Abstract
Some microbes, among them a few species of cyanobacteria, are able to excavate carbonate minerals, from limestone to biogenic carbonates, including coral reefs, in a bioerosive activity that directly links biological and geological parts of the global carbon cycle. The physiological mechanisms that enable such endolithic cyanobacteria to bore, however, remain unknown. In fact, their boring constitutes a geochemical paradox, in that photoautotrophic metabolism will tend to precipitate carbonates, not dissolve them. We developed a stable microbe/mineral boring system based on a cyanobacterial isolate, strain BC008, with which to study the process of microbial excavation directly in the laboratory. Measurements of boring into calcite under different light regimes, and an analysis of photopigment content and photosynthetic rates along boring filaments, helped us reject mechanisms based on the spatial or temporal separation of alkali versus Acid-generating metabolism (i.e., photosynthesis and respiration). Instead, extracellular Ca2+ imaging of boring cultures in vivo showed that BC008 was able to take up Ca2+ at the excavation front, decreasing the local extracellular ion activity product of calcium carbonate enough to promote spontaneous dissolution there. Intracellular Ca2+ was then transported away along the multicellular cyanobacterial trichomes and excreted at the distal borehole opening into the external medium. Inhibition assays and gene expression analyses indicate that the uptake and transport was driven by P-type Ca2+-ATPases. We believe such a chemically simple and biologically sophisticated mechanism for boring to be unparalleled among bacteria.
Keywords: bioerosion, calcium metabolism, endoliths, microbialites
Endolithic cyanobacteria that bore their way into carbonate minerals, known as euendoliths, or true endoliths (1), constitute a major erosive force contributing to the morphogenesis of coastal (2) and terrestrial (3) limestones, the destruction of coral reefs and other biological carbonates (4, 5), and the obliteration of carbonate sands (6). They also represent a pest of commercial relevance for bivalve aquaculture (7). Despite their environmental relevance, the mechanism that enables them to dissolve carbonates, even in waters supersaturated with respect to calcite and aragonite, has remained elusive (8–13). It is in fact a geochemical paradox subject to speculation, in that cyanobacterial autotrophy will tend to increase pH and precipitate carbonates, not dissolve them (10). In the absence of moving or hard parts, etching must be clearly chemical. At circumneutral pH the dissolution reaction takes the following form: CaCO3 (s) + H+ ↔ Ca2+ + HCO3−. The reaction shows that one can promote dissolution through acidification, which was long taken to be the basis of a potential excavation mechanism (9, 14), even though it cannot work well for cyanobacteria. Alternative mechanisms to explain the paradox of boring phototrophs have more recently been proposed (10). Some put forward an alternative way of acid generation that does not collide with acid-consuming CO2 fixation. One postulates the agency of heterotrophic bacteria hosted within the borehole. Others call for the separation of photosynthesis and boring activities in space or time. A mechanism based on shifting the dissolution equilibrium by lowering Ca2+ concentration, obvious upon inspection of the aforementioned dissolution reaction, has also been proposed (10). The lack of cultured cyanobacteria that could bore in a sustained fashion under laboratory conditions effectively prevented the experimental assessment of any of these models (9, 10). Only a few species of cyanobacteria are euendoliths, mainly in the morphogenera Hyella, Solentia, Plectonema, and Mastigocoleus, but the exact phylogenetic position of these cyanobacteria has not been resolved. We developed a stable microbe/mineral boring system based on a cyanobacterial isolate, strain BC008 (15). It likely represents one of the most common cyanobacterial euendoliths, the filamentous, true-branching, heterocystous Mastigocoleus testarum (14). We used this system to conduct growth experiments, as well as in vivo, real-time microscopic examination of the microbe/mineral system in action. Complementing the study with a molecular genetic investigation of the capabilities of BC008, we were able to gain significant insights into the question of how cyanobacteria bore.
