Abstract
The all-trans-retinoic acid (atRA) isomer, 9-cis-retinoic acid (9cRA), activates retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in vitro. RARs control multiple genes, whereas RXRs serve as partners for RARs and other nuclear receptors that regulate metabolism. Physiological function has not been determined for 9cRA, because it has not been detected in serum or multiple tissues with analytically validated assays. Here, we identify 9cRA in mouse pancreas by liquid chromatography/tandem mass spectrometry (LC/MS/MS), and show that 9cRA decreases with feeding and after glucose dosing and varies inversely with serum insulin. 9cRA reduces glucose-stimulated insulin secretion (GSIS) in mouse islets and in the rat β-cell line 832/13 within 15 min by reducing glucose transporter type 2 (Glut2) and glucokinase (GK) activities. 9cRA also reduces Pdx-1 and HNF4α mRNA expression, ∼8- and 80-fold, respectively: defects in Pdx-1 or HNF4α cause maturity onset diabetes of the young (MODY4 and 1, respectively), as does a defective GK gene (MODY2). Pancreas β-cells generate 9cRA, and mouse models of reduced β-cell number, heterozygous Akita mice, and streptozotocin-treated mice have reduced 9cRA. 9cRA is abnormally high in glucose-intolerant mice, which have β-cell hypertropy, including mice with diet-induced obesity (DIO) and ob/ob and db/db mice. These data establish 9cRA as a pancreas-specific autacoid with multiple mechanisms of action and provide unique insight into GSIS.
Keywords: retinol, vitamin A, rexinoids
Impaired glucose-stimulated insulin secretion (GSIS) develops through multiple mechanisms, including actions of metabolic hormones and inflammatory cytokines, products of metabolic overload, and endoplasmic reticulum stress; however, mechanisms of GSIS and impaired glucose tolerance remain incompletely understood (1–4). Also uncertain is the contribution of impaired glucose tolerance to diminished pancreatic β-cell function and mass associated with type 2 diabetes (5). GSIS relies on the pancreas, and pancreas development, islet formation, and function require normal vitamin A nutriture (6–8). Vitamin A restriction during development impairs islet development and promotes glucose intolerance in adult rodents. On the other hand, restricting vitamin A in mature diabetes-prone rats reduces diabetes and insulitis, possibly through enhancing glucose sensing and metabolism. All-trans-retinoic acid (atRA), an activated metabolite of vitamin A, regulates pancreas development, and atRA does not enhance the incidence of diabetes in diabetes-prone rats fed a vitamin A-deficient diet (7, 9, 10). Although the contribution of vitamin A to pancreas development through atRA seems clear, mechanisms whereby vitamin A affects mature pancreas function have not been determined in depth, nor have the specific vitamin A metabolites been identified that contribute to GSIS control.
atRA induces differentiation and regulates cell processes by activating the nuclear receptors RAR α, -β, and -γ, which regulate transcription and translation (11). atRA does not activate the nuclear receptors RXR α, -β and -γ, which serve as obligatory partners for RAR and numerous other nuclear receptors that regulate metabolism and energy balance (12). 9-Cis-retinoic acid (9cRA), an atRA isomer, binds both retinoic acid receptors (RARs) and retinoid X receptors (RXRs) with high affinity in vitro and has diverse pharmacological actions distinct from atRA (13). For example, treating embryo day-11 pancreas organ cultures with 9cRA inhibits stellate cell activation more potently and quickly than atRA and inhibits acini differentiation, but prompts ductal differentiation and endocrine maturation (9, 10). atRA, in contrast, induces acini rather than ductal differentiation. As a panagonist of six nuclear receptors, 9cRA has undergone extensive pharmacological assessment. As the drug alitretinoin, it is effective against chronic hand dermatitis and T-cell lymphoma (14). Used systemically, it alters energy metabolism (15). 9cRA also shows promise in reducing ischemic brain injury in a rat model and in immunosuppressing human dendritic cells (16, 17). Regardless of the pharmacological utility of 9cRA, sensitive assays capable of quantifying individual RA isomers in biological matrices have not detected 9cRA in serum and in a variety of tissues (18, 19). This leaves uncertain whether 9cRA functions in vivo as an activated vitamin A metabolite with discrete physiological functions.
We applied a liquid chromatography/tandem mass spectrometry (LC/MS/MS) assay developed to distinguish and quantify RA isomers in biological matrices to identify retinoids in the pancreas and detected not only atRA, but also 9cRA. Pancreas 9cRA, but not atRA, reacts within minutes to blood glucose fluctuations and attenuates the impact of glucose on GSIS through multiple mechanisms, including rapid action. These data validate 9cRA as a naturally occurring metabolite of vitamin A with a physiological function unique among retinoids, broaden insight into mechanisms of GSIS, and provide unique perspective into vitamin A and islet function.
Results
9cRA as an Endogenous Pancreas Retinoid.
