Abstract
In a natural environment, foragers constantly face the risk of encountering predators. Fear is a defensive mechanism evolved to protect animals from danger by balancing the animals’ needs for primary resources with the risk of predation, and the amygdala is implicated in mediating fear responses. However, the functions of fear and amygdala in foraging behavior are not well characterized because of the technical difficulty in quantifying prey–predator interaction with real (unpredictable) predators. Thus, the present study investigated the rat's foraging behavior in a seminaturalistic environment when confronted with a predator-like robot programmed to surge toward the animal seeking food. Rats initially fled into the nest and froze (demonstrating fear) and then cautiously approached and seized the food as a function of decreasing nest−food and increasing food−robot distances. The likelihood of procuring food increased and decreased via lesioning/inactivating and disinhibiting the amygdala, respectively. These results indicate that the amygdala bidirectionally regulates risk behavior in rats foraging in a dynamic fear environment.
Keywords: brain lesions, decision making, loss aversion, risk taking
Many organisms forage for resources (such as food, water, mate, and shelter) and information about their environment. Although essential for survival, foraging behavior also increases the likelihood of encountering dangers such as predators (1). Fear is a defensive mechanism thought to have evolved to protect animals from danger by balancing the animal's primary needs with the risk of predation (2–5). In 1939, Kluver and Bucy (6) revealed that lesions in the medial temporal lobe altered fear behavior in rhesus monkeys. Subsequent studies have identified the amygdala as the crucial brain structure concerned with fear in animals, including humans (7–9).
The contemporary views on the role of the amygdala in fear derived largely from animal studies using the Pavlovian fear-conditioning paradigm (10, 11), where an initially innocuous conditioned stimulus (CS; e.g., tone, light, context) is contingently paired with an aversive unconditioned stimulus (US; e.g., electric shock), which reflexively activates unconditioned responses (URs). The subject learns rapidly that a CS signals the impending US by displaying conditioned responses (CRs; e.g., freezing, potentiated startle, autonomic changes) that mimic fear URs. A large body of evidence from lesion, pharmacological, neurophysiological, molecular, and imaging studies point to the amygdala as the key structure subserving fear conditioning (12–17; but see ref. 18 for an alternative view). In humans, the amygdala also has been implicated in recognizing fear in facial expressions (9, 19). Nonetheless, how the amygdala's involvement in these fractional fear responses translates to behavior in the natural environment remains unclear.
Ethobehavioral studies have shown that rats display situation-specific defensive behaviors when encountering bigger animals (such as a cat or a human at varying proximity) (20, 21), predatory odors (such as the fox odor component 2,3,5-trimethyl-3-thiazoline or a cat odor) (22, 23), and moving objects (such as a toy humanoid robot hovering on a string) (24). Here, we investigated the functions of fear and amygdala in the rat's foraging behavior in a seminaturalistic environment when confronted with a predator-like Robogator (LEGO Mindstorms robot) programmed to surge toward the animal as it emerges from the nesting area in search of food (Fig. 1).
Fig. 1.
Foraging experimental design. (A) Baseline days. Snapshots show a rat emerging from a nest into a foraging area to search for pellets placed 25.4, 50.8, and 76.2 cm away. Once the animal returns with the food to the nest, the gateway closed until the next foraging trial. (B) Test day. Snapshots show the same rat foraging for a pellet 76.2 cm from the nest when confronted with a Robogator for the first time. Each time the animal approached the vicinity of the pellet (about ≤25 cm), the Robogator surged, snapped its jaws once, and returned to its original position. The rat immediately fled to the nesting area. A stationary Robogator did not obstruct foraging. (Video clips are available at http://faculty.washington.edu/jeansokk/Robogator.html).
Results
Fear, Amygdala, and Foraging Behavior.
Rats, implanted with lesion electrodes in the amygdalae and food-restricted, quickly learned to search for food pellets placed 25.4, 50.8, or 76.2 cm away from the acclimated nesting area in a large, open field (Fig. 1A). Upon procuring the pellet, the animals instinctively returned to the nest for consumption (Fig. S1). After 5–7 d of baseline foraging, the Robogator was positioned in the opposite end of the open field (Fig. 1B). As the animal emerged from the nest and approached the pellet, the Robogator surged a set distance (∼23 cm) toward the pellet, snapped its jaws, and then returned to its starting position. Upon the first encounter with the Robogator, all rats instantly fled into the nest and froze (an overt demonstration of fear). Flight to a familiar enclosure and freezing also have been reported as responses to a compound (tone + light) fear CS in rats (25). When animals reemerged from the nest, they made slow, cautious attempts toward the food. Each time the rat directly or circuitously approached the food, the Robogator surged, reliably evoking escape behavior in the animal (Fig. S2).
