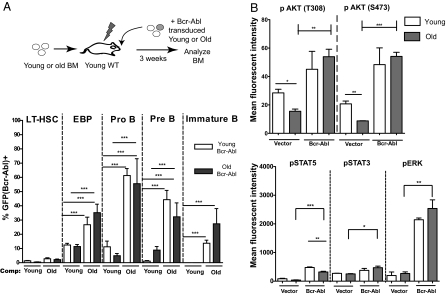
We asked whether age-related reductions in fitness of BM B progenitors affects selection for cells expressing Bcr-Abl. To this end, we harvested BM progenitors from young or old donor mice and transduced them with murine stem cell virus-ires-GFP (MiG) vectors expressing p190 Bcr-Abl. Transduced cells were transplanted into irradiated young recipients that were pretransplanted 4 d earlier with excess young or old competitor BM. The purpose of pretransplantation (“prepopulation”) was to create a more competitive context by limiting the numbers of free niches available for the engraftment of retrovirally transduced cells. Notably, in these recipients, most hematopoiesis is of the “competitor” age and genotype. p190 Bcr-Abl–expressing progenitors (both old and young) underwent a substantially higher expansion in EBP, pro-B cell, pre–/immature B-cell, and mature B-cell pools in recipients prepopulated with old BM competitor cells relative to young cells [Fig. 2Aand Fig. S4 (flow cytometric profiles)]. Thus, the expression of p190 Bcr-Abl conferred a significantly stronger competitive advantage within aged B-progenitor pools compared with young B-progenitor pools. Note that the age of the B progenitors transduced with Bcr-Abl did not determine the extent of competitive expansion; rather, the age of the competing progenitors did. Contributions of Bcr-Abl–expressing cells to myeloid and HSC-enriched pools in the BM were not age-dependent (Fig. 2A and Fig. S5A), suggesting that a similar number of old and young multipotent progenitors were transduced with MiG-p190 Bcr-Abl. Moreover, the preferential expansion in the B-cell lineage was not attributable to lower engraftment of the transduced cells into mice prepopulated with young BM cells or to differential transduction of old vs. young progenitors, because contributions of vector-expressing cells to hematopoiesis were similar for old and young transduced BM (Fig. S5B). Importantly, age-related defects of B-cell progenitor fitness and increased selective advantage of cells that express Bcr-Abl could be observed in the less complex context of in vitro culture (Fig. S6).
Fig. 2.
Aged hematopoiesis selects for p190 Bcr-Abl–expressing B progenitors, and defective signaling in old B progenitors is restored by Bcr-Abl expression. (A) Young, lethally irradiated, BALB/c recipients were each injected with 2 × 106 unsorted BM cells from either young or aged competitors, denoted as “comp” at the base of the graph. Four days later, mice were transplanted with 1 × 105 MiG-p190 Bcr-Abl–transduced c-Kit–selected BM cells from young (white bars) or old (gray bars) donors, ∼98% of which were uninfected. The percentage of GFP+ cells among HSCs (CD150+CD48negLinneg), EBPs, pro-B cells, pre-B cells/immature B cells, and mature B cells in BM of mice transplanted with Bcr-Abl–transduced cells was determined as in Fig. 1 by flow cytometry at 3 wk posttransplantation. (B) Sublethally irradiated young mice were transplanted with ∼1 × 105 MiG or MiG-p190 Bcr-Abl–transduced c-Kit–selected BM cells from young or old donors. BM cells were transduced at high efficiency and transplanted without competitor BM to maximize the fraction of progenitors expressing Bcr-Abl or vector in recipient mice. At 3 wk posttransplantation, recipient mice were killed and BM cells were analyzed for P-Akt (T308 or S473), P-STAT3, P-STAT5, and P-Erk, together with surface staining for B220, Mac1, CD3, and Ter119, by flow cytometry. The detection of the indicated phosphorylated effector protein within B-lineage cells (B220+MacnegCD3negTer119neg) is graphed for both vector and Bcr-Abl (GFP+)–expressing cells. One-way ANOVA was used for statistical analyses. *P values between 0.05 and 0.01; **P values between 0.01 and 0.001; ***P < 0.001.