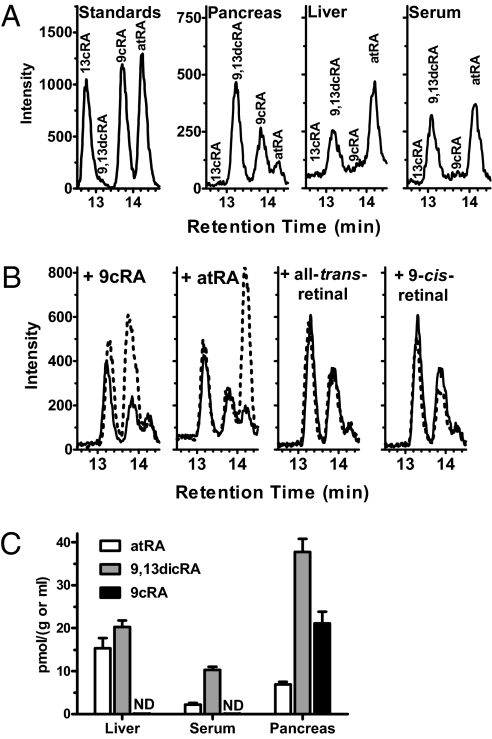
We applied a sensitive LC/MS/MS assay to compare pancreas RA isomers to those in serum and liver, because the endocrine pancreas expresses nuclear receptors that recognize RA isomers and responds to retinoid-induced signaling, and liver serves as the principal storage site of retinoids and contributes to retinoid homeostasis (20, 21). Consistent with previous work, prominent physiological RA isomers in serum and liver included atRA and 9,13-di-cis-RA (9,13dcRA), an RA isomer without known biological activity, but 9cRA was not detected (18, 19). In contrast, we identified 9cRA in pancreas, along with atRA and 9,13dcRA (Fig. 1A). We confirmed that analysis did not generate 9cRA by adding retinoids to pancreata before homogenization, extraction, and assay (Fig. 1B). Only 9cRA increased the 9cRA signal, excluding oxidation of the RA precursor retinal and/or isomerization of atRA during analyses as sources of 9cRA. Concentrations of 9cRA in pancreas occur within the range of concentrations of other RA isomers in tissues and serum (Fig. 1C). These data provide an analytically rigorous identification of 9cRA as a naturally occurring retinoid. If 9cRA occurs in the tissues assayed other than pancreas, amounts would be <0.05 pmol/g, on the basis of the LC/MS/MS assay's limit of detection in biomatrices.
Fig. 1.
9cRA occurs in pancreas. (A) Representative LC/MS/MS chromatograms of RA isomers from analyses of mouse pancreas, liver, and serum. (B) Representative LC/MS/MS chromatograms of pancreatic RA isomers before (solid lines) and after addition (dashed lines) of retinoids before homogenization, extraction, and analysis. Each chromatogram shows one of triplicate analyses. (C) Quantification of RA isomers in mouse liver, serum, and pancreas: ND, not detected; eight mice per group (SEM).
9cRA Varies with Fasting, Feeding, and Glucose Challenge.
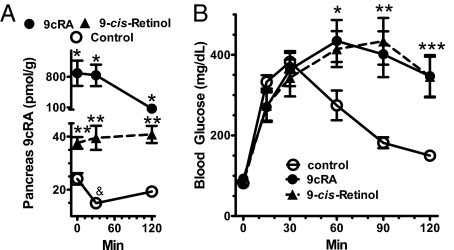
The fasted-to-fed transition resulted in a 36% decrease in 9cRA, which accompanied the increase in blood glucose and serum insulin, but caused no changes in pancreas atRA, 9,13dcRA, or retinol (Fig. 2A and Fig. S1). Consistent with this observation, challenging fasted mice with a bolus of glucose decreased 9cRA >80% within 15 min, coinciding with the rapid rise in blood glucose (Fig. 2B). 9cRA recovered markedly by 30 min and continued to rise thereafter. In contrast, glucose challenge had no impact on pancreas atRA or 9,13dcRA. During glucose challenge, 9cRA correlated inversely with serum insulin, further suggesting a contribution to pancreas function consistent with decreasing GSIS (Fig. 2C). In addition, exogenous 9cRA reduced serum insulin during glucose challenge (Fig. 2D).
Fig. 2.
9cRA reflects fasting vs. feeding. (A) Blood glucose, serum insulin, and pancreas RA isomers in fed or 12-h fasted mice. Data are means of three experiments with 6–10 mice per group per experiment; *P ≅ 0.03, **P < 0.004, ***P < 0.003 vs. fed values. (B) 9cRA and atRA responses to a glucose challenge (2 g/kg glucose). Values are means of two to five experiments with 5–10 mice per point per experiment, except 9,13dcRA (one experiment, 10 mice per time); *P < 0.05 from 0 time. The three glucose values after 0 min differ from control; *P < 0.05. (C) Inverse relationship between pancreas 9cRA and serum insulin after a glucose challenge: the slope differs significantly from 0, P = 0.02; 10 mice per point. (D) 9cRA hinders insulin secretion: 9cRA (0.5 mg/kg in 60 μL DMSO) or vehicle alone were injected i.p. in mice 15 min before an i.p dose of glucose (0.5 g/kg). Data are means of six to seven mice; *P < 0.05. All data ± SEM.
9cRA Promotes Glucose Intolerance.
