Abstract
Group I metabotropic glutamate receptors (mGluR1/5) are important to synaptic circuitry formation during development and to forms of activity-dependent synaptic plasticity. Dysregulation of mGluR1/5 signaling is implicated in some disorders of neurodevelopment, including fragile X syndrome, the most common inherited form of intellectual disabilities and leading cause of autism. Site(s) in the intracellular loops of mGluR1/5 directly bind caveolin-1, an adaptor protein that associates with membrane rafts. Caveolin-1 is the main coat component of caveolae and organizes macromolecular signaling complexes with effector proteins and membrane receptors. We report that long-term depression (LTD) elicited by a single application of the group I mGluR selective agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) was markedly attenuated at Schaffer collateral-CA1 synapses of mice lacking caveolin-1 (Cav1−/−), as assessed by field recording. In contrast, multiple applications of DHPG produced LTD comparable to that in WT mice. Passive membrane properties, basal glutamatergic transmission and NMDA receptor (NMDAR)-dependent LTD were unaltered. The remaining LTD was reduced by anisomycin, an inhibitor of protein synthesis, by U0126, an inhibitor of MEK1/2 kinases, and by rapamycin, an inhibitor of mammalian target of rapamycin (mTOR), suggesting mediation by the same mechanisms as in WT. mGluR1/5-dependent activation (phosphorylation) of MEK and extracellular signal-regulated kinase (ERK1/2) was altered in Cav1−/− mice; basal phosphorylation was increased, but a single application of DHPG had no further effect, and after DHPG, phosphorylation was similar in WT and Cav1−/− mice. Taken together, our findings suggest that caveolin-1 is required for normal coupling of mGluR1/5 to downstream signaling cascades and induction of mGluR-LTD.
Group I metabotropic glutamate receptors (mGluRs), mGluR1 and mGluR5, are G protein-coupled receptors enriched at excitatory synapses throughout the brain, where they regulate neuronal excitation (1, 2). mGluR1/5s play an important role in the establishment of synaptic circuitry during brain development (3, 4) and in activity-dependent forms of synaptic plasticity, including long-term potentiation and long-term depression as occur in associative learning (5–8). Dysregulated mGluR1/5 signaling is implicated in neurological, psychiatric, and cognitive disorders (8), including fragile X syndrome, the most common inherited cause of intellectual disabilities (9, 10). The broad spectrum of physiological and pathological functions in which mGluRs participate is related to their capacity to initiate diverse signaling events by G protein-dependent and -independent mechanisms (11, 12).
Membrane rafts (13, 14) and caveolae (15) are specialized membrane domains of the plasma membrane, enriched in cholesterol and glycosphingolipids, that serve as platforms to compartmentalize specific signaling activities at the cell surface. Caveolin-1 is an adaptor protein that interacts with an array of receptors, including mGluR1 and mGluR5 (16–18) and effector proteins, and modifies signaling strength and duration by regulating the activity of its interacting partners (19–21). Binding to caveolin-1 attenuates the rate of mGluR1 constitutive internalization and attenuates mGluR1-mediated activation of extracellular signal-regulated kinase (ERK) signaling (16). Cortical neurons from Cav1−/− mice show enhanced basal ERK1/2 phosphorylation and prolonged phosphorylation/activation of ERK1/2 in response to stimulation by the group I mGluR-selective agonist DHPG (16). However, the full impact of caveolin-1 on synaptic function and plasticity is unclear.
Activation of group I mGluRs elicits long-term depression (mGluR-LTD) at Schaffer collateral-CA1 (Sch-CA1) synapses, a form of NMDA receptor (NMDAR)-independent synaptic plasticity (22) that requires de novo protein synthesis in the adult (23–27). mGluR-LTD induced by DHPG is absent in the mGluR5 KO mouse (24). In the present study, we examined mGluR-LTD at these synapses in mice lacking caveolin-1 (Cav1−/−). Cav1−/− mice are viable and fertile (28, 29), although they develop pathological features including vascular and pulmonary dysfunction and impaired liver regeneration (15). They do not show gross neuroanatomical abnormalities, but they do exhibit motor and behavioral deficits, including decreased exploratory activity, impaired spatial memory, and increased anxiety (30, 31).
Here, we report that mGluR-LTD at Schaffer collateral-CA1 synapses of Cav1−/− mice induced by a single brief (5 min) application of (RS)-3,5-dihydroxyphenylglycine (DHPG) was markedly reduced relative to that in wild-type (WT) mice. Increasing the duration of DHPG application had little or no effect on the magnitude of LTD. Multiple applications of DHPG increased the LTD; the saturating level was the same in Cav1−/− mice as in WT, but more applications were required. Basal glutamatergic synaptic transmission, presynaptic function, and NMDAR-dependent LTD were normal. Basal phosphorylation of MEK and ERK1/2, signaling kinases required for the induction of mGluR-LTD, were elevated in the hippocampus of Cav1−/− mice, but were not further increased by brief application of DHPG. Together, these findings suggest that caveolin-1 orchestrates signaling events required for efficacious induction of mGluR-LTD at CA1 synapses.