Defective Signaling in Old B Progenitors Is Restored by Bcr-Abl Expression.
Given that Bcr-Abl is strongly selected for within aged B-progenitor pools, we asked whether Bcr-Abl expression could reverse impaired signaling in old progenitors. We determined the activation status of Akt, STAT3, STAT5, and Erk in BM progenitors expressing vector or Bcr-Abl at 3 wk posttransplantation. As observed for B progenitors isolated directly from young and old mice (Fig. 1B), Akt activation and, to a lesser extent, STAT5 activation were decreased in old B progenitors, indicating that these defects are hematopoietic-autonomous, because they were observed in young recipient mice (Fig. 2B and Fig. S7). Importantly, Bcr-Abl expression in both young and old progenitors led to potent activation of Akt and STAT5 (as well as Erk). Notably, the expression of constitutively active STAT5 in old EBPs has been shown to improve B-cell generation in vitro (5).
Decreased IL-7R–Mediated Signaling Promotes the Selection of Bcr-Abl–Expressing Cells.
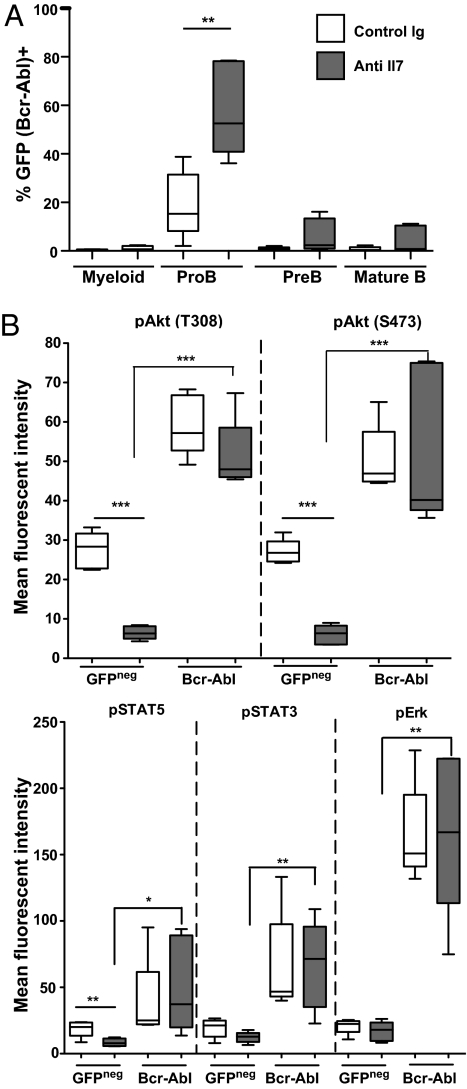
In vitro studies show that aged pro-B cells exposed to limiting IL-7 proliferate less than young pro-B cells, despite similar IL-7R expression levels (5, 20). Moreover, administration of antagonistic anti–IL-7 antibody to young mice has been shown to recapitulate the peripheral B-cell repertoire skewing observed in old mice (6). We asked whether reductions in IL-7R–mediated signaling would be sufficient to promote the expansion of Bcr-Abl–expressing B progenitors. Young BM progenitors transduced with p190 Bcr-Abl were transplanted into irradiated young recipients, which were then treated with anti–IL-7 or vehicle for 3 wk, at which point, contributions of Bcr-Abl+ cells to hematopoietic lineages and effects on cell signaling were determined. IL-7 neutralization resulted in a significant increase in the percentages of Bcr-Abl–expressing pro-B cells but not myeloid cells (Fig. 3A). In addition, neutralization of IL-7 resulted in substantial decreases in Akt and STAT5 activation but not STAT3 or ERK activation in nontransduced B progenitors (Fig. 3B). Notably, Bcr-Abl expression resulted in significant increases in the activation of Akt, STAT5, STAT3, and Erk under conditions of reduced IL-7 signaling. Thus, impairing IL-7–mediated signaling phenocopies aged B lymphopoiesis in several ways: reduced signaling via Akt and STAT5 and increased selection for Bcr-Abl expression, which can restore signaling.