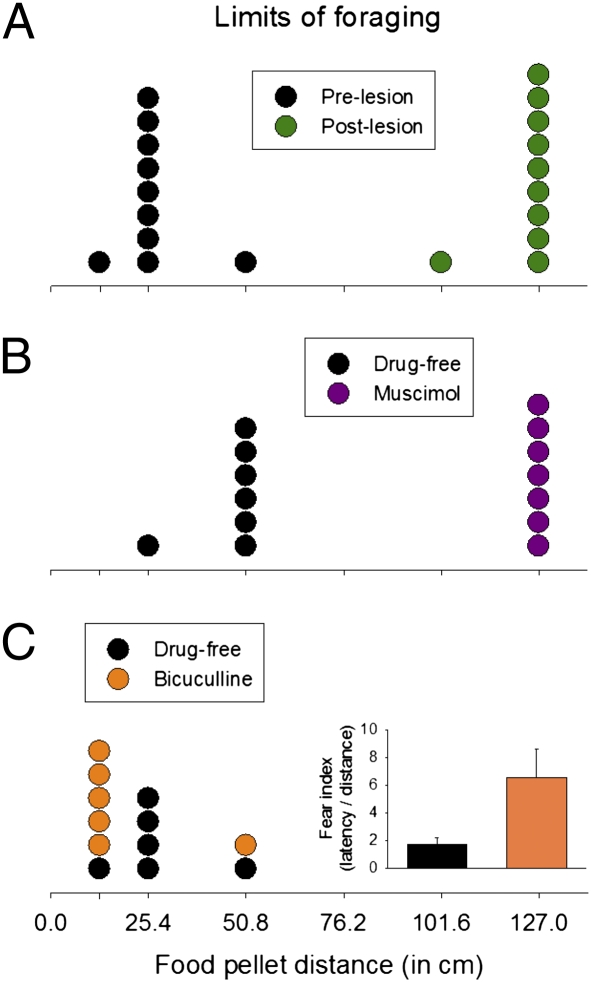
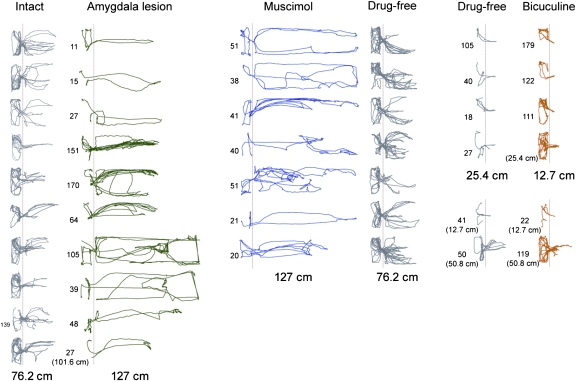
Whether the animal acquired the pellet depended on the nest–food distance. Although none of the animals procured the pellet at 76.2 cm within the 3-min allotted time, one rat succeeded at 50.8 cm, eight rats succeeded at 25.4 cm, and one rat retrieved the pellet at 12.7 cm (Fig. 2A). This result indicates that the rats encoded a spatial (or distance) gradient of fear near the Robogator. Afterward, under light halothane anesthesia, electrolytic lesions were made in the amygdalae (Fig. S3). The lesion resulted in dramatic changes in foraging behavior the next day, because nine animals obtained the pellet placed up to 127 cm away, and one animal succeeded at 101.6 cm. Amygdalar-lesioned rats paused momentarily until the Robogator retracted from its surge before snatching the pellet; a few animals closely explored the Robogator (which prolonged the latency) before seizing the pellet. All lesioned rats returned to the nest for consumption. Using the food procurement distances as ordinal data (Fig. 3), a sign test confirmed that the maximum distances of successful foraging behavior were significantly greater after lesions than before amygdalar lesions (S10 = 0, P < 0.002, two-sided).
Fig. 2.