Results
mGluR-LTD at Sch-CA1 Synapses Is Impaired in Cav1−/− Mice.
Activation of group I mGluRs (mGluR1/5) with the selective agonist DHPG (32) elicits a form of homosynaptic long-term depression (chemical mGluR-LTD) of synaptic transmission at Schaffer collateral to CA1 (Sch-CA1) pyramidal cell synapses that is independent of NMDA receptor (NMDAR) activation (24, 33). To examine whether caveolin-1 has a role in mGluR-LTD, we applied DHPG (100 μM, 5 min) to hippocampal slices from WT and Cav1−/− mice aged 20–34 d (Fig. 1A). In WT slices, DHPG produced a robust depression of evoked field potential (fEPSP) slope values to 76 ± 3% of pre-DHPG baseline, assessed at 50–55 min after DHPG (n = 10; Fig. 1A), consistent with previous reports (24). In contrast, in slices from Cav1−/− mice, DHPG produced less mGluR-LTD (depression of fEPSPs to 90 ± 3% of baseline, n = 10, P < 0.01 Cav1−/− vs. WT; Fig. 1A). The short-term depression of fEPSPs, measured at 4–6 min after onset of DHPG application, was also decreased in Cav1−/− compared with WT mice (Cav1−/− to 67 ± 4%, n = 10, vs. WT to 51 ± 4%, n = 10; P < 0.05 Cav1−/− vs. WT). Attenuation of short- and long-term synaptic depression in response to DHPG was also observed in slices from P10-11 Cav1−/− mice (Fig. S1), indicating that caveolin-1 also affects mGluR-LTD at earlier ages. However, the mechanisms are likely to be different, because at this early age, mGluR-dependent LTD is presynaptic and not dependent on protein synthesis (34). Application of DHPG in the presence of the selective mGluR1 antagonist LY367385 (100 μM) together with the mGluR5 antagonist MPEP (10 μM) to Cav1−/− slices failed to induce LTD of fEPSPs (responses were 99 ± 2% of baseline, n = 6; Fig. 1B), consistent with a requirement for group I mGluR activation. Together, these findings indicate that both short- and long-term mGluR1/5-dependent synaptic depression induced by a single application of DHPG are markedly decreased at CA1 synapses of mice lacking caveolin-1.
Fig. 1.
DHPG-induced LTD at Sch-CA1 synapses is attenuated in Cav1−/− mice, whereas NMDAR-dependent LTD is normal. The fEPSPs in the CA1 region were evoked by stimulation of Schaffer collateral axons; LTD was induced by bath-application of RS-DHPG (100 μM, 5 min). The fEPSP slope (mean ± SEM) is plotted as the percent of pre-DHPG baseline. Insets show representative fEPSPs (average of three to five traces) at times indicated by numbers (baseline: thick gray line, posttreatment, thin black line). (A) LTD induced by DHPG (5 min) was attenuated in Cav1−/− mice compared with that in WT mice (for each genotype n = 5 mice, n = 10 slices, P < 0.01). (B) Incubation with mGluR1 (LY367385, 100 μM) and mGluR5 (MPEP, 10 μM) antagonists blocked DHPG-induced LTD in Cav1−/− mice (n = 3 mice, n = 6 slices). (C) LTD induced by low-frequency stimulation (LFS; 1 Hz, 20 min) of Schaffer collateral axons. The fEPSP slope (mean ± SEM) is plotted as the percent of the pre-LFS baseline. LFS-induced LTD was not altered in Cav1−/− mice compared with WT (WT, n = 9; Cav1−/−, n = 8; P = 0.342). (D) LFS-induced LTD was blocked by D-APV in Cav1−/− mice (n = 6).
NMDAR-LTD at Sch-CA1 Synapses Is Normal in Cav1−/− Mice.
To examine whether caveolin-1 is required for mGluR-independent forms of LTD, we examined NMDAR-LTD at CA1 synapses of Cav1−/− mice. We delivered low-frequency stimulation (LFS; 1,200 pulses at 1 Hz) to Schaffer collateral axons and recorded fEPSCs in area CA1 (35–37). LFS induced robust depression of fEPSPs in slices from both WT and Cav1−/− mice, as assessed 35–40 min after stimulation (WT depression to 83 ± 5% of baseline, n = 9; Cav1−/− depression to 79 ± 4% of baseline, n = 8; P > 0.05 Cav1−/− vs. WT, P < 0.001 Cav1−/− or WT vs. baseline; Fig. 1C). To confirm the dependence of this form of synaptically induced LTD on activation of NMDARs, LFS was delivered in the presence of the selective NMDAR antagonist D-APV (50 μM). Application of D-APV abolished LFS-LTD in slices from Cav1−/− mice (Fig. 1D). Collectively, these observations indicate that the cellular machinery underlying this form of LTD remains intact in Cav1−/− mice.