Fig. 3.
Impairing IL-7–mediated signaling promotes selection for Bcr-Abl–expressing B progenitors. BALB/c mice were irradiated and prepopulated with young BM cells and then transplanted 4 d later with c-Kit–selected progenitors transduced with p190 Bcr-Abl as in Fig. 2A. Starting the next day, mice were injected i.p. with control IgG or anti–IL-7 neutralizing antibodies at 0.5 mg per mouse every 4 d for 3 wk. (A) Percentage of GFP+ cells among B220+ BM B progenitors was determined as in Fig. 2A by flow cytometry at 3 wk posttransplantation. (B) At 3 wk posttransplantation, BM cells were analyzed for P-Akt, P-STAT3, P-STAT5, and P-Erk in GFP+Bcr-Abl+ and nontransduced (GFPneg) B220+ BM progenitors as in Fig. 2B. Graphing was as in Fig. 1A, and a two-tailed Student's t test was used for statistical analyses. *P values between 0.05 and 0.01; **P values between 0.01 and 0.001; ***P < 0.001.
Aged Hematopoiesis Promotes Bcr-Abl–Dependent Leukemogenesis.
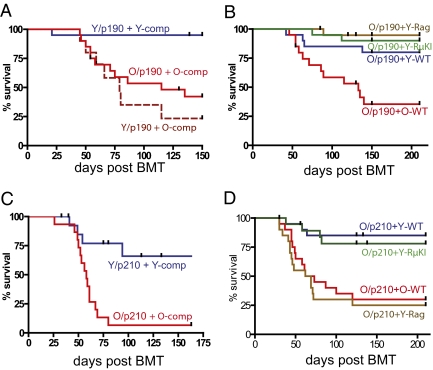
We next asked whether the increased competitive expansion of p190 Bcr-Abl–expressing B progenitors translates into increased leukemogenesis. Indeed, when MiG-p190–transduced young or old BM progenitors were transplanted into recipients prepopulated with either young or old BM competitors, p190 Bcr-Abl induced leukemias with much greater efficiency in the old background relative to the young, independent of the age of the Bcr-Abl–expressing progenitors (Fig. 4A). For p190-induced leukemias, morbid mice mostly exhibited aggressive B-lineage leukemias (Table S1), with high BM and lymph node blast counts. These leukemias were mostly pro-B cell–like (B220+CD19+CD43+AA4.1loIL7R+KitloIgMneg; Fig. S8A), with rearranged D-J heavy chains (Fig. S8B). Whereas the initial (3 wk posttransplantation) Bcr-Abl–driven B-progenitor expansion was polyclonal, as judged by heavy chain rearrangements, the resulting leukemias were mostly monoclonal (Fig. S8B). These results suggest that aging hematopoiesis augments Bcr-Abl–initiated leukemogenesis by increasing the initial expansion of Bcr-Abl–expressing B-progenitor clones, which, in turn, increases the probability that one of these clones transitions to leukemia following the acquisition of additional genetic and/or epigenetic events.
Fig. 4.