The inverse relationship between serum insulin and pancreas 9cRA during the glucose tolerance test (GTT), and ability of 9cRA to reduce serum insulin, prompted testing whether 9cRA decreases glucose disposal. Mice were injected with 9-cis retinol, a potential precursor of 9cRA, or 9cRA before a GTT. Mice injected with 9-cis retinol responded with a 2- to 2.7-fold increase in pancreas 9cRA, sustained at least 120 min (Fig. 3A). Mice injected with 9cRA responded with an ∼30-fold increase in pancreas 9cRA, which declined by 120 min to ∼fourfold above control. Note that the decrease in endogenous 9cRA in the control (dosed only with glucose) at 30 min reflected the same degree of decrease (∼40%) observed at 30 min in the GTT experiment of Fig. 2B. Increases in pancreas 9cRA caused by both 9-cis retinoids resulted in glucose intolerance, such that 120 min after the glucose challenge, blood glucose was at least twofold higher than in vehicle-dosed mice (Fig. 3B). The lowest concentrations of 9cRA (40 nM) achieved after dosing either 9cRA or 9-cis retinol arrested glucose disposal to the same extent as the higher concentrations achieved, indicating a dose–response relationship with a maximum effect near or below 40 nM 9cRA. These data suggest that the physiological decrease in pancreas 9cRA as glucose increases permits optimum insulin secretion, and reveal that 9-cis retinol can serve as a precursor to 9cRA.
Fig. 3.
Exogenous 9cRA induces glucose intolerance. (A) Increases in total pancreas 9cRA after dosing with 9-cis retinol or 9cRA (0.5 mg/kg in 100 μL DMSO). 9-cis retinol and 9cRA were injected 60 and 15 min before glucose, respectively. Glucose (2 g/kg) was injected at 0 min: five to eight mice per group; *P ≅ 0.01 and **P < 0.003 vs. vehicle control; &P < 0.005 vs. 0 min. (B) GTT in mice dosed with 9-cis retinol or 9cRA: five to seven mice per group; *P < 0.04, **P < 0.002, ***P < 0.005 vs. control. Mice were dosed with retinoids as described in A. All data are ±SEM.
9cRA Rapidly Attenuates Glucose Sensing and Insulin Secretion.
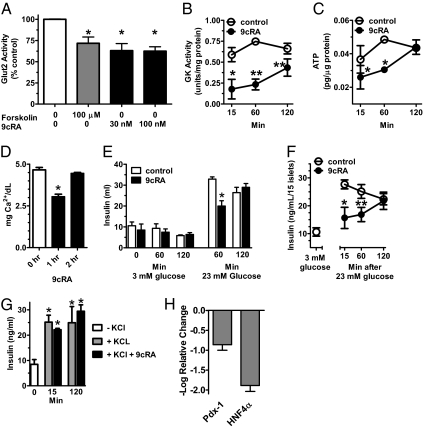
Glucose uptake by the pancreas rapidly affects both Glut2 and GK activities through posttranslational mechanisms (22, 23). Although GK activity limits the rate of glucose uptake by the β-cell, Glut2 contributes more than passive glucose transport by signaling glucose concentrations: phosphorylation decreases both the transport and signaling functions of Glut2 (22, 24). Forskolin, which stimulates cAMP synthesis and induces protein kinase A-dependent Glut2 phosphorylation in β-cells, decreases Glut2 activity. We repeated the experiment published with forskolin and compared its effect to that of 9cRA in the 832/13 β-cell line, established as a model of GSIS (25) (Fig. 4A). We duplicated the published results with forskolin and found that 9cRA decreased glucose-stimulated Glut2 activity ∼40% within 15 min, similar to the impact of forskolin. Thirty nanomolar of 9cRA was as effective as 100 nM, consistent with the results of Fig. 3, which indicated a marked effect of 40 nM of 9cRA on glucose disposal.
Fig. 4.
9cRA attenuates pancreas glucose sensing. Pancreas 832/13 β-cells and islets were preincubated 2 h with 3 mM glucose. At 0 min, the medium was exchanged for medium containing 23 mM and agents indicated for the duration of experiments. (A) 9cRA reduces Glut2 activity in 832/13 cells after 15 min incubation: 4–8 replicates per group; *P ≤ 0.0003 vs. no addition. (B) 9cRA reduces GK activity in 832/13 cells: 3–7 replicates per group, *P < 0.02, **P < 0.002 vs. control. (C) 9cRA reduces ATP content in 832/13 cells: 2–4 replicates per group, *P < 0.008 vs. control. (D) 9cRA decreases Ca2+ influx into 832/13 cells: 2 replicates per group; *P < 0.02. (E) 9cRA decreases GSIS by 832/13 cells: 3–11 replicates per group; *P < 0.01 vs. control. (F) 9cRA decreases GSIS by pancreatic islets. The graph shows baseline insulin secretion during 3 mM glucose and the effect of 9cRA on stimulation of insulin secretion by 23 mM glucose: 8–9 replicates per group; *P < 0.02, **P < 0.002 vs. control. (G) 9cRA does not affect KCl-stimulated insulin secretion from islets; *P < 0.02 vs. 0 time. (H) 9cRA reduces Pdx-1 and HNF4α mRNA after 2 h in 832/13 cells: 3 replicates per group. One hundred nanomolar 9cRA was used in all experiments, unless noted otherwise. All data are ±SEM.