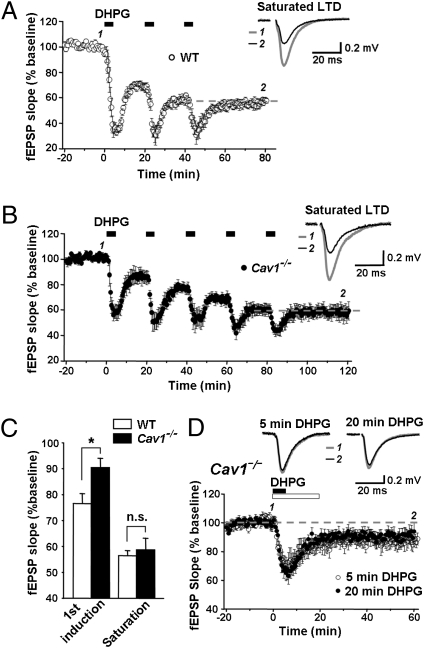
Saturating Levels of mGluR-LTD Are the Same in WT and Cav1−/− Mice.
DHPG-induced LTD at Sch-CA1 synapses is a saturable form of plasticity that shares a common expression mechanism, internalization of AMPA receptors, with NMDAR-independent LTD induced by paired-pulse low-frequency stimulation (24). To further investigate the properties of the reduced mGluR-LTD in Cav1−/− mice, we used repeated applications of DHPG. In WT mice, two consecutive applications of DHPG produced maximal depression of the fEPSPs (Fig. 2A), in corroboration of others (24). In contrast, four applications of DHPG were required to achieve maximal LTD in slices from Cav1−/− mice (Fig. 2B). Importantly, the maximal depression measured 30–35 min after final application of DHPG in slices of Cav1−/− mice was as great as that observed in WT slices (P > 0.05 Cav1−/− 59 ± 5%, n = 5, vs. WT 56 ± 2%, n = 6; Fig. 2C).
Fig. 2.
The saturated level of mGluR-LTD does not differ in WT and Cav1−/− mice. fEPSPs in CA1 were evoked by stimulation of Schaffer collaterals. Insets show representative fEPSPs (average of three to five traces) at indicated times (baseline, thick gray traces; post treatment, thin black traces). (A–C) mGluR-LTD in Cav1−/− mice was saturated by four applications of DHPG (B), whereas two applications were sufficient to saturate LTD in WT (A). Saturated levels were comparable in both. (C) Summary graph of the magnitude of LTD after one application of DHPG and at saturation. The magnitude of LTD induced by one application of DHPG is significantly less in Cav1−/− than in WT mice (n = 10, P < 0.01 WT vs. Cav1−/−), whereas LTD at saturation is not significantly different (Cav1−/−, n = 5; WT, n = 6; P > 0.05). (D) Prolonged stimulation with DHPG did not enhance LTD in Cav1−/− mice. LTD was induced by bath-application of RS-DHPG (100 μM) for 5 (filled bar) or 20 min. The fEPSP slope (mean ± SEM) is plotted as the percent of pre-DHPG baseline. DHPG applied for 20 min did not significantly enhance LTD compared with DHPG applied for 5 min (n = 10, P > 0.05). The LTD data for 5-min DHPG application in Cav1−/− mice are the same as in Fig. 1A.
The observed attenuation of mGluR-LTD in Cav1−/− mice could be due to insufficient stimulation of mGluR1/5 by the 5-min exposure to DHPG. To test this possibility, we examined whether a single but longer lasting application of DHPG (20 min; ref. 33) enhanced the magnitude of LTD in slices from Cav1−/− mice. Although slightly increased, LTD induced by 20 min of DHPG did not significantly differ from that induced by 5 min of DHPG (data from Fig. 1A included in Fig. 2D; 20 min of DHPG, 85 ± 4%, n = 10, 20 min vs. 5 min; P > 0.05), indicating that prolonged exposure to the agonist does not further engage the molecular machinery underlying LTD. These findings are consistent with a model whereby coupling of mGluR1/5 to downstream signaling pathways mediating mGluR-LTD is impaired in mice lacking caveolin-1.
Basal Transmission at Ionotropic Glutamate Receptors Is Normal in Cav1−/− Mice.
To examine whether the impaired mGluR-LTD in slices of Cav1−/− mice is associated with deficits in basal glutamatergic neurotransmission, we measured paired-pulse facilitation (PPF; ref. 38), and AMPAR- and NMDAR-EPSCs at Sch-CA1 synapses (Fig. 3). No significant differences were observed in PPF (Fig. 3A), the EPSC amplitude-membrane voltage relation (Fig. 3B), the AMPAR-EPSC rectification index (measured in 50 μM D-APV, Fig. 3C), or the NMDAR/AMPAR ratio (assessed at +40 mV as the ratio of the peaks of the D-APV sensitive and insensitive components; Fig. 3D) in Cav1−/− vs. WT slices. The membrane properties of CA1 neurons including resting potential, input resistance (Rin), and input time constant under voltage clamp were not significantly different in Cav1−/− vs. WT slices (Table S1). Furthermore, the magnitude of DHPG-induced, Ca2+-dependent inward current (39–41) did not differ between Cav1−/− and WT mice (Fig. S2). Collectively, these findings indicate that mGluR-independent forms of LTD and basal AMPA/NMDA transmission at Sch-CA1 excitatory synapses were not significantly altered in Cav1−/− mice. Thus, the role of caveolin-1 at CA1 synapses may be selective for mGluR-dependent plasticity.