Results and Discussion
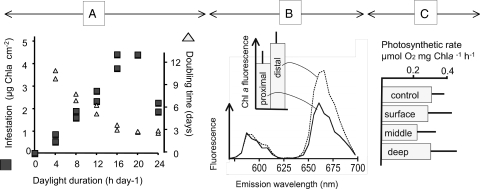
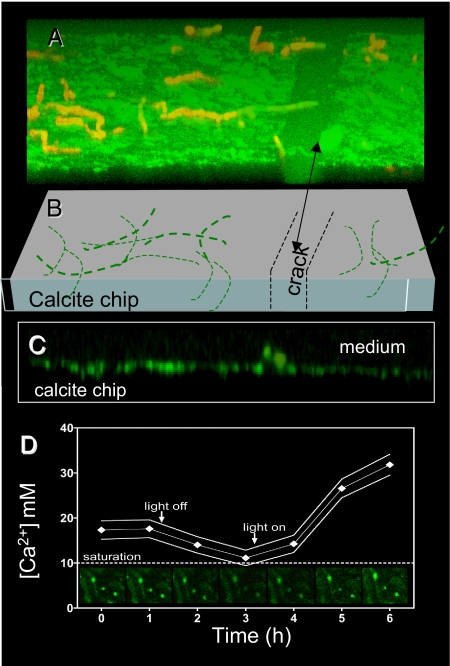
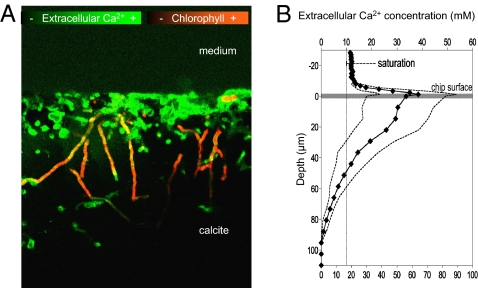
BC008 (Fig. 1A) could bore into calcite chips in the laboratory and could routinely be grown on millimeter-sized, sterilized, illuminated crystalline blocky calcite chips immersed in liquid medium, which allowed for real-time microscopic observation of boring (Fig. 1B), and simple experimentation. The strain was demonstrably axenic (free of contaminants; Materials and Methods describes related tests). Thus, one can simply reject the alleged implication of heterotrophic bacteria in the boring process. To test for an excavation mechanism based on temporal separation between photosynthesis (in the daytime) and boring (at night, as a result of acidification caused by respiration of glycogen reserves), we measured BC008’s rates of growth and excavation as a function of the duration of illumination period during growth (Fig. 2A). Although boring rates were optimal in the presence of a short daily dark phase, excavation could proceed unimpeded under constant illumination. A night period and the temporal cessation of photosynthesis was thus not a requirement for boring. A possible spatial separation of boring and photosynthesis along the cyanobacterial trichomes could alternatively be at play. In this scenario, cells at the boring front would function heterotrophically, producing net acidification of the medium by releasing CO2, at the expense of photosynthate transported from the cells at the lagging end. Precedent for such metabolic segregation can be found in heterocysts, specialized cells of some filamentous cyanobacteria. Heterocysts lose autotrophy and the ability to evolve oxygen, turning into a photoheterotrophic metabolism sustained with carbon fixed in neighboring cells, to optimize nitrogen fixation in an oxic environment (16). We tested the prediction that if apical cells were photosynthetically inactive, they should display pigmentation changes similar to those found in heterocysts (i.e., lowered levels of photosystem II and phycobiliproteins) or an overall decrease in all photosynthetic pigments. By using laser scanning confocal fluorescence microscopy (LSCM), we probed for such changes in actively boring filaments, but could not detect any (Fig. 2B). This speaks against a boring mechanism based on spatial separation of photosynthesis and heterotrophy. We also tested the prediction that if apical, boring cells were not photosynthetic, we should be able do detect directly a relative decrease in photosynthetic rates between populations of cells proximal to the boring front versus those that are distal to it. For this we “disinterred” boring BC008 populations by an artificial dissolution of the solid calcite chip using an EDTA perfusion method (17). As we dissolved the calcite chip, boring filaments of BC008 became exposed, and could be sequentially harvested into three discrete populations (surface, middle, and deep). Specific photosynthetic rates were not significantly different from those of free-living controls, indicating that the procedure did not affect