In 15 min, 9cRA inhibited GK activity 70%, which persisted until 60 min in 832/13 β-cells treated with 23 mM of glucose (Fig. 4B). By 120 min, 9cRA inhibition of GK activity decreased to 34%. Despite the changes in Glut2 and GK activities, 9cRA did not affect Glut2 or GK mRNA by 15 min (Fig. S2). 9cRA decreased ATP in 832/13 β-cells 30–40% from 15 through 60 min after introduction of 23 mM of glucose and ATP recovered by 120 min (Fig. 4C). After 60 min of 9cRA exposure in cells treated with 23 mM of glucose, 9cRA reduced 832/13 β-cell intracellular Ca2+ 35%; Ca2+ returned to control levels by 2 h (Fig. 4D). Consistent with its impact on glucose sensing, 9cRA reduced GSIS 40% in 832/13 β-cells after 60 min incubation with 23 mM of glucose, but did not impair baseline insulin secretion in the presence of 3 mM of glucose (Fig. 4E). In pancreatic islets, 9cRA reduced GSIS 33–43% induced by 15–60 min incubation with 23 mM of glucose, with islet sensitivity returning by 120 min (Fig. 4F). A rise in ATP closes the β-cell K+ATP channel, which allows Ca2+ influx. This process serves as a “triggering signal” to initiate the first phase of GSIS (2). KCl induces insulin release in the absence of high glucose by circumventing the ATP effect on the K+ channel. 9cRA had no impact on KCl-stimulated insulin secretion from islets (Fig. 4G). To provide preliminary insight into the possible roles of RAR and RXR in short-term 9cRA action, the effects of the RAR panagonist TTNPB and the RXR panagonist bexarotene (BXR) were tested on GSIS in 832/13 cells (Fig. S3) (26, 27). In contrast to 9cRA, neither decreased the fold increase in insulin secretion after 1-h incubation with 23 mM of glucose vs. 3 mM, but both decreased basal insulin secretion stimulated by 3 mM.
After 2 h, 9cRA decreased expression of Pdx-1 and HNF4α mRNA, 7- and 77-fold, respectively (Fig. 4H). Pdx-1 induces glucokinase, Glut2, and insulin gene expression in the mature pancreas (28, 29). HNF4α regulates insulin release through controlling mitochondrial metabolism of glucose and HNF1α, Glut2, and insulin gene expression (30, 31).
Pancreas β-Cells Produce 9cRA.
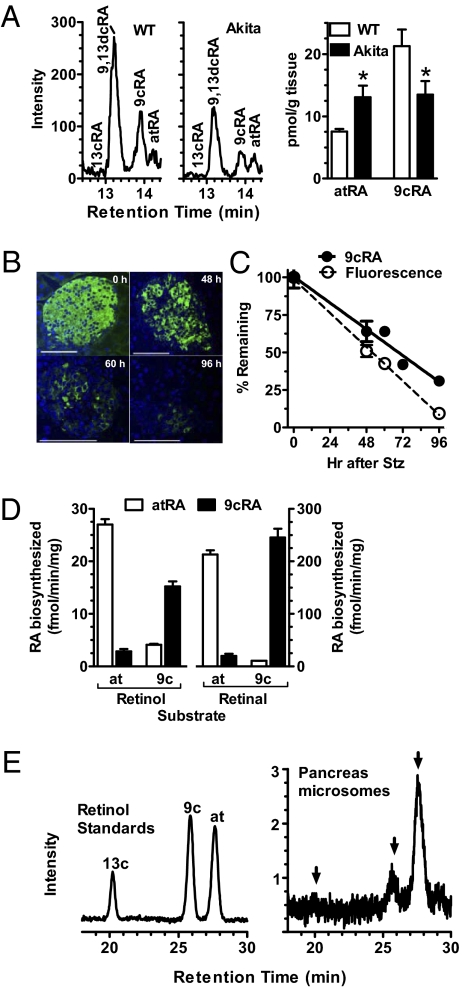
We analyzed pancreata from mice with decreased numbers of β-cells to identify sources of 9cRA. A point mutation in the insulin 2 (Ins2) gene of Ins2Akita mice induces β-cell apoptosis, which reduces the number of β-cells, indicated by a 46% reduction in pancreas insulin (32). Pancreata from fed heterozygous Akita mice had 40% lower 9cRA than wild type (WT), consistent with the decrease in β-cells (Fig. 5A and Fig. S4). In contrast, pancreas atRA increased and retinol did not differ from WT, demonstrating a unique relationship between 9cRA and β-cells. To confirm this insight, we injected mice with streptozotocin (Stz), which causes β-cell necrosis (33). 9cRA in pancreas of Stz-treated mice decreased with time in direct proportion to the decrease in β-cells, assessed by insulin content (Fig. 5 B and C). Seventy-two hours after Stz dosing, β-cell numbers decreased 67%, accompanied by a 58% decrease in 9cRA, impaired glucose tolerance, and elevated nonfasting blood glucose (Fig. 5C and Fig. S5). By 96 h, β-cells decreased 95% and 9cRA deceased 70%, consistent with β-cells serving as a major source of pancreas 9cRA. On the basis of the 9cRA remaining after β-cell destruction, other pancreas cells may contribute ∼20–25% to the 9cRA pool.
Fig. 5.
Pancreas β-cells produce 9cRA. (A) Representative LC/MS/MS chromatograms and quantification of RA isomers in pancreas of WT and Akita mice: 8 mice per group; *P < 0.05 vs. WT. (B) Immunohistochemistry showing loss of insulin in pancreas with time after a Stz dose. (Scale bars, 100 μm.) (C) Effect of Stz on β-cell numbers (3–6 islets) and pancreas 9cRA (9–18 mice per group). (D) Biosynthesis of RA isomers from retinol and retinal isomers by the pancreas β-cell line 832/13: 3 replicates/substrate. (E) HPLC of retinol standards and representative analyses of pancreas microsomes. Arrows denote elution positions of 13-cis-, 9-cis-, and all-trans-retinol, respectively. All data are ±SEM.