Fig. 3.
Basal glutamatergic synaptic transmission is not altered in Cav1−/− mice. (A–C) EPSCs were evoked in the presence of D-APV (50 μM). (A) Paired-pulse facilitation was not altered in Cav1−/− neurons: Representative paired-pulse EPSCs at indicated interstimulus intervals in WT (Top) and Cav1−/− (Middle) mice. Mean PPF ratio is plotted as a function of interstimulus interval (Bottom; WT, n = 13; Cav1−/−, n = 8). (B) Representative EPSC traces (Upper) and current–voltage relation (Lower) in 50 μM D-APV in Cav1−/− mice. The EPSC reversal potential was near 0 mV. (C) Representative EPSC traces in D-APV (50 μM) at −60 and +40 mV in Cav1−/− mice (Left) and summary graph (Right) of rectification index in WT (n = 20) and Cav1−/− mice (n = 19; P > 0.05). (D) Summary graph of NMDAR/AMPAR ratio (Right). The D-APV-sensitive current (Subtracted) recorded at +40 mV was obtained by subtracting the EPSC before D-APV (Control) from that after D-APV (WT, n = 17; Cav1−/−, n = 15; P = 0.581). The bar graph shows the ratio of the EPSP peaks.
Reduced mGluR-LTD in Cav1−/− Mice Is Protein Synthesis-Dependent and Requires Signaling via ERK and Mammalian Target of Rapamycin (mTOR).
In adult mice, induction of mGluR-LTD requires stimulation of de novo protein synthesis (7, 23, 34) and depends on signaling via the ERK (42, 43) and mTOR (44, 45) pathways. To test for dependence on protein synthesis in Cav1−/− mice, hippocampal slices were incubated with the protein synthesis inhibitor anisomycin (25 μM, 40–60 min). Anisomycin did not significantly affect DHPG-induced short-term depression (vehicle LTD to 62 ± 7% of baseline, n = 8, anisomycin to 65 ± 5% of baseline, n = 8, P > 0.05 anisomycin vs. vehicle; measured at 4–6 min after onset of DHPG) but suppressed LTD at 50–55 min after DHPG (anisomycin-treated Cav1−/− slices depression to 98 ± 1% of baseline, n = 8; vehicle-treated slices depression to 88 ± 4% of baseline, n = 8, P < 0.05 anisomycin vs. vehicle, Fig. 4A).
Fig. 4.
The attenuated mGluR-LTD in CA1 of Cav1−/− mice depends on protein synthesis and requires activation of ERK and mTOR. Procedures and display as in Fig. 1A, but comparing Cav1−/− slices with and without drug. (A) Preincubation with the protein synthesis inhibitor anisomycin reduced DHPG-LTD compared with vehicle (vehicle LTD to 88 ± 4% of baseline, n = 8, P < 0.05; anisomycin LTD to 98 ± 1% of baseline, n = 8; P < 0.05 anisomycin vs.vehicle). (B) Preincubation with the MEK inhibitor U0126 reduced residual DHPG-LTD compared with vehicle (vehicle LTD to 88 ± 4% of baseline, n = 8, P < 0.05; U0126 LTD to 95 ± 4% of baseline, n = 8; P < 0.05 U0126 vs. vehicle). (C) Preincubation with the mTOR signaling inhibitor rapamycin reduced DHPG-LTD compared with vehicle (vehicle LTD to 89 ± 4% of baseline, n = 8, P < 0.05; rapamycin LTD to 96 ± 3% of baseline, n = 8; P < 0.05 rapamycin vs. vehicle).
To examine whether ERK signaling is required for the residual mGluR-LTD observed in Cav1−/− mice, we incubated hippocampal slices with the selective MEK inhibitor U0126 (20 μM, 40–60 min). U0126 had little or no effect on short-term depression but reduced DHPG-elicited LTD assessed at 50–55 min after initiation of DHPG application (U0126-treated Cav1−/− slices depression to 97 ± 4% of baseline, n = 8; vehicle-treated slices depression to 88 ± 4% of baseline, P < 0.05 U0126 vs. vehicle; Fig. 4B). To examine whether mGluR1/5 signaling via mTOR is also required for the reduced mGluR-LTD in Cav1−/− mice, we incubated hippocampal slices with rapamycin (20 nM, 40–60 min), an inhibitor of mTOR signaling. Blockade of mTOR greatly reduced mGluR-LTD in Cav1−/− mice (rapamycin-treated Cav1−/− slices depression to 96 ± 3% of baseline, n = 8; vehicle treated slices depression to 89 ± 4% of baseline, P < 0.05 rapamycin vs. vehicle at 50–55 min after DHPG; Fig. 4C). Together, these findings indicate that the residual mGluR-LTD in Cav1−/− mice requires activation of ERK and mTOR signaling and the same cellular mechanisms engaged by group I mGluRs at WT Sch-CA1 synapses.