photosynthesis. We could detect neither a significant difference among any of these fractions nor any decreasing trends with depth (Fig. 2C), thus invalidating the prediction. We then turned to testing a mechanism based on Ca2+ transport, whereby Ca2+ could be taken up by the boring cells to lower the extracellular ion activity product of CaCO3 below that of calcite saturation, promoting localized, spontaneous mineral dissolution. Ca2+ must then be transported internally across cells and excreted at the lagging end of the filament into the external liquid medium. Here, one can make two predictions. First, Ca2+ should accumulate above saturation in the liquid medium close to the solid's surface as carbonates are bored, and second, Ca2+ should be depleted below saturation equilibrium deep in the borehole, in the extracellular space close to the boring front. We could in fact detect and quantify such departures from equilibrium, when boring BC008 was imaged in real time under the LSCM in the presence of a fluorescent Ca2+-sensitive reporter dye (Fig. 3A; Materials and Methods). At the boundary layer between solid and liquid, Ca2+ reached concentrations several fold that of (calcite) saturation. Active Ca2+ transport into this layer is required to maintain a steady supersaturation against diffusive losses. No such accumulations occurred in sterile chips, or in BC008-infested chips that had been bleach-killed before imaging, proving that transport was mediated by the cyanobacteria. Ca2+ buildup, in fact, required illumination of the sample, the magnitude of supersaturation being a function of the intensity of illumination supplied (Fig. 3D). Buildup would relax back to equilibrium saturation within 1 to 2 h of darkening, pointing to the coupling of photosynthetic energy in the process. Lengthwise imaging of boring filaments and boreholes (Fig. 4A) revealed that the Ca2+ within the borehole interstitial space departed from dissolution equilibrium at both ends. There was clear supersaturation close to the surface, as we had found at the chip's boundary layer, but we also could detect severe undersaturation in the extracellular space at the boring front, often lower than detection limits. This shows that the Ca2+ mass transfer could not be extracellular, as this would imply movement up against a concentration gradient, which is not thermodynamically allowed. The Ca2+ missing from the deep interstitial space had only one place to go: inside the apical cells, and from there along the cyanobacterial filament, to be released to the extracellular space close to the borehole entrance. A transcellular Ca2+ transport is thus at play during boring. Diffusion eventually accomplishes net mass transfer from the boundary layer to the bulk medium.
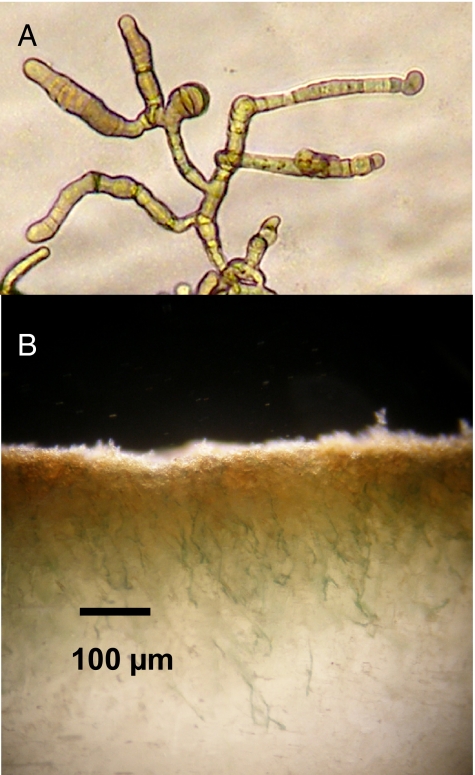
Fig. 1.
Strain BC008 and its ability to bore. (A) Photomicrograph of culture grown in liquid. (B) Close-up of a heavily infested calcite chip (infestation started from top). Single green filaments can be observed deep in the crystal matrix. Notice the reprecipitation of a yellow, light-scattering micrite layer close to the surface.
Fig. 2.