Aged lymphopoiesis promotes Bcr-Abl–initiated leukemia. (A) Young or old progenitors transduced with MiG-p190 Bcr-Abl (designated Y/p190 or O/p190) were transplanted into recipients prepopulated with either old or young BM (Y-comp or O-comp), as in Fig. 2A. Kaplan–Meier curves are plotted for leukemia-free survival (n >15 per group). comp, competitor. Two experiments are combined (P < 0.001 for Y + Y-comp vs. O + O-comp or Y + O-comp using the log-rank test). (B) Similar leukemia-free survival curves are shown, but all mice received p190 Bcr-Abl–transduced old progenitors, whereas the genotype or age of the BM (“competitor”) used to prepopulate recipients varied (n = 20 per group). Leukemia-free survival differences between mice receiving old competitors (O-WT) relative to Rag2−/−, RμKI, and young competitors are significant at P < 0.001, P < 0.01, and P < 0.05, respectively (log-rank test). (C and D) Experimental design was as in A and B, except that progenitors were transduced with MiG-p210 Bcr-Abl. (C) Difference between the curves is significant at P < 0.0002. (D) Comparisons of O/p210 + Y-WT or O/p210 + Y-RμKI vs. O/p210 + O-WT or O/p210 + Y-Rag are significant at P < 0.0001.
To determine if the observed age dependence of leukemogenesis is indeed attributable to defects in B-cell lymphopoiesis and to dissect which stage of B-cell lymphopoiesis is critical for the suppressive effect on leukemogenesis, we performed competitive BMT experiments using competitors from young mice with stage-specific defects in B lymphopoiesis, including Rag2−/− (pro-B cell block) and Rag2−/− μ-heavy chain knock-in (RμKI; pre-B cell block) mice (24). Rag2−/− and RμKI competitors were as effective as young WT competitors at preventing p190 Bcr-Abl–induced leukemias in old progenitors (Fig. 4B), suggesting that age-dependent selection for p190 Bcr-Abl at the pro-B stage or earlier underlies increased leukemogenesis.
Similar to p190 Bcr-Abl, p210 Bcr-Abl also induced leukemias with much greater potency in the aged hematopoietic system than in young mice (Fig. 4C). Again, both young WT and young RμKI BM effectively prevented p210 Bcr-Abl–induced leukemias initiated in old progenitors (Fig. 4D). Interestingly, Rag2−/− BM was as ineffective as old BM in preventing p210-initiated leukemias, contrasting with its ability to inhibit p190-initiated leukemogenesis. Thus, because Rag2−/− progenitors cannot progress to the pre-B cell stage, effective competition at the pre-B cell stage appears necessary to limit p210-initiated leukemias. It is also notable that most leukemias initiated by p210 in old progenitors with Rag2−/− competitors were myeloid (Table S1), contrasting with the predominance of B-lineage leukemias in other groups. Regardless, we can conclude that aging strongly promotes both p190- and p210-initiated leukemias (albeit with at least partially distinct mechanisms) attributable, in large part, to increased selection for Bcr-Abl at early B-progenitor stages.
Discussion
Data presented here demonstrate that aging increases the competitive advantage conferred by expression of Bcr-Abl in aged B-cell progenitors. The simplest explanation for this increased selective advantage, consistent with results presented here, is that aging results in reduced receptor-mediated kinase signaling in B progenitors, providing selective pressure for the expression of oncogenes like Bcr-Abl that restore signaling. Given that IL-7 is critical for B lymphopoiesis, that IL-7 signals via Akt and STAT5 (23), and that Bcr-Abl potently activates these signaling pathways, reduced signaling via Akt and STAT5 likely contributes to reduced B-progenitor fitness and increased adaptation by Bcr-Abl with age. Indeed, impairing IL-7–mediated signaling promoted selection for Bcr-Abl–expressing B progenitors in vivo. Nonetheless, the aging phenotype is clearly complex, and aging-associated fitness reductions in B progenitors as well as mechanisms for adaptation conferred by Bcr-Abl expression will certainly involve other factors beyond altered kinase signaling.