Limits of foraging distance. (A) A frequency histogram of maximum successful foraging distances before (black; test day 1) and after (green; test day 2) amygdalar lesions. (B) Maximum successful foraging distances under intraamygdalar muscimol (purple; test day 1) and drug-free (black; test day 2) conditions. (C) Maximum successful foraging distances under drug-free (black; test day 1) and with intraamygdalar bicuculline (orange; test day 2) conditions. Inset shows fear index (latency/distance) between drug-free and bicuculline tests.
Fig. 3.
Track plots. (Left) Animals before and after amygdalar lesions. (Middle) Animals with intraamygdalar muscimol and drug-free conditions. (Right) Animals with drug-free and intraamygdalar bicuculline conditions. Numbers next to plots denote the latency (in seconds) to procure the food pellet successfully. The absence of a number signifies unsuccessful foraging. Dotted vertical lines demarcate the nest–foraging area boundary.
Reversible Inactivation of the Amygdala and Foraging Behavior.
To determine whether the lesion effects resulted from damage to the intrinsic amygdalar neurons or to the fibers of passage and whether reexposure to the Robogator on test day 2 might have reduced fear via an habituation (extinction)-like process, rats with guide cannulae in the amygdala (Fig. S3) were infused with the GABAA receptor agonist muscimol (0.876 nmol, 0.1 μL per side) 15–30 min before their first encounter with the Robogator. All rats showed virtually no escape response to the Robogator; they simply approached and paused (or explored) the Robogator before seizing the food placed farthest from the nest (i.e., 127 cm) and returning to the nest (Fig. 2B). On the following day, the animals were retested without muscimol. All seven rats failed to procure the food pellet placed 76.2 cm away within the criterion time. However, six rats seized the pellets placed 50.8 cm away, and one rat was able to take the pellet placed 25.4 cm away. A sign test showed that the maximum distances of successful foraging behavior were extended significantly in the intraamygdalar muscimol condition compared with the no-drug condition (S7 = 0, P < 0.02, two-sided) (Fig. 3). The fact that more animals secured pellets placed 50.8 cm away in the day 2 no-muscimol condition than in the day 1 prelesion condition (Fig. 2A) suggests that amygdalar-independent learning (e.g., familiarity with the Robogator) occurred in the intraamygdalar muscimol animals during day 1.
Disinhibition of the Amygdala and Foraging Behavior.
Because amygdalar lesions/inactivations blocked the rat's fear of the Robogator, we then tested whether intraamygdalar infusions of the GABAA receptor antagonist bicuculline methiodide (100 pmol, 0.2 μL per side) produced heightened fear. Previous studies have found that bicuculline infusions into the amygdala produce anxiogenic responses (26, 27), presumably by blocking the endogenous GABA inhibitory effects in the amygdala (28). Following the infusion, five of six animals showed increased latencies and/or shortened distances to secure pellets compared with the drug-free condition on the previous day (Fig. 2C). A paired t test, with latency/distance (s/cm) as a fear index, indicated that intraamygdalar bicuculline significantly elevated the fear of foraging compared with the drug-free condition (t6 = 2.78, P = 0.039, two-sided) (Fig. 3).
After testing with the Robogator, rats with lesions and cannulae implants underwent a standard contextual fear conditioning (Fig. S4). Amygdalar-lesioned animals displayed neither postshock freezing during training nor conditioned freezing during the next test day. Amygdalar-cannulated animals showed robust postshock freezing (when trained drug-free), virtually no conditioned freezing when tested with muscimol infusions (day 2), and strong conditioned freezing when retested drug-free (day 3). These results indicate that amygdalar lesions and muscimol infusions used in the present study were comparably effective to those used in fear-conditioning studies (29, 30).
Discussion
Most laboratory studies of fear assess the animal's tendency to emit specific CRs (e.g., freezing) or instrumental responses (e.g., avoidance) following a dermal pain caused by electric shock in small enclosures (10–18). Although effective in producing robust fear responses, this approach limits or does not address the functional aspect of fear as defensive behavior. In contrast, the present study used an ethologically plausible seminaturalistic environment (see also refs. 4 and 5) and a programmed predator-like robot (Robogator) to investigate the functions of fear and amygdala on rats’ foraging behavior. The Robogator effectively mimicked a naturalistic threat because its size is relatively larger than the rat, and its shape (with eyes, moving jaw, and tail) and surging action simulate a predatory strike. The utilization of a robot, moreover, allowed relatively reliable and quantitative interaction with the rats, which is not possible with real (but unpredictable) predatory animals.