Evidence against separation between photosynthetic and boring activities in strain BC008. (A) growth rate (as doubling time, right axis) in liquid culture, and the intensity of infestation on blocky calcite chips attained at 5 wk of incubation (left axis), both as a function of the length of the illumination period when grown on a day/night cycle. (B) Cell-specific photosynthetic pigment content measured in situ during boring using fluorescence emission spectroscopy of confocal optical sections with excitation at 488 nm. Lines show average (n = 5) emission spectra of single cells for cells close to the boring front (proximal) compared with cells close to the surface of the solid (distal). Inset: Chlorophyll a-specific emission at 685 nm (error bars are 1 SD); differences are not significant (t test; P = 0.234). (C) Photosynthetic rates of boring BC008 cells retrieved from layers close to the surface of chips compared with those in the middle and deep zones and those from nonboring cultures (control). No significant differences were found (P > 0.33 in all t tests; n = 3).
Fig. 3.
Real-time free Ca2+ supersaturation around boreholes during boring of BC008 on calcite. LSCM Images are plane view from live illuminated cultures boring into calcite chips in custom-designed stage incubation chambers. (A) Three-dimensional reconstruction from single optical sections recorded parallel to the chip surface shown at an elevation angle of 30°. Green fluorescence denotes dissolved Ca2+ concentration reported by the externally supplied fluorophore. Red autofluorescence is from photosynthetic pigments (chlorophyll a and phycobilins) and tracks cyanobacterial filaments. (B) In an interpretation of A, thick lines denote that some filaments naturally stick out of the chip and thin lines denote filaments inside the chip. Note that Ca2+ inside a natural crack in the chip is much closer to saturation. (C) Vertical cross-section of the solid/liquid interface reconstructed from data in A (only the green channel is shown). (D) Temporal dynamics of Ca2+ concentration measured around boreholes after changes in stage illumination. Solid lines delimit 2 SD around the mean of n = 10 boreholes. Some source images are shown in the sequence of insets. Ca2+ decreased to saturation within 2 h of turning light (10 W m−2) off. Higher Ca2+ supersaturation was reached within 2 h of turning on illumination at 30 W m−2.
Fig. 4.
Evidence for a boring mechanism based on transcellular Ca2+ transport. (A) LSCM calcium mapping of a heavily infested chip taken along the main boring direction shows supersaturation at the boundary layer (particularly in the heavily eroded surface) and within the boreholes close to the surface, and undersaturation around the deeper parts of the boreholes. (B) Quantitative data for Ca2+ concentration along the boreholes of single filaments measured in lightly bored chips (solid line is the average of 10 filaments; SDs shown as dashed lines); the dotted vertical line marks expected calcite-saturation equilibrium.
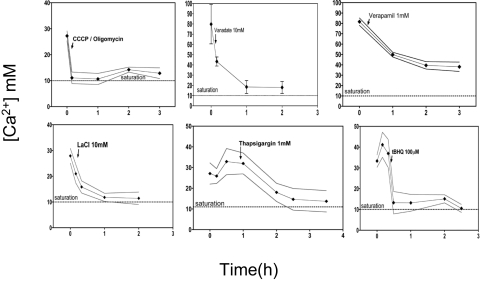
We used time-course incubations similar to those shown in Fig. 3D, with the solid/liquid boundary Ca2+ supersaturation level as a proxy for boring activity, to further probe the nature of the Ca2+ transport mechanism, among those known to exist (18). Addition of a mixture of ATP-generating inhibitors (CCCP and oligomycin) completely abolished boring, confirming the tight coupling to cellular energy availability (Fig. 5). Verapamil, a Ca2+ channel blocker, affected, but did not abolish, boring in the short term. Addition of specific inhibitors of P-type cation-transporting ATPases, such as La3+ or vanadate (19), also caused fast relaxation of surface Ca2+ supersaturation, suggesting that this type of transporters are central to the process. The addition of either of two specific inhibitors of Ca2+-transporting P-type ATPases, thapsigargin and tert-butylhydroquinone, also resulted in complete inhibition of Ca2+ extrusion. In parallel controls, none of these compounds affected photosynthesis of BC008 except very transiently. This implies that Ca2+-transporting P-type ATPases, which couple directly the extrusion of Ca2+ across the plasma membrane to the hydrolysis of ATP and the counter transport of protons, play a key role in the mechanism behind cyanobacterial boring. To test this further, we probed for the presence and regulation of P-type Ca2+ transporter genes in BC008. An