Although aging-related changes of B lymphopoiesis are influenced by systemic and microenvironmental factors (4), our studies focused on hematopoietic-autonomous contributions to aging-associated leukemogenesis, because all recipient mice were young. Of note, recipient mice were irradiated to allow for engraftment of transplanted BM, and irradiation-induced changes in the cellular microenvironment can influence tumorigenesis (25). Still, the effects of irradiation should be the same across experimental groups; yet, young and old progenitors exhibited clear differences in fitness, cell signaling, and oncogenic adaptation within young irradiated hosts as well as in ex vivo culture. The hematopoietic-autonomous nature of the aging-related defects observed in our experiments is further supported by the ability of young competitors to limit expansion of Bcr-Abl–expressing cells and leukemogenesis within aged B-cell progenitor pools. Together with the observation that the early expansions of Bcr-Abl–expressing progenitors are polyclonal, these data argue against the involvement of preaccumulated oncogenic mutations as being responsible for the age-dependent increase in Bcr-Abl–driven leukemias (at least in our model).
Although our results argue for the importance of changes autonomous to the hematopoietic system in aging-related leukemogenesis, these changes likely reflect only one of multiple mechanisms linking aging and increased incidence of malignancies. One consequence of aging is the accumulation of senescent cells in many tissues (8, 26), and senescent cells secrete a number of active molecules that can promote tumor growth (27). Another important hallmark of aging is the frequent persistence of chronic inflammation, which is a well-established tumor promoter (28). Furthermore, decreased progenitor cell fitness, and consequent inadequate production of mature progeny, should lead to increased homeostatic demand, resulting in higher levels of prosurvival growth factor signaling and, thereby, increased thresholds for triggering apoptosis or senescence by oncogenic mutations. Finally, the accumulation of genetic and epigenetic alterations over time results in increased heritable diversity within stem and progenitor cell populations of aged individuals, thus increasing the frequency of potentially oncogenic events that are adaptive within aged tissues. As a result, aging should alter the fitness landscape as well as the epigenetic/mutational diversity of tissues, providing contexts conducive to somatic evolution.
Although p210 Bcr-Abl more commonly leads to myeloid leukemias in humans, dominant promotion of B leukemias by both p190 and p210 in mice in our model may, in part, reflect the selective decline in B lymphopoiesis in aging mice. Notably, our results differ from those reported by Dorshkind and colleagues (29): although young BM cells transduced with MiG-p210 give rise to both myeloid and lymphoid leukemias in recipient mice, old p210-transduced BM cells give rise exclusively to myeloid leukemias, coinciding with defective B lymphopoiesis in aged mice. The resistance of aged B progenitors to transformation by Bcr-Abl was proposed to derive from increased expression of the Ink4a and Arf genes in old progenitors (30). The Dorshkind group used high transduction efficiency protocols with MiG-p210 (leading to 100% penetrant leukemias with young and old donor BM), priming with 5-fluorouracil [which is known to promote myeloid leukemias (31)], and the C57BL/6 background, and these differences could account for discrepancies in leukemia lineage bias. Of note, BALB/c mice, which were used in our studies, are hypomorphic for Ink4a (32).
The use of BM competitors blocked at different stages of lymphopoiesis in our studies provides further insight into why aged hematopoiesis promotes Bcr-Abl–initiated leukemias. Because Rag2−/− and RμKI mice are unable to generate mature T and B cells, the ability of these progenitors to prevent Bcr-Abl–mediated leukemias argues against the involvement of decreased immune surveillance in augmented leukemogenesis in the context of old hematopoiesis (at least in our model). Given that p190 and p210 fusions are predominantly associated with B-cell ALLs and CML in humans, respectively, the differential ability of Rag2−/− BM to provide competition for p190 and p210 Bcr-Abl–initiated progenitors is intriguing. One speculative possibility is that for both p190 and p210, young B progenitors up to the pro-B stage can effectively compete with Bcr-Abl–expressing progenitors at similar stages. Nevertheless, p210 but not p190 may allow progenitors (perhaps myeloid progenitors) to adapt to vacant pre-B niches that cannot be occupied by the deficient Rag2−/− competitors. Notably, myeloid progenitors can compete with pre-B cells for niches, and the numbers of available myeloid niches is increased in Rag1−/− mice (33).