The β-cell line 832/13 generated 9cRA and atRA from their respective 9-cis- or all-trans-retinol and retinal precursors at similar rates (Fig. 5D). Pancreas microsomes contain both 9-cis- and all-trans-retinol (33 ± 1.4 and 76 ± 2 pmol/g protein, respectively; three replicate analyses of a five-pancreata pool) (Fig. 5E). Thus, β-cells have the capacity and a substrate to biosynthesize 9cRA.
Elevated 9cRA in Models of Glucose Intolerance.
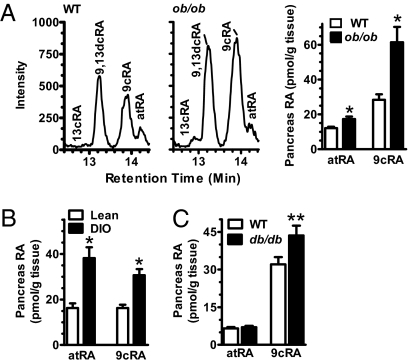
To determine whether mouse models of glucose intolerance are accompanied by 9cRA increases, we assayed pancreata from ob/ob and db/db mice and mice with diet-induced obesity (DIO) (34). Ob/ob mice lack leptin, are obese, and have high blood glucose and serum insulin (Fig. S6 A and B). Ob/ob mice had 2.2-fold higher 9cRA than WT controls (Fig. 6A). Mice with DIO were glucose intolerant, weighed ∼50% more than controls, and had ∼twofold higher 9cRA than controls (Fig. 6B and Fig. S6 C and D). Db/db mice, which lack the leptin receptor and have elevated blood glucose and serum insulin (Fig. S6E), had 34% higher 9cRA than lean controls (Fig. 6C). Although atRA also increased in pancreas of ob/ob mice, the increase was modest, and atRA did not increase in pancreas of db/db mice. In DIO pancreata, the atRA increase exceeded the 9cRA increase. Neither the atRA nor the 9cRA changes in pancreas correlated with changes in retinol. Thus, 9cRA was the only endogenous retinoid assayed (9cRA, atRA, and retinol) that changed consistently in pancreas with changes in glucose tolerance, regardless of the underlying cause of impaired glucose tolerance.
Fig. 6.
Increased pancreas 9cRA accompanies glucose intolerance. (A) Representative LC/MS/MS chromatograms and RA isomers in pancreas of fed WT and ob/ob mice: 8 mice per group; *P < 0.008 vs. WT. (B) Increased 9cRA in pancreas of mice with DIO: 8–10 mice per group: *P < 0.001 vs. lean. (C) RA isomers in pancreas of fed WT and db/db mice: 8 mice per group; **P = 0.033. All data are ±SEM.
Discussion
With an analytically rigorous assay, we determined that 9cRA is a naturally occurring retinoid in pancreas. This result addresses the long-standing questions of whether or not 9cRA contributes to the biological activity of vitamin A in vivo, and if so, in what capacity. We found that 9cRA localizes to the pancreas, where it attenuates sensitivity to glucose through a rapid reduction of Glut2 and GK activities, without a concomitant rapid change in their mRNA. Reduction of glucose uptake and phosphorylation led to reduced ATP and insulin release. In the longer term, 9cRA reduces transcription of two genes required for insulin synthesis and secretion—Pdx-1 and HNF4α. Notably, defects in either of these two genes and the gene that expresses GK cause the monogenic diseases known as maturity onset diabetes of the young (MODY): defects in HNF4α, GK, and Pdx-1 cause MODY1, 2, and 4, respectively (35, 36). Therefore, at least three of six well-characterized MODY are caused by defects in genes regulated by 9cRA or in a gene that encodes a protein regulated by 9cRA. These data clarify insight into the effects of vitamin A on pancreas function, i.e., atRA programs pancreas development and 9cRA attenuates GSIS in the developed pancreas. This is consistent with need for vitamin A during pancreas development and with alleviation of glucose intolerance by restricting vitamin A in the mature pancreas. The observation of 9cRA in the pancreas justifies future work to determine when the pancreas begins producing 9cRA, to clarify its function during pancreas development.
β-cells, which are integral to GSIS, serve as a major source of 9cRA, and 9cRA effects on β-cell function were observed by 15 min. These data indicate that 9cRA acts as an autacoid in pancreas: it is generated in situ and executes rapid local actions of relatively brief duration. Rapid actions of retinoids are not unprecedented, although they have not been investigated as extensively as their transcriptional effects. 9cRA reportedly stimulates phosphorylation of p38 mitogen-activated protein kinase (37). Recent work has shown that atRA has rapid, nongenomic actions on neurite outgrowth and endothelial cell growth (38, 39)—indicating that retinoids, like several steroid hormones, do not solely regulate transcription (40–43).
The acute, nongenomic effects reported here for 9cRA differ from the effects of synthetic RXR analogs, known as rexinoids, in rodent diabetic models (44). Longer-term systemic treatment with rexinoids has been reported to promote insulin sensitization, presumably through cumulative effects on diverse receptors distributed throughout multiple tissues. In the case of the more RXR selective retinoids, insulin sensitization occurs by activating peroxisome proliferator-activated receptors, presumably mainly in adipose (45).