inspection of publicly available genomes reveals that, although these are common, they are not universal among cyanobacteria. Homologues putatively encoding for such transporters were retrieved from databases, aligned, and used to design 11 oligonucleotide primer pairs targeting the conserved Ca2+-binding, ATP-binding, and phosphorylation coding regions (SI Text). These were then used to PCR-amplify putative Ca2+ ATPases from genomic DNA of BC008. Five pairs gave product. After cloning the products and sequencing 19 clones, we found evidence for only two distinct genes, JGE01 and JGE04, with high homology to known cyanobacterial Ca2+-P-type ATPases (SI Text). We then proceeded to study their expression by using quantitative PCR applied to reversely transcribed mRNA preparations of BC008 cells (20). We compared cultures grown in liquid without any calcite versus filaments that were boring into calcite. Preparations of biomass close to the chip's surface (0–50 μm deep) and deep within it (50–100 μm), and a fraction containing the deepest filaments (100–150 μm), obtained after calcite dissolution with EDTA (17), showed that both genes were, in general, up-regulated (Fig. 6) with respect to free-living cultures, which is consistent with their involvement in boring.
Fig. 5.
Effect of inhibitors on BC008 boring into calcite chips. All experiments measured the level of Ca2+ supersaturation at the chip's surface as a proxy for boring activity using Ca2+ imaging under LSCM as in Fig. 3D. Inhibitors were added at the time marked by the arrows in each panel. CCCP is a proton motive force inhibitor and oligomycin inhibits ATP synthase. Vanadate and lanthanum ions are typical inhibitors of P-type ATPases. Thapsigargin and tert-butylhydroquinone (t-BHQ) are known inhibitors of Ca2+-transporting P-type ATPases. Verapamil is a Ca2+ channel blocker.
Fig. 6.
Level of overexpression of two putative P-type Ca2+ ATPase genes detected in BC008, JGE01, and JGE04, with respect to the levels found in nonboring, free-living cultures, as measured by quantitative RT-PCR. Results are from three depths in a single chip, and with an analytical replication of three for each depth. Only overexpression levels that were statistically significant (P < 0.05; pair-wise fixed reallocation randomization test) are presented; an asterisk indicates overexpression that was not highly significant (P = 0.117). Lines on the bars indicate 95% confidence intervals for the mean ratio. Uncorrected PCR data for all samples and controls can be found in SI Text.
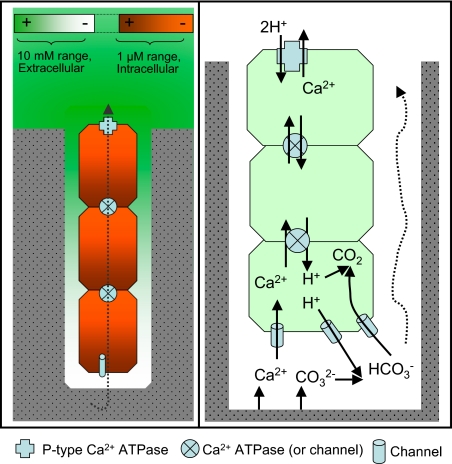
A model as to how calcium transport and specific calcium transporters may be involved in boring, as well as to how this process may interact with photoautotrophy, is presented in Fig. 7. Based on the literature, the model assumes that all P-type Ca2+ ATPases pump Ca2+ out of the cytoplasm and require a counter transport of protons (21). Intense extrusion of intracellular Ca2+ by P-type ATPases strategically anchored on the plasma membrane of the distal cells, close to the borehole entrance, keep intracellular concentrations within the typical physiological micromolar (22) range and establish an intracellular diffusive mass transport down the concentration gradient that brings Ca2+ along the trichome toward this region. Either through active transport with additional ATPases located at the cross walls, or through channels that allow facilitated diffusion of Ca2+ and protons (the first variant is depicted in Fig. 7, as it is consistent with the vertical profile of gene expression shown in Fig. 6), counter-migration propagates transcellularly to the apical cell. Strategically placed Ca2+ channels allow the down-gradient entry of extracellular Ca2+ into the apical cell, lowering interstitial extracellular concentration below that of calcite saturation, and promoting mineral dissolution. The involvement of channels is consistent with the moderately negative effect of specific inhibitors on boring. Counter transported protons (two per Ca2+) promote the conversion of carbonate ions released from calcite into CO2, most likely in concert with the carbon-concentrating mechanism (as shown). This CO2 can then be used in photosynthesis. Although the participation of P-type Ca2+ ATPases and the transcellular calcium transport have been demonstrated, the exact cellular distribution and the specific nature of some of the transporters remain to be determined with certainty.