We have previously proposed that stem and progenitor cells are highly adapted to their niches in a young healthy individual, minimizing selective pressure for adaptive changes (12). Cellular competition in young healthy progenitor pools appears to be tumor-suppressive, and the competitive elimination (at least partially genetically determined) of less fit cells appears to be important for the culling of damaged cells (34–36). When cellular fitness is reduced as a result of cell-intrinsic changes or alterations in the microenvironment, however, a progenitor cell population will no longer possess maximal fitness and certain oncogenic mutations will have an increased chance of being adaptive, and hence advantageous. Results presented here provide support for this model. We propose that in young lymphoid progenitors, efficient receptor signaling (e.g., via IL-7R) provides the “right amount” of activation of downstream effectors for optimal B-progenitor fitness. In this context, the activation of downstream effectors by Bcr-Abl expression will lead to excessive signaling beyond the levels needed for optimal fitness. In old B progenitors, however, receptor signaling is deficient; thus, Bcr-Abl is probably adaptive, in part, by increasing signaling via effectors shared by receptors like IL-7R. In simplistic terms, fitness reductions in aged progenitor pools create “room for improvement.” Although other aspects of aging certainly contribute to increasing cancer incidence, data presented here implicate oncogenic adaptation within poorly fitting progenitor pools in the strong association between age and tumorigenesis.
Materials and Methods
Young and old female BALB/c mice were purchased from the National Institute of Aging or the National Cancer Institute. Rag2−/− and RμKI mice (congenic in BALB/c) were kindly provided by Roberta Pelanda (National Jewish Health, Denver, CO). Flow cytometric analyses of phosphorylated signaling proteins (37) were as performed previously, with modifications. Additional flow cytometric analyses, retroviral transduction, BMT (omitting 5-fluorouracil priming), and pathological examinations were performed as described by Bilousova et al. (13). Statistical analysis was performed using GraphPad Prism 5 software. Detailed descriptions of experimental procedures are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Roberta Pelanda for her critical review of this manuscript, and the Cancer Center Flow Cytometry and Tissue Culture Cores (supported by National Institutes of Health Grant 2 P30-CA46934). These studies were supported by National Institutes of Health Grants RO1-CA109657 and P20-CA103680 (to J.D.) and Grant T32-AI07405 (to C.J.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005486107/-/DCSupplemental.
References
- 1.Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 2.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 3.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 4.Allman D, Miller JP. The aging of early B-cell precursors. Immunol Rev. 2005;205:18–29. doi: 10.1111/j.0105-2896.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Lescale C, et al. Reduced EBF expression underlies loss of B cell potential of hematopoietic progenitors with age. Aging Cell. 2010;9:410–419. doi: 10.1111/j.1474-9726.2010.00566.x. [DOI] [PubMed] [Google Scholar]
- 6.Guerrettaz LM, Johnson SA, Cambier JC. Acquired hematopoietic stem cell defects determine B-cell repertoire changes associated with aging. Proc Natl Acad Sci USA. 2008;105:11898–11902. doi: 10.1073/pnas.0805498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balducci L, Beghe’ C. Cancer and age in the USA. Crit Rev Oncol Hematol. 2001;37:137–145. doi: 10.1016/s1040-8428(00)00109-8. [DOI] [PubMed] [Google Scholar]
- 8.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg RA. The Biology of Cancer. New York: Garland Science; 2007. Multi-step tumorigenesis. [Google Scholar]
- 10.Lodish H, et al. Molecular Cell Biology. 6th Ed. New York: Freeman; 2008. Cancer. [Google Scholar]
- 11.Sieber OM, Tomlinson SR, Tomlinson IP. Tissue, cell and stage specificity of (epi)mutations in cancers. Nat Rev Cancer. 2005;5:649–655. doi: 10.1038/nrc1674. [DOI] [PubMed] [Google Scholar]