Our findings indicate that rats’ foraging behavior is regulated by fear and the amygdalar activity. Specifically, the Robogator's brief surge action pattern evoked a coordinated set of defensive behaviors (i.e., fleeing and freezing in the nest) followed by risk-assessment behavior comprised of stretched posture anchored near the gateway (to scan and monitor the foraging area) before cautiously venturing out toward the food pellet until the surging Robogator retriggered the rat's defensive behavior. However, the Robogator's disrupting effects on the animal's foraging varied as a function of nest−food and food−robot distances. Although none of the rats were able to sequester the pellet placed 76.2 cm from the nest, all animals were successful when the food was placed ≤25.4 cm away. This adaptive adjustment of foraging behavior by fear is in accord with the models of “predatory imminence” (4) and “(risk assessment-based) antipredator defensive behavior” (5) that postulate fear responses as a coordinated reaction to the specific threat situation and its perceived proximity.
Fleeing followed by freezing in the nest are URs, because they are both innately and reliably elicited by the surging Robogator US (but see ref. 25). However, the changes in rats’ venturing behavior with repeated experience with the Robogator indicate that learning is involved also. For instance, the foraging area may become a contextual (spatial) CS associated with the fear generated by the Robogator US that evokes an instrumental avoidance response (e.g., 18, 31). Future studies need to reveal the extraamygdalar learning components and how they supply the animals with “a representation of the causal structure” (32) in the foraging environment with a potential threat.
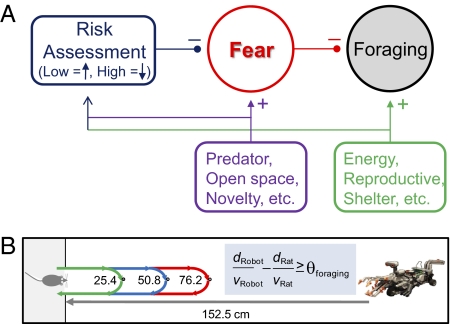
What, then, is the basis of the rats’ decision to accept the risk and attain pellets placed ≤25.4 cm but not 76.2 cm from its nest? A motivational hypothesis (2, 4, 5) would posit that the farther the food is from the nest, the more strongly the fear motivation for self-preservation inhibits the hunger motivation for foraging (Fig. 4A). To expand this qualitative approach food−avoid predator conflict, we suggest incorporating a simple quantitative model of predation risk assessment based on the animal's velocity (VA, ∼88 cm/s; Fig. S4), the Robogator's velocity (VR, ∼75 cm/s), the distance the animal traverses from nest-to-food-to-nest (dN-F-N), and the distance from the Robogator's position to the nest (dR-N) (Fig. 4A). The likelihood of foraging then is determined by a simple comparator mechanism that gauges the margin of safety based on the animal's estimate of the time to reach the food and carry it back to the nest (tA = dN-F-N/VA) contrasted with the animal's estimate of the time needed for the Robogator to reach the nest from its position (tR = dR-N/VR). Foraging is likely to occur if the margin of safety is significantly greater than a threshold (tR − tA ≥ θsafety), and foraging does not occur if the tR − tA ≤ θsafety. For example, when the food is placed at 76.2 cm, the estimated time for the animal to complete the pellet procurement is ∼1.73 s (excluding the delay in seizing the pellet), and the estimated time for the Robogator to reach the nest is ∼1.81 s. At this distance, the margin of safety is too narrow and may lead to a fatal outcome for the rat. In contrast, when the food is placed 25.4 cm away, the estimated time for food procurement is dramatically shortened, creating a much wider margin of safety. The proposed model of predation risk assessment, which is essentially a “two trains” puzzle (33), is simply a scheme to illustrate how time to respective goals (distance/velocity) of prey and predator can be used to conceptualize risk-taking behavior of prey. Whether this simple model is valid will require detailed parametric studies (e.g., determining whether θsafety is a linear or nonlinear function of the Robogator's size and location) and elaborations (e.g., identifying neurocomputational processes of perceived speeds of both the animal itself and the predator) (1, 4, 20, 21). Nonetheless, the fact that rats do not simply avoid foraging altogether in the presence of the Robogator but instead make repeated efforts (from different directions) to procure the food indicates the utilization of risk assessment on the part of the animal. Perhaps timing or anticipatory control mechanisms—hypothesized in other behaviors such as interval timing in associative learning (34–36)—might have relevance to the present finding of predation risk-taking and foraging behavior.