Basal Phosphorylation of MEK and ERK1/2 Is Elevated in the Hippocampus of Cav1−/− Mice, but Not Further Increased by Brief Application of DHPG.
Caveolin-1 acts as an adaptor for macromolecular signaling complexes within cholesterol-rich membrane microdomains and can modify signal intensity and duration by regulating the activity of some of its interacting partners, including ERK2 (46). We examined the ability of mGluR1/5 to activate ERK signaling in the hippocampus of mice lacking caveolin-1. DHPG (100 μM, 5 min) or vehicle was applied to acute hippocampal slices and the levels of phosphorylated MEK and ERK1/2 (which are the active forms of these enzymes) were assessed in lysates of control and treated slices by Western blot analysis. Application of DHPG to WT slices rapidly induced phosphorylation of MEK and ERK1/2 (Fig. 5 A and B), in corroboration of others (42, 43, 47, 48). In Cav1−/− slices, basal levels of pMEK, pERK1, and pERK2 (relative to the unphosphorylated forms) were markedly increased compared with levels in WT slices (Fig. 5 A and B). Application of DHPG to Cav1−/− slices induced little or no change in the already elevated levels of pMEK, pERK1, or pERK2, and the levels of activated/phosphorylated forms after DHPG were similar in DHPG-treated WT and Cav1−/− slices (P > 0.05; Fig. 5 A and B). These findings indicate that in the hippocampus of Cav1−/− mice, the rapid activation of ERK signaling by group I mGluRs is impaired or perhaps occluded by increased basal phosphorylation and are consistent with a model whereby uncoupling of mGluR1/5 from downstream intracellular signaling contributes to the markedly attenuated mGluR-LTD at CA1 synapses of Cav1−/− mice in response to a brief application of DHPG.
Fig. 5.
mGluR1/5 signaling to MEK and ERK is impaired in Cav1−/− mice. (A and B) Phosphorylation of MEK and ERK1/2 in hippocampal slices from Cav1−/− and WT mice under basal conditions and after application of DHPG (100 μM). Representative immunoblots and summary data for total and p-MEK and p-ERK1/2. (A) Total and phosphorylated MEK under basal or DHPG-stimulated conditions (WT, n = 15; Cav1−/−, n = 10). DHPG increased p-MEK in WT. Basal phosphorylation was greater in Cav1−/− mice, and unaltered by DHPG. (B) Total and phosphorylated ERK1/2 under basal and DHPG-stimulated conditions. DHPG increased ERK1/2 phosphorylation in WT. Basal phosphorylation was greater in Cav1−/− mice and was unaltered by DHPG. Phosphorylation was comparable in WT and Cav1−/− mice after DHPG treatment. Unpaired Student's t test for comparisons of basal levels between groups; paired Student's t test, for comparisons of basal vs. DHPG levels within each group, n = 10. *P < 0.05, **P < 0.01.
Surface Expression of Group I mGluRs Is Not Altered in Cav1−/− Mice.
A possible explanation for deficits in DHPG-elicited LTD and/or DHPG-dependent activation of ERK signaling is that surface expression of mGluR1/5 is reduced in mice lacking caveolin-1, although lack of change in DHPG induced inward current makes reduced surface expression unlikely. We used biotinylation to examine surface expression of mGluR5, the most prevalent group I mGluR in CA1 pyramidal neurons (49, 50), and required for DHPG-induced LTD at Sch-CA1 synapses (24). Hippocampal slices were labeled with cell-impermeable biotin under resting conditions to label surface proteins, and total and biotinylated surface mGluR5 was assessed by Western blot analysis. Surface mGluR5 abundance did not significantly differ in Cav1−/− vs. WT slices (Cav1−/− 91 ± 7% of WT, n = 7 for WT, 8 for Cav1−/−, P > 0.05; Fig. S3A). Because biotinylation experiments do not distinguish between synaptic and extrasynaptic receptors, we examined receptor abundance in synaptoneurosomes, enriched in synaptic proteins (Fig. S3B) and total homogenates in WT and Cav1−/− mice. Western blot analysis with antibodies against mGluR5 (WT 1.3 ± 0.3 vs. Cav1−/− 1.4 ± 0.2; mean ± SD, n = 3, P > 0.05) or mGluR1 (WT 1.0 ± 0.3 vs. Cav1−/− 1.0 ± 0.3; mean ± SD, n = 3, P > 0.05) showed that the relative abundance of mGluR5 or mGluR1 did not differ significantly in synaptic membrane preparations from WT vs. Cav1−/− mice (Fig. S3C); moreover, the total abundance of mGluR5 or mGluR1 was not significantly affected by the absence of caveolin-1 (Fig. S3C). These findings indicate that impaired DHPG-LTD and DHPG-induced activation of ERK signaling in mice lacking caveolin-1 are unlikely to arise from deficits in mGluR1/5 expression levels.