By attenuating insulin secretion and biosynthesis, 9cRA functions physiologically to prevent hypoglycemia and to allow blood glucose levels raised (from glycogenolysis and gluconeogenesis) in response to decreasing blood glucose to increase to a greater extent than possible otherwise. Ultimately, reestablished higher blood glucose decreases 9cRA, sensitizing the pancreas for optimum insulin secretion. Animal models of diabesity had both high pancreas 9cRA and high serum insulin—an abnormal occurrence. Either 9cRA target resistance or resistance of 9cRA concentration changes to glucose levels could contribute to the phenotypes manifested in these models and to impaired glucose secretion. Alternatively, the β-cell hyperplasia that occurs in these models would contribute to, or underlie, increased 9cRA (46).
Occurrence of 9cRA in pancreas, along with nondetectable concentrations in serum and a host of other tissues, support the deduction on the basis of genetic evidence that 9cRA does not serve as a universal RXR-activating ligand in vivo (47). Validation of 9cRA as an autacoid in pancreas provides framework for evaluating whether it functions via RARs and/or RXRs and for evaluating potential cross-signaling between atRA and 9cRA. The outcome of a potential 9cRA/atRA interaction with pancreas RARs cannot be predicted simply because both retinoids bind to the same receptor. Nuclear receptor action depends on the ligand bound and the nucleotide sequence of the gene's response element—both influence receptor conformation, affinity for a particular response element, and function (48, 49).
Autacoid action in pancreas appears specific to 9cRA. Pancreas atRA did not change with an increase in blood glucose after the fasted-to-the-fed transition, or after glucose injection, indicating that atRA does not regulate short-term modulation of GSIS. The increase in atRA in the pancreas during DIO and in ob/ob mice seems a compensating reaction to obesity, because disrupting atRA biogenesis by ablating Rdh1 enhances adiposity, whereas chronic dosing of atRA to mice with DIO reduces weight by ∼15% (15, 50).
In summary, this work demonstrates that 9cRA occurs as an endogenous pancreas retinoid, establishes a function for 9cRA in GSIS, and provides unique insight into retinoid function and glucose homeostasis. Of interest are the likely nongenomic effects of 9cRA. These studies provide insight into an essential element of pancreas-mediated glucose homeostasis, which should prove useful for understanding mechanisms of GSIS and diabesity.
Methods
Animals.
Male C57BL/6 mice were used, unless noted otherwise, in accordance with institutional guidelines. Mice were fed either a stock diet with 30 IU/g vitamin A (Global 18% protein rodent diet 2018S; Harlan Laboratories Teklad) or an AIN-93G purified rodent diet with 30 IU/g vitamin A (119139; Dyets). The fatty acyl composition of the stock diet was (% of diet): 16:0, 0.7%; 18:0, 0.2%; 18:1,1.2%; 18:2, 3.1%; and 18:3, 0.3%. The fatty acid content of the AIN-93G diet has been published (51). These diets are referred to as vitamin A-ample diets and produced similar results. Seven- to 12-wk-old WT, ob/ob mice, and mice heterozygous for the Akita spontaneous mutation (Ins2Akita) were purchased from The Jackson Laboratory. To establish DIO, 1-mo-old mice were fed a high-fat diet (HFD) for 5 mo. The HFD was an AIN-93G purified rodent diet (diet no. 180614; Dyets) with 50% fat-derived calories. Lard served as the fat source. The fatty acyl composition of the lard was: 14:0, 1.5%; 16:0, 25.6%; 16:1, 3.4%; 18:0, 13.1%; 18:1, 44.1%; 18:2, 10.8%; and 18:3, 1.5%. To deplete β-cells, Stz (170 mg/kg) was dosed i.p. once in 10 mM sodium citrate, pH 4.5. For GTTs, glucose (2 g/kg) was dosed i.p. in saline. The db/db mice were 5 mo old and fed ad libitum. Insulin levels in this db/db colony at this age average threefold higher than controls, P < 0.01 (52). Fasting was done for 12–16 h. Mice were killed during the early light cycle.
Retinoids and Retinoid Quantification.
Retinoids were purchased from Sigma, except for 9-cis retinol, which was synthesized and characterized as described (18). Retinoids were used under yellow light as described (19, 53). Samples were harvested under yellow lights, frozen immediately in liquid nitrogen, and kept at −80 °C until extraction and retinoid quantification within 1 d by LC/MS/MS using selected reaction monitoring (RA isomers) or HPLC/UV (retinol isomers) as described in detail (19, 53).
β-Cell Line Function Assays.
The pancreatic β-cell line 832/13 was cultured in growth medium (RPMI 1640 with 11 mM glucose and 10% FCS, 10 mM Hepes, 2 mM l-glutamine, 1 mM sodium pyruvate, and 50 mM β-mercaptoethanol, 100 units/mL penicillin and 100 mg/mL streptomycin at 37 °C) under 5% CO2 in 100-mm Petri dishes as described (25). The medium was refreshed every 2–3 d. Cells were subcultured when they approached ≥70% confluence. Endogenous 9cRA in 832/13 cells at the time of experiments was <0.04 ± 0.0012 pmol/106 cells (n = 9 plates).