Fig. 4.
Hypothetical accounts of foraging behavior under predatory threat. (A) A qualitative model posits that a foraging hungry animal is effectively in an approach food–avoid predator conflict situation. (B) A simple quantitative model suggests that the animal's foraging behavior varies as a function of the subject's speed (VA), the predator's speed (VR), the distance the subject navigates to acquire food and back to safety (dN-F-N), and the pursuit distance of the predator (dR-N). (See Discussion for explanation.)
Consistent with the motivational notion of fear inhibiting hunger for foraging, the propensity to secure food increased dramatically when the amygdala was lesioned or inactivated. With lesions/inactivation, rats evidently were not frightened by the Robogator's sudden movement; they momentarily paused but did not run back to the nest. This effect was not nonspecific, because the animals’ behavior of taking the pellet back to the nest for consumption was unaffected. Conversely, with pharmacological disinhibitions of the amygdala, animals’ fear of the Robogator increased, as evidenced by increased latency and/or decreased distance required to procure the pellet. These results indicate that the amygdala bidirectionally regulates predation risk–foraging behavior in a dynamic fear environment. Without the amygdala and consequently devoid of fear, the animal's foraging behavior becomes perilously maladaptive. Conversely, an overactive amygdala will hinder foraging behavior even under safe circumstances and thereby reduce the animal's biological fitness.
A functional MRI study (37) initially reported increased amygdalar blood flow/oxygenation in normal subjects engaged in a prospective negative monetary outcome task, implicating the amygdala in human risky decisions and loss aversion (38). In accordance, a recent study found that two human subjects with focal bilateral lesions of the amygdala (caused by Urbach–Wiethe disease) displayed a significant reduction in loss aversion in a gambling task (39). By demonstrating the involvement of amygdala in risk behavior related to predation in foraging rats, the present study raises an intriguing possibility that the phenomenon of loss aversion embodies the basic survival instinct in animals and therefore can be investigated at molecular-genetic levels using rodents.
In conclusion, the utilization of rats’ foraging behavior in a seminaturalistic, dynamic fear environment presents an avenue for investigating the neurobiology of fear and predation-risk behaviors. This approach might be useful in revealing how the amygdala and its associated circuitry are involved in other risk-taking and thrill-seeking behaviors in humans (40), in screening drug effects that may not translate accurately to human conditions based on conventional fear-conditioning studies, and in addressing the neuronal basis of the basic approach–avoid conflicts that may contribute to human psychopathologies.
Materials and Methods
Subjects.
Experimentally naïve male Charles River Long-Evans rats (initially weighing 275–300 g) were housed individually in the Department of Psychology animal care facility at the University of Washington (accredited by the Association for Assessment and Accreditation of Laboratory Animal Care) and were maintained on a reverse 12-h light-dark cycle (lights on at 1900 hours). Animals were placed on a standard food-deprivation schedule with free access to water to reach gradually and then maintain 80–85% of their normal weight. Experiments were conducted during the dark phase of the cycle, in strict compliance with the University of Washington Institutional Animal Care and Use Committee guidelines.
Amygdala Surgery.
Under anesthesia (30 mg/kg ketamine and 2.5 mg/kg xylazine, i.p.), rats were mounted in a stereotaxic instrument (Stoelting) and were implanted chronically with either custom-made electrolytic electrodes or guide cannulae (Plastics One Inc.) bilaterally into the amygdalae. Lesion electrodes were made of two stainless steel insect pins (#00) insulated with epoxy (except for ∼0.5 mm at the tip), with the tips spaced ∼0.8 mm laterally and ∼1.0 mm vertically. Stereotaxic coordinates for lesion electrodes were (referenced from bregma) anteroposterior (AP) – 2.5; mediolateral (ML) ± 4.0 (4.8) and ± 7.4 (8.4) mm. Stereotaxic coordinates for 26-gauge guide cannulae (Plastics One Inc.,) were AP – 2.5, ML ± 4.5–5.0 and ± 7.4 mm. Implanted lesion electrodes and cannulae were cemented to the skull with four anchoring screws. All rats were given 5–7 d of surgical recovery and daily handling before the experimental procedures began.