Discussion
In this study, we document a role for caveolin-1 in mGluR-LTD at Sch-CA1 synapses. In mice lacking caveolin-1, the magnitude of LTD induced by a single brief (5 min) application of DHPG at Sch-CA1 synapses was markedly reduced, as determined by field recordings. Prolonged (20 min) application did not significantly increase the magnitude of LTD. However, the effects of repetitive applications summated, and the maximum LTD was the same in WT and Cav1−/− mice. Thus, the machinery for mGluR LTD was present, although it took repeated application of DHPG to achieve the same degree of depression. [WT mice may differ in the effect of prolonged DHPG application; Volk et al. (51) reported that WT mice showed greater LTD in response to 20-min application of DHPG than to 5-min application.] Furthermore, Cav1−/− mice exhibited normal NMDAR-dependent LTD, which requires an NMDAR-mediated rise in postsynaptic Ca2+. Both forms of LTD are mediated by persistent internalization of synaptic AMPARs (52, 53), but mGluR LTD requires protein synthesis, whereas NMDA LTD does not (23). The deficit in mGluR-LTD in absence of caveolin-1 was unlikely to be caused by deficits in basal presynaptic function, because paired-pulse facilitation and the AMPAR- and NMDAR-mediated components of the EPSC were not altered in CA1 neurons of Cav1−/− mice. Furthermore, passive membrane properties and DHPG-induced depolarization, mediated via activation of nonselective cation channels, were not altered in CA1 neurons of Cav1−/− mice. These findings restrict the possible mechanisms underlying the deficit in mGluR LTD in Cav1−/− mice.
One hypothesis to explain the difference in mGluR-LTD in Cav1−/− versus WT mice is that mGluR1/5 expression at hippocampal synapses was reduced (or compromised) in absence of caveolin-1. Several lines of evidence argue against this possibility. First, surface expression of mGluR5, the most abundant group I mGluR subtype present in CA1 pyramidal neurons (49, 50, 54), was not detectably altered in Cav1−/− vs. WT hippocampal slices. Second, the relative abundance of mGluR5 (and mGluR1) in synaptoneurosomes was similar in Cav1−/− and WT mice, suggesting normal expression of the receptors at synaptic sites. Third, the amplitude of inward current elicited by bath-applied DHPG was not altered in CA1 neurons lacking caveolin-1, suggesting that the number of functional group I mGluRs expressed at the plasma membrane was not altered. Acute manipulation of caveolin-1 expression or mutations in caveolin-1 binding motifs present within the intracellular loops of mGluR1 alter the kinetics of mGluR1 constitutive internalization, thereby affecting receptor expression at the cell surface (16). The observation that mGluR5 surface expression was not detectably altered in Cav1−/− mice under basal conditions is consistent with findings by others that compensatory trafficking mechanisms are operative in cells derived from Cav1−/− mice (55).
An attractive scenario is that in absence of caveolin-1, the ability of mGluR1/5 to engage intracellular signaling cascades that mediate induction of mGluR-LTD is compromised. Whereas DHPG-LTD does not require a rise in postsynaptic Ca2+ (33) or retrograde signaling by endocannabinoids (34), it does require rapid ERK- (42, 43) and mTOR-dependent (44, 45) de novo protein synthesis (23–27, 34). We found that the small remaining DHPG-LTD at CA1 synapses of Cav1−/− mice also required de novo protein synthesis and activation of ERK and mTOR signaling. These observations suggest that the mechanisms and signaling pathways underlying the induction of DHPG-LTD are preserved in the absence of caveolin-1 but can be activated only at a reduced level in response to mGluR1/5 stimulation. Caveolin-1 possesses the dual capacity of promoting the formation of macromolecular signaling complexes (by binding both receptors and effectors) and inhibiting the activity of some signaling effectors with which it interacts; for example, G protein alpha subunits (56), PKC (57), Src (58), and ERK (59). Our findings showed that in Cav1−/− hippocampal slices, the basal level of phosphorylated/activated MEK and ERK1/2 was enhanced compared with WT and that application of DHPG (adequate to induce hyperpolarization and reduced LTD) failed to induce a further increase in phosphorylation. A possible interpretation of these findings is that the absence of caveolin-1 impairs the rapid assembly of functional macromolecular signaling complexes that are required for stimulation of de novo protein synthesis and mGluR-LTD (25–27). Loss of coupling could be due to increased desensitization of a receptor property not measured by the inward current or by desensitization further downstream in the signaling cascade.
An alternative possibility is that reduced mGluR1/5-dependent activation of ERK and mTOR signaling arises from a “ceiling” effect imposed by the high basal level of hyperactive kinases. Enhanced basal phosphorylation/hyperactivation of ERK1/2 in Cav1−/− mice has been reported in both nonneuronal cells (59) and cortical neurons (16, 21) and might arise as a consequence of inhibition of phosphatase activity and/or loss of caveolin-1 inhibitory actions on kinases. We propose that the intrinsic property of caveolin-1 to act as a “dynamic” adaptor for macromolecular signaling complexes modulates the potency and temporal expression of signaling initiated by group I mGluRs that underlies mGluR-LTD.
Materials and Methods
Details are in SI Materials and Methods.