To assay Glut2, GK, and ATP, cells were transferred to 12-well plates until reaching 85% of confluence. Medium with 5 mM glucose was substituted for growth medium for 16 h. Before assays, this medium was substituted with 2 mL HBSS containing 0.2% fatty-acid free BSA, pH 7.2 (sensing buffer), and 3 mM glucose for 2 h (except for the glucose uptake assay). Experiments were then initiated by changing the sensing buffer to 0.8 mL per well of either fresh secretion buffer with 3 mM glucose or 23 mM glucose and/or 100 nM 9cRA, delivered in 5–10 μL DMSO, or vehicle alone. Incubations were done at 37 °C under yellow light.
9cRA Biosynthesis.
832/13 cells were cultured in 12- or 6-well plates. At confluence, the growth medium was replaced with serum-free medium (11 mM glucose) and retinoids (1 μM) in DMSO (0.1% vol/vol) or vehicle alone were added and incubated for 1 h. Cells were lysed using Reporter lysis buffer (Promega) and combined with their medium for retinoid analyses.
Insulin Secretion Studies.
Islets were isolated by the University of California, San Francisco Diabetes and Endocrinology Research Center. Fifteen islets of similar size were incubated at 37 °C in 12-well plates with 5 mM d-glucose in RPMI1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM Hepes, 2 mM l-glutamine, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol, and 5% CO2. After 16 h, islets were washed and transferred to 1.5-mL tubes in secretion medium (HBSS with 20 mM Hepes and 1% BSA, pH 7.2) containing 3 mM glucose. After 2 h, secretion medium was replaced with fresh secretion medium containing 3 or 23 mM glucose with 100 nM 9cRA or vehicle alone (DMSO). Separate groups of islets were incubated in secretion medium containing 3 mM glucose with 35 mM KCl and 90 mM NaCl. Media were centrifuged for 2 min at 1,000 × g to remove nonadherent cells and frozen at −80 °C. Endogenous 9cRA in islets at the time of the experiment (after isolation and handling of islets) did not exceed 0.12 ± 0.008 pmol per plate (n = 5).
Statistics.
Data are means ± SEM. Statistical significance was assessed by two-tailed, unpaired Student's t tests for comparison of two groups, or by two-way ANOVA for comparison of two curves.
Supplementary Material
Acknowledgments
We thank Gregory Szot (University of California, San Francisco Diabetes Center) for islet isolation, and Ron Tilton (University of Texas Medical Branch, Galveston, TX) for tissue samples from db/db mice. M.A.K. received support from a Ruth Kirschstein National Research Service Award Postdoctoral Fellowship (DK066924). A.E.F., C.R.K., and K.M.O. received support from National Institutes of Health (NIH) Predoctoral Training Grant DK061918. This work was funded in part by NIH Grant DK47839 (to J.L.N.). A.P., M.P., and E.C. were visiting scholars from the Department of Pharmaco-Biology, University of Calabria, Italy. A.P. and M.P. were supported by grants from Ministero Università e Ricerca Scientifica, Italy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008859107/-/DCSupplemental.
References
- 1.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: Many choices on the menu. Genes Dev. 2007;21:1443–1455. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MV, et al. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab. 2008;295:E1287–E1297. doi: 10.1152/ajpendo.90604.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muoio DM, Newgard CB. Mechanisms of disease: Molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 4.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 5.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 6.Driscoll HK, et al. Vitamin A status affects the development of diabetes and insulitis in BB rats. Metabolism. 1996;45:248–253. doi: 10.1016/s0026-0495(96)90062-1. [DOI] [PubMed] [Google Scholar]
- 7.Matthews KA, Rhoten WB, Driscoll HK, Chertow BS. Vitamin A deficiency impairs fetal islet development and causes subsequent glucose intolerance in adult rats. J Nutr. 2004;134:1958–1963. doi: 10.1093/jn/134.8.1958. [DOI] [PubMed] [Google Scholar]
- 8.McCarroll JA, et al. Vitamin A inhibits pancreatic stellate cell activation: Implications for treatment of pancreatic fibrosis. Gut. 2006;55:79–89. doi: 10.1136/gut.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadison A, et al. Retinoid signaling directs secondary lineage selection in pancreatic organogenesis. J Pediatr Surg. 2001;36:1150–1156. doi: 10.1053/jpsu.2001.25734. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, et al. Retinoid signaling controls mouse pancreatic exocrine lineage selection through epithelial-mesenchymal interactions. Gastroenterology. 2002;123:1331–1340. doi: 10.1053/gast.2002.35949. [DOI] [PubMed] [Google Scholar]
- 11.Noy N. Ligand specificity of nuclear hormone receptors: Sifting through promiscuity. Biochemistry. 2007;46:13461–13467. doi: 10.1021/bi7018699. [DOI] [PubMed] [Google Scholar]
- 12.Germain P, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58:760–772. doi: 10.1124/pr.58.4.7. [DOI] [PubMed] [Google Scholar]
- 13.Ahuja HS, Szanto A, Nagy L, Davies PJ. The retinoid X receptor and its ligands: Versatile regulators of metabolic function, cell differentiation and cell death. J Biol Regul Homeost Agents. 2003;17:29–45. [PubMed] [Google Scholar]
- 14.Cheng C, Michaels J, Scheinfeld N. Alitretinoin: A comprehensive review. Expert Opin Investig Drugs. 2008;17:437–443. doi: 10.1517/13543784.17.3.437. [DOI] [PubMed] [Google Scholar]