Lesion and Drug Infusion.
Amygdalar lesions were made under light halothane anesthesia (a gas mask was placed on the animal while it was gently restrained) (41, 42) by passing constant current (1 mA, 10 s; Grass Medical Instruments) through each tip. Muscimol-free base and bicuculline methiodide (Sigma-Aldrich) dissolved in artificial cerebrospinal fluid (pH ∼7.4) were microinfused into the amygdala bilaterally by backloading the drugs up a 33-gauge infusion cannula into polyethylene (PE 20) tubing connected to 10-μL Hamilton microsyringes (Hamilton Company). The infusion cannula protruded 1 mm beyond the guide cannula. Infusion volumes of 0.1 μL and 0.2 μL per side were delivered for muscimol and bicuculline, respectively, using a Harvard PHD2000 syringe pump (Harvard Apparatus) at a rate of 0.1 μL/min. The infusion cannula remained in place for 30 s after the infusion. The dosages and volumes of muscimol (0.876 nmol, 0.1 μL per side) and bicuculline (100 pmol, 0.2 μL per side) used are well within the intraamygdalar infusion parameters used in fear conditioning (43, 44) and anxiety (26, 27) studies, as well as in recent in fluorescent imaging of muscimol spread in the amygdala (45, 46).
Foraging Apparatus.
A custom-built seminaturalistic apparatus consisted of a nesting area (29.21 cm length × 57.12 cm width × 59.69 cm height; equipped with a water bottle; 16.2 Lux illuminance) with a remotely controlled gateway (10 cm × 10 cm) to an adjacent foraging area (201.93 cm length × 58.42 cm width × 60.96 cm height; 56.7 Lux; 60 dB white noise, A scale). The ANY-maze video tracking system (Stoelting Co.), with a video feed from an ultra-digital wireless camera (LW2101; Lorex Technology Inc.) affixed over the apparatus and connected to a Sony HD DVD recorder (RDR-HX900), was used to capture video images and automatically track the animal's movement (30 frames/s) from both nesting and foraging areas.
Robot Device.
A Mindstorms robot (LEGO Systems), in a figure of Robogator on wheels (66.04 cm length, 17.78 cm width, 15.24 cm height), was programmed to surge 23 cm (at a velocity of ∼75 cm/s), snap its jaw once (at an angular velocity 44.4 rpm), and return to its starting position. A wireless mini-video camera (RC-12; RF Systems), attached next to the Robogator's jaw, was used to transmit a live video of the rat's response to the robot wirelessly to a digital video recorder.
Behavioral Procedures.
Rats maintained on 80–85% normal body weight underwent successive stages of habituation, baseline foraging, and robot encounter.
Habituation days.
Animals were placed in the nesting area for 30 min/d for 3 consecutive days with three food pellets (grain-based, 2.0–2.5 g) in the nest to acclimatize to the nesting area.
Baseline days.
After a minute in the nesting area (no food pellets), the gateway to the foraging area opened, and the animal was allowed to explore and search for a food pellet placed 25.4 cm from the nest area (first trial). As soon as the animal took the pellet back inside the nest, the gateway closed. Once the animal finished consuming the pellet, the second foraging trial (with the pellet placed 50.8 cm from the nest area) and then the third foraging trial (with the pellet placed 76.2 cm from the next area) started in the same manner. Animals underwent 5–7 consecutive days of baseline foraging.
Robot encounter days.
There were two consecutive robot test days with the Robogator placed at the opposite end of the foraging area. The food pellet was placed at the 76.2-cm location on the first encounter with the Robogator. After the gateway opened, each time the animal approached the vicinity (∼25 cm) of the pellet, the Robogator surged 23 cm toward the pellet, snapped its jaws once, and returned to its original position. Animals were permitted 3 min to procure the pellet. If the rat was unsuccessful, the gate was closed with the animal inside the nesting area, and the food pellet was placed 25.4 cm closer to the nest on the following trial. If the rat successful, the pellet was placed farther from the nest (in 25.4-cm steps; maximum distance, 127 cm) on succeeding trials. The furthermost nest–food foraging distance and the latency required for the animal to procure the pellet successfully (i.e., the time from the gate opening to the rat's returning to the nest with the pellet) served as the dependent variables.