- 12.Marusyk A, DeGregori J. Declining cellular fitness with age promotes cancer initiation by selecting for adaptive oncogenic mutations. Biochim Biophys Acta. 2008;1785:1–11. doi: 10.1016/j.bbcan.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bilousova G, Marusyk A, Porter CC, Cardiff RD, DeGregori J. Impaired DNA replication within progenitor cell pools promotes leukemogenesis. PLoS Biol. 2005;3:e401. doi: 10.1371/journal.pbio.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172–183. doi: 10.1038/nrc1567. [DOI] [PubMed] [Google Scholar]
- 15.Vickers M. Estimation of the number of mutations necessary to cause chronic myeloid leukaemia from epidemiological data. Br J Haematol. 1996;94:1–4. doi: 10.1046/j.1365-2141.1996.d01-1751.x. [DOI] [PubMed] [Google Scholar]
- 16.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 17.Greer JP, et al., editors. Wintrobe's Clinical Hematology. Philadelphia: Lippincott Williams & Wilkins; 2004. 11th Ed, Vol 2. [Google Scholar]
- 18.Van Etten RA. Studying the pathogenesis of BCR-ABL+ leukemia in mice. Oncogene. 2002;21:8643–8651. doi: 10.1038/sj.onc.1206091. [DOI] [PubMed] [Google Scholar]
- 19.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 20.Stephan RP, Lill-Elghanian DA, Witte PL. Development of B cells in aged mice: Decline in the ability of pro-B cells to respond to IL-7 but not to other growth factors. J Immunol. 1997;158:1598–1609. [PubMed] [Google Scholar]
- 21.Riley RL, Blomberg BB, Frasca D. B cells, E2A, and aging. Immunol Rev. 2005;205:30–47. doi: 10.1111/j.0105-2896.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 22.Sherwood EM, Blomberg BB, Xu W, Warner CA, Riley RL. Senescent BALB/c mice exhibit decreased expression of lambda5 surrogate light chains and reduced development within the pre-B cell compartment. J Immunol. 1998;161:4472–4475. [PubMed] [Google Scholar]
- 23.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: Intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 24.Pelanda R, et al. Receptor editing in a transgenic mouse model: Site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 25.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment—Tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–875. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 26.Dimri GP, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrinello S, Coppe JP, Krtolica A, Campisi J. Stromal-epithelial interactions in aging and cancer: Senescent fibroblasts alter epithelial cell differentiation. J Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 29.Signer RA, Montecino-Rodriguez E, Witte ON, McLaughlin J, Dorshkind K. Age-related defects in B lymphopoiesis underlie the myeloid dominance of adult leukemia. Blood. 2007;110:1831–1839. doi: 10.1182/blood-2007-01-069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Signer RA, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Ilaria RL, Jr, Million RP, Daley GQ, Van Etten RA. The P190, P210, and P230 forms of the BCR/ABL oncogene induce a similar chronic myeloid leukemia-like syndrome in mice but have different lymphoid leukemogenic activity. J Exp Med. 1999;189:1399–1412. doi: 10.1084/jem.189.9.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang S, Ramsay ES, Mock BA. Cdkn2a, the cyclin-dependent kinase inhibitor encoding p16INK4a and p19ARF, is a candidate for the plasmacytoma susceptibility locus, Pctr1. Proc Natl Acad Sci USA. 1998;95:2429–2434. doi: 10.1073/pnas.95.5.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J Exp Med. 2005;201:1771–1780. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marusyk A, et al. Irradiation alters selection for oncogenic mutations in hematopoietic progenitors. Cancer Res. 2009;69:7262–7269. doi: 10.1158/0008-5472.CAN-09-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8:e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6:309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van De Wiele CJ, et al. Thymocytes between the beta-selection and positive selection checkpoints are nonresponsive to IL-7 as assessed by STAT-5 phosphorylation. J Immunol. 2004;172:4235–4244. doi: 10.4049/jimmunol.172.7.4235. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.