- 15.Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen H, et al. 9-Cis-retinoic acid reduces ischemic brain injury in rodents via bone morphogenetic protein. J Neurosci Res. 2009;87:545–555. doi: 10.1002/jnr.21865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zapata-Gonzalez F, et al. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: Inhibition of peroxisome proliferator-activated receptor gamma blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol. 2007;178:6130–6139. doi: 10.4049/jimmunol.178.10.6130. [DOI] [PubMed] [Google Scholar]
- 18.Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–369. doi: 10.1042/BJ20041867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napoli JL. Retinoic acid: Its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol. 1999;63:139–188. doi: 10.1016/s0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- 21.Chuang JC, Cha JY, Garmey JC, Mirmira RG, Repa JJ. Research resource: Nuclear hormone receptor expression in the endocrine pancreas. Mol Endocrinol. 2008;22:2353–2363. doi: 10.1210/me.2007-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorens B, et al. Protein kinase A-dependent phosphorylation of GLUT2 in pancreatic beta cells. J Biol Chem. 1996;271:8075–8081. doi: 10.1074/jbc.271.14.8075. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, et al. Conformational transition pathway in the allosteric process of human glucokinase. Proc Natl Acad Sci USA. 2006;103:13368–13373. doi: 10.1073/pnas.0605738103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferber S, et al. GLUT-2 gene transfer into insulinoma cells confers both low and high affinity glucose-stimulated insulin release. Relationship to glucokinase activity. J Biol Chem. 1994;269:11523–11529. [PubMed] [Google Scholar]
- 25.Hohmeier HE, et al. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 26.Pignatello MA, Kauffman FC, Levin AA. Multiple factors contribute to the toxicity of the aromatic retinoid, TTNPB (Ro 13-7410): Binding affinities and disposition. Toxicol Appl Pharmacol. 1997;142:319–327. doi: 10.1006/taap.1996.8047. [DOI] [PubMed] [Google Scholar]
- 27.Boehm MF, et al. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 28.Ashizawa S, Brunicardi FC, Wang XP. PDX-1 and the pancreas. Pancreas. 2004;28:109–120. doi: 10.1097/00006676-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Babu DA, Deering TG, Mirmira RG. A feat of metabolic proportions: Pdx1 orchestrates islet development and function in the maintenance of glucose homeostasis. Mol Genet Metab. 2007;92:43–55. doi: 10.1016/j.ymgme.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartoov-Shifman R, et al. Activation of the insulin gene promoter through a direct effect of hepatocyte nuclear factor 4 alpha. J Biol Chem. 2002;277:25914–25919. doi: 10.1074/jbc.M201582200. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem. 2000;275:35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 33.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- 34.Leibel RL, Chung WK, Chua SC., Jr. The molecular genetics of rodent single gene obesities. J Biol Chem. 1997;272:31937–31940. doi: 10.1074/jbc.272.51.31937. [DOI] [PubMed] [Google Scholar]
- 35.Hattersley AT, Pearson ER. Minireview: Pharmacogenetics and beyond: The interaction of therapeutic response, beta-cell physiology, and genetics in diabetes. Endocrinology. 2006;147:2657–2663. doi: 10.1210/en.2006-0152. [DOI] [PubMed] [Google Scholar]
- 36.Bell GI, Polonsky KS. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414:788–791. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 37.Teruel T, Hernandez R, Benito M, Lorenzo M. Rosiglitazone and retinoic acid induce uncoupling protein-1 (UCP-1) in a p38 mitogen-activated protein kinase-dependent manner in fetal primary brown adipocytes. J Biol Chem. 2003;278:263–269. doi: 10.1074/jbc.M207200200. [DOI] [PubMed] [Google Scholar]
- 38.Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARalpha associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sidell N, et al. Retinoic acid is a cofactor for translational regulation of vascular endothelial growth factor in human endometrial stromal cells. Mol Endocrinol. 2010;24:148–160. doi: 10.1210/me.2009-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 41.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 42.Grossmann C, Gekle M. New aspects of rapid aldosterone signaling. Mol Cell Endocrinol. 2009;308:53–62. doi: 10.1016/j.mce.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee R, et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 45.Leibowitz MD, et al. Biological characterization of a heterodimer-selective retinoid X receptor modulator: Potential benefits for the treatment of type 2 diabetes. Endocrinology. 2006;147:1044–1053. doi: 10.1210/en.2005-0690. [DOI] [PubMed] [Google Scholar]
- 46.Lindström P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–685. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calléja C, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meijsing SH, et al. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;324:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JC, et al. Novel arylpyrazole compounds selectively modulate glucocorticoid receptor regulatory activity. Genes Dev. 2006;20:689–699. doi: 10.1101/gad.1400506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang M, Hu P, Krois CR, Kane MA, Napoli JL. Altered vitamin A homeostasis and increased size and adiposity in the rdh1-null mouse. FASEB J. 2007;21:2886–2896. doi: 10.1096/fj.06-7964com. [DOI] [PubMed] [Google Scholar]
- 51.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(5, Suppl):838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 52.Yin D, et al. Recovery of islet beta-cell function in streptozotocin-induced diabetic mice: An indirect role for the spleen. Diabetes. 2006;55:3256–3263. doi: 10.2337/db05-1275. [DOI] [PubMed] [Google Scholar]
- 53.Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.