Histology.
At the completion of behavioral testing, animals were overdosed with Buthanesia and perfused intracardially with 0.9% saline followed by 10% buffered formalin. The brains were removed and stored in 10% formalin overnight and then kept in 30% sucrose solution until they sank. Transverse 50-μm sections were taken through the extent of the lesion and cannulae, mounted on gelatin-coated slides, and stained with cresyl violet and Prussian blue dyes (cf. refs. 29 and 46).
Statistical Analyses.
The maximum distances (12.7, 25.4, 50.8, 76.2, 101.6, and 127 cm) at which the animals successfully procured pellets within the 3-min criterion were scored as ordinal data and analyzed with a sign test (47). For data with overlapping distances (i.e., the bicuculline experiment), a paired t test was performed on fear index (computed as latency/distance, s/cm) measures. The baseline pellet procurement latency and freezing data were analyzed using repeated-measures ANOVA.
Supplementary Material
Acknowledgments
We thank Paul E. M. Phillips and Ross A. McDevitt for comments on the manuscript, Truc T. Hang and Eun Joo Kim for assistance in the experiment, and Jeremy J. Kim for the idea of using the Mindstorms robot. This study was supported by the National Research Foundation of Korea Grant MEST 2009-008145 funded by the Korean government, by Grant 2009K001284 from the Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology, the Republic of Korea, by the Seoul Broadcasting System Foundation (to J.-S.C.), and by National Institutes of Health Grant MH64457 and by the James McKeen Cattell Sabbatical Award (to J.J.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010079108/-/DCSupplemental.
References
- 1.Stephens DW, Krebs JR. Foraging Theory. Princeton, NJ: Princeton Univ Press; 1986. [Google Scholar]
- 2.Bolles RC. Species-specific defense reactions and avoidance learning. Psychol Rev. 1970;77:32–48. [Google Scholar]
- 3.Seligman MEP. On the generality of the laws of learning. Psychol Rev. 1970;77:406–418. [Google Scholar]
- 4.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Beecher MD, editor. Evolution and Learning. Hillsdale, NJ: Erlbaum; 1988. pp. 185–211. [Google Scholar]
- 5.Blanchard RJ, Blanchard DC. Anti-predator defense as models of fear and anxiety. In: Brain PF, Blanchard RJ, Mainardi D, editors. Fear and Defense. Chur, Switzerland: Harwood Academic Publishers; 1990. pp. 89–108. [Google Scholar]
- 6.Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 7.MacLean PD, Delgado JMR. Electrical and chemical stimulation of frontotemporal portion of limbic system in the waking animal. Electroencephalogr Clin Neurophysiol. 1953;5:91–100. doi: 10.1016/0013-4694(53)90056-x. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 9.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 10.LeDoux JE. Emotion, memory and the brain. Sci Am. 1994;270:50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- 11.Fanselow MS. What is conditioned fear? Trends Neurosci. 1984;7:460–462. [Google Scholar]
- 12.Maren S, Fanselow MS. The amygdala and fear conditioning: Has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 13.Davis M. Neurobiology of fear responses: The role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 14.Paré D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 15.Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: A cellular and molecular perspective. Trends Neurosci. 2001;24:540–546. doi: 10.1016/s0166-2236(00)01969-x. [DOI] [PubMed] [Google Scholar]
- 16.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci Biobehav Rev. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGaugh JL. Memory—a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 19.Adolphs R, et al. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard RJ, Blanchard DC. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(Suppl):S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 21.Blanchard RJ, Blanchard DC, Hori K. An ethoexperimental approach to the study of defense. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S, editors. Ethoexperimental Approaches to the Study of Behavior. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1989. pp. 114–136. [Google Scholar]
- 22.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: Some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Dielenberg RA, Hunt GE, McGregor IS. “When a rat smells a cat”: The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience. 2001;104:1085–1097. doi: 10.1016/s0306-4522(01)00150-6. [DOI] [PubMed] [Google Scholar]