Drugs.
Agents used were obtained from Tocris Cookson and diluted from stock solutions immediately before use. Lidocaine N-ethyl bromide (QX-314, a quaternary form of lidocaine; Sigma Aldrich) was used in the internal solution for whole-cell patch-clamp recording.
Mice and Slice Preparation.
All procedures involving animals were carried out in accordance with the guidelines of the National Institute of Health for the care and use of laboratory animals and were approved by the Animal Institute of the Albert Einstein College of Medicine. Acute hippocampal slices were prepared by using standard procedures from 20- to 34 d-old Cav1−/− mice [made in B6129SF2/J and backcrossed for five generations with WT C57BL/6J (Jackson Laboratory)].
Electrophysiology.
For extracellular field recordings, pipettes (1–2 MΩ) filled with external solution were placed in the stratum radiatum of area CA1. The fEPSPs were evoked by square pulses (10–100 μA, 100 μs) to Schaffer collateral/commissural afferents with a concentric bipolar tungsten stimulating electrode. Baseline presynaptic stimulation was delivered once every 30 s at an intensity yielding 40–60% of the maximal response. Baseline slope was calculated from the mean slope values recorded for 20 min before drug application or synaptic conditioning.
For whole-cell voltage-clamp recordings from CA1 pyramidal neurons, slices were maintained at 32 °C and perfused with external solution (2.5–3 mL/min) containing picrotoxin (100 μM, to block inhibitory synaptic transmission), and neurons were visualized by infrared–differential interference contrast (IR–DIC) microscopy.
Biochemical Analysis of Surface Receptors.
After 2 h to recover from slicing 400-μm-thick hippocampal slices were incubated with 1 mg/mL sulfo-NHS-LC-biotin (Pierce) (30 min at 4 °C), washed, and homogenized in lysis buffer. After centrifugation, equal amounts of protein per condition were incubated with 80-μL Neutravidin beads (Pierce) for 2 h at 4 °C, centrifuged, and washed. Bound proteins were eluted in sample buffer (with 100 mM DTT) at 70 °C for 20 min, and total and biotinylated proteins were fractionated by SDS/PAGE, transferred to membranes, probed with anti-mGluR5 (LabVision) or anti-actin antibodies (LabVision), and visualized by enhanced chemiluminescence. Band densities were measured with the NIH Image/Image J software.
Synaptoneurosomes.
Synaptoneurosomes (SNs) were prepared according to ref. 60. Briefly, mouse cerebral cortices were homogenized and centrifuged and the supernatants were overlaid onto Percoll gradients. The SN fraction was isolated at the 15 and 23% interface.
MEK, ERK1/2 Phosphorylation.
Hippocampal slices (400 μm thick) were incubated at room temperature in normal external recording solution bubbled with 95% O2/5% CO2 for 120 min. Control and DHPG-treated slices were snap-frozen in dry ice and stored at −80 °C. After thawing on ice, the tissue was homogenized in lysis buffer and proteins were resolved by SDS/PAGE, and transferred to membranes. Membranes were probed with anti–p-MEK (Ser217/221), anti-MEK, anti–p-ERK1/2 (Thr202/Tyr204), and anti-ERK1/2. Detection and quantification of band densities were as described above.
Statistics.
Data are expressed as mean ± SE. Student's t tests and ANOVA were used as appropriate. Significance was taken as P < 0.05.
Supplementary Material
Acknowledgments
We thank Adrianna Latuszek for excellent technical assistance and Reed Carroll, Pablo Castillo, and Andrés Chávez for insightful scientific discussion. This work was supported by the Japanese Society for Promotion of Sciences Young Scientist Grant-in-Aid 21791592 (to Y.T.), NIH Grants NS55363 (to M.V.L.B.), NS20752 (to R.S.Z.), and MH082870 (to A.F.); and a FRAXA Research Foundation grant (to A.F.). M.V.L.B. is the Sylvia and Robert S. Olnick Professor of Neuroscience and Distinguished Professor. R.S.Z. is the F. M. Kirby Professor of Neural Repair and Protection.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015553107/-/DCSupplemental.