- 24.Tiffany PB, Mollenauer S, Plotnik R, White M. Olfactory bulbectomy: Emotional behavior and defense responses in the rat. Physiol Behav. 1979;22:311–317. doi: 10.1016/0031-9384(79)90092-1. [DOI] [PubMed] [Google Scholar]
- 25.de Oca BM, Minor TR, Fanselow MS. Brief flight to a familiar enclosure in response to a conditional stimulus in rats. J Gen Psychol. 2007;134:153–172. doi: 10.3200/GENP.134.2.153-172. [DOI] [PubMed] [Google Scholar]
- 26.Sanders SK, Shekhar A. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 1995;52:701–706. doi: 10.1016/0091-3057(95)00153-n. [DOI] [PubMed] [Google Scholar]
- 27.Soltis RP, Cook JC, Gregg AE, Sanders BJ. Interaction of GABA and excitatory amino acids in the basolateral amygdala: Role in cardiovascular regulation. J Neurosci. 1997;17:9367–9374. doi: 10.1523/JNEUROSCI.17-23-09367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanselow MS, Kim JJ. The benzodiazepine inverse agonist DMCM as an unconditional stimulus for fear-induced analgesia: Implications for the role of GABAA receptors in fear-related behavior. Behav Neurosci. 1992;106:336–344. doi: 10.1037//0735-7044.106.2.336. [DOI] [PubMed] [Google Scholar]
- 29.Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 30.Muller J, Corodimas KP, Fridel Z, LeDoux JE. Functional inactivation of the lateral and basal nuclei of the amygdala by muscimol infusion prevents fear conditioning to an explicit conditioned stimulus and to contextual stimuli. Behav Neurosci. 1997;111:683–691. doi: 10.1037//0735-7044.111.4.683. [DOI] [PubMed] [Google Scholar]
- 31.Choi J-S, Cain CK, LeDoux JE. The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem. 2010;17:139–147. doi: 10.1101/lm.1676610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–352. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- 33.Weisstein EW. Two trains puzzle. MathWorld-A Wolfram Web Resource. 1999. http://mathworld.wolfram.com/TwoTrainsPuzzle.html.
- 34.Balsam PD, Gallistel CR. Temporal maps and informativeness in associative learning. Trends Neurosci. 2009;32:73–78. doi: 10.1016/j.tins.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balci F, Freestone D, Gallistel CR. Risk assessment in man and mouse. Proc Natl Acad Sci USA. 2009;106:2459–2463. doi: 10.1073/pnas.0812709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilhardi P, Keen R, MacInnis MLM, Church RM. How rats combine temporal cues. Behav Processes. 2005;69:189–205. doi: 10.1016/j.beproc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Kahn I, et al. The role of the amygdala in signaling prospective outcome of choice. Neuron. 2002;33:983–994. doi: 10.1016/s0896-6273(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 38.Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–292. [Google Scholar]
- 39.De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci USA. 2010;107:3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C-S, Chao HH-A, Lee T-W. Neural correlates of speeded as compared with delayed responses in a stop signal task: An indirect analog of risk taking and association with an anxiety trait. Cereb Cortex. 2009;19:839–848. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCormick DA, Steinmetz JE, Thompson RF. Lesions of the inferior olivary complex cause extinction of the classically conditioned eyeblink response. Brain Res. 1985;359:120–130. doi: 10.1016/0006-8993(85)91419-2. [DOI] [PubMed] [Google Scholar]
- 42.Brunzell DH, Kim JJ. Fear conditioning to tone, but not to context, is attenuated by lesions of the insular cortex and posterior extension of the intralaminar complex in rats. Behav Neurosci. 2001;115:365–375. [PubMed] [Google Scholar]
- 43.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19:RC48:1–5. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maren S, Yap SA, Goosens KA. The amygdala is essential for the development of neuronal plasticity in the medial geniculate nucleus during auditory fear conditioning in rats. J Neurosci. 2001;21:RC135:1–6. doi: 10.1523/JNEUROSCI.21-06-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen TA, et al. Imaging the spread of reversible brain inactivations using fluorescent muscimol. J Neurosci Methods. 2008;171:30–38. doi: 10.1016/j.jneumeth.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Graham LK, Yoon T, Kim JJ. Stress impairs optimal behavior in a water foraging choice task in rats. Learn Mem. 2010;17:1–4. doi: 10.1101/lm.1605510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Howell DC. Statistical Methods for Psychology. Belmont, CA: Thomson Wadsworth; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.