References
- 1.Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: Prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 3.Wijetunge LS, Till SM, Gillingwater TH, Ingham CA, Kind PC. mGluR5 regulates glutamate-dependent development of the mouse somatosensory cortex. J Neurosci. 2008;28:13028–13037. doi: 10.1523/JNEUROSCI.2600-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kano M, et al. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGluR1. Neuron. 1997;18:71–79. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- 5.Bellone C, Lüscher C, Mameli M. Mechanisms of synaptic depression triggered by metabotropic glutamate receptors. Cell Mol Life Sci. 2008;65:2913–2923. doi: 10.1007/s00018-008-8263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwyl R. Metabotropic glutamate receptor-dependent long-term potentiation. Neuropharmacology. 2009;56:735–740. doi: 10.1016/j.neuropharm.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Waung MW, Huber KM. Protein translation in synaptic plasticity: mGluR-LTD, Fragile X. Curr Opin Neurobiol. 2009;19:319–326. doi: 10.1016/j.conb.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dölen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–1508. doi: 10.1113/jphysiol.2008.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dölen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niswender CM, Conn PJ. Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: Intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 14.Allen JA, Halverson-Tamboli RA, Rasenick MM. Lipid raft microdomains and neurotransmitter signalling. Nat Rev Neurosci. 2007;8:128–140. doi: 10.1038/nrn2059. [DOI] [PubMed] [Google Scholar]
- 15.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29:3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong YH, et al. Agonist-induced internalization of mGluR1alpha is mediated by caveolin. J Neurochem. 2009;111:61–71. doi: 10.1111/j.1471-4159.2009.06289.x. [DOI] [PubMed] [Google Scholar]
- 18.Burgueño J, et al. Mutual regulation between metabotropic glutamate type 1alpha receptor and caveolin proteins: From traffick to constitutive activity. Exp Cell Res. 2004;300:23–34. doi: 10.1016/j.yexcr.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 20.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277:41295–41298. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 21.Head BP, et al. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 22.Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- 23.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 24.Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis—dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 25.Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang DO, Martin KC, Zukin RS. Spatially restricting gene expression by local translation at synapses. Trends Neurosci. 2010;33:173–182. doi: 10.1016/j.tins.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drab M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 29.Razani B, et al. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 30.Trushina E, Du Charme J, Parisi J, McMurray CT. Neurological abnormalities in caveolin-1 knock out mice. Behav Brain Res. 2006;172:24–32. doi: 10.1016/j.bbr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Gioiosa L, et al. Altered emotionality, spatial memory and cholinergic function in caveolin-1 knock-out mice. Behav Brain Res. 2008;188:255–262. doi: 10.1016/j.bbr.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Ito I, et al. 3,5-Dihydroxyphenyl-glycine: A potent agonist of metabotropic glutamate receptors. Neuroreport. 1992;3:1013–1016. [PubMed] [Google Scholar]
- 33.Fitzjohn SM, et al. A characterisation of long-term depression induced by metabotropic glutamate receptor activation in the rat hippocampus in vitro. J Physiol. 2001;537:421–430. doi: 10.1111/j.1469-7793.2001.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nosyreva ED, Huber KM. Developmental switch in synaptic mechanisms of hippocampal metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2005;25:2992–3001. doi: 10.1523/JNEUROSCI.3652-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9:967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 37.Kemp N, McQueen J, Faulkes S, Bashir ZI. Different forms of LTD in the CA1 region of the hippocampus: Role of age and stimulus protocol. Eur J Neurosci. 2000;12:360–366. doi: 10.1046/j.1460-9568.2000.00903.x. [DOI] [PubMed] [Google Scholar]
- 38.Wu LG, Saggau P. Presynaptic calcium is increased during normal synaptic transmission and paired-pulse facilitation, but not in long-term potentiation in area CA1 of hippocampus. J Neurosci. 1994;14:645–654. doi: 10.1523/JNEUROSCI.14-02-00645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Partridge LD, Valenzuela CF. Ca2+ store-dependent potentiation of Ca2+-activated non-selective cation channels in rat hippocampal neurones in vitro. J Physiol. 1999;521:617–627. doi: 10.1111/j.1469-7793.1999.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Congar P, Leinekugel X, Ben-Ari Y, Crépel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crépel V, Aniksztejn L, Ben-Ari Y, Hammond C. Glutamate metabotropic receptors increase a Ca(2+)-activated nonspecific cationic current in CA1 hippocampal neurons. J Neurophysiol. 1994;72:1561–1569. doi: 10.1152/jn.1994.72.4.1561. [DOI] [PubMed] [Google Scholar]
- 42.Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hou L, Klann E. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2004;24:6352–6361. doi: 10.1523/JNEUROSCI.0995-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma A, et al. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelman JA, et al. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- 47.Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hou L, et al. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Shigemoto R, et al. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romano C, et al. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 51.Volk LJ, Daly CA, Huber KM. Differential roles for group 1 mGluR subtypes in induction and expression of chemically induced hippocampal long-term depression. J Neurophysiol. 2006;95:2427–2438. doi: 10.1152/jn.00383.2005. [DOI] [PubMed] [Google Scholar]
- 52.Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. doi: 10.1038/35072500. [DOI] [PubMed] [Google Scholar]
- 53.Citri A, Malenka RC. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 54.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 55.Damm EM, et al. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–488. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li S, et al. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995;270:15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- 57.Oka N, et al. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J Biol Chem. 1997;272:33416–33421. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- 58.Li S, Couet J, Lisanti MP. Src tyrosine kinases, Galpha subunits, and H-Ras share a common membrane-anchored scaffolding protein, caveolin. Caveolin binding negatively regulates the auto-activation of Src tyrosine kinases. J Biol Chem. 1996;271:29182–29190. doi: 10.1074/jbc.271.46.29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen AW, et al. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol. 2003;284:C457–C474. doi: 10.1152/ajpcell.00380.2002. [DOI] [PubMed] [Google Scholar]
- 60.Dunkley PR, et al. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction: Homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.