Fig. 7.
Working model for cyanobacterial excavation of calcium carbonates. Left: Concentrations and ranges of extracellular Ca2+ (measured; green intensity) and intracellular Ca2+ (inferred, red intensity) around the boring system, as well as the major direction of net Ca2+ movement. Right: Inferred nature and distribution of transporter components (legend at bottom), and the fate of Ca2+ and CO32− after calcite dissolution.
The mechanism delineated here is attractive because it can explain several geological phenomena associated with modern and fossil bioeroded substrates, in that the extreme local supersaturation that occurs close to the lagging end of a boring filament can account for the reprecipitation of carbonates as poorly crystalline phases (the micrite “rind” in Fig. 1) (23–25), or as alternative polymorphs such as aragonite or vaterite (26). This may also explain how reprecipitation at the lagging end may cause microbial borers to act as cements agent in sedimentary structures when growth allows them to cross between mineral grains (27). Our model also opens biological questions as to how boring cyanobacteria might cope with altered intracellular Ca2+, which is otherwise kept low and tightly regulated (22). Because a lowering of ocean calcium carbonate saturation state driven by anthropogenic CO2 emissions (28) will lower the bioenergetic cost of boring, a global increase in cyanobacterial bioerosion rates in shallow waters can be logically predicted, an outcome for which some experimental evidence is already available (29), likely affecting already impacted (30) coral reefs, as well as the bivalve fisheries industry (7).
Materials and Methods
Strain BC008 (15) was isolated from a marine snail shell in Cabo Rojo, Puerto Rico (latitude N 17° 56' 1.89”, longitude W 67° 11' 32.64”). Cultures were kept boring on small calcite chips, submerged in liquid sterile medium [Provasoli enriched seawater (PES)] (31) containing 30 gL−1 of Instant Ocean salts (Spectrum Brands) at pH 8.3. BC008 was obtained here in axenic (pure) culture by micromanipulation of individual filaments under the dissecting microscope. Axenicity was eventually verified by meeting the following criteria: (i) absence of heterotrophic colonies on old-growth BC008 agar plates; (ii) absence of colony growth after 3 d of plating filaments of BC008 in PES agar supplemented with peptone, glucose, and yeast extract; (iii) absence of visible bacteria, other than BC008, in mature liquid cultures when observed under 1,000-fold magnification, phase contrast, in wet mounts; and (iv) presence of a single high-quality sequence after PCR amplification of a 1,100-bp-long fragment of the 16S rRNA gene using universal bacterial primers. Forward and reverse Sanger sequences were aligned with MEGA 4.0 to obtain a final sequence of 1,094 bp, which expectedly blasted to the heterocystous cyanobacteria. Photosynthetic rates were determined as O2 evolution of dilute cell suspensions illuminated at a light intensity of 500 μmol photon m−2 s−1 in a liquid-phase chamber (Hansatech), in which partial O2 pressure was monitored with a Clark-type electrode, calibrated using air-saturated PES medium and a sodium-dithionite solution (zero O2). For each value reported, the average of three consecutive rate determinations was used. Rates are expressed per unit mass of chlorophyll a, the concentration of which was determined spectrophotometrically in each cell suspension after centrifugation and pellet extraction in 90% acetone. For microscopy experiments, calcite chips of appropriate size were cleaved manually from commercial blocky calcite, sterilized by immersion in ethanol, added into tissue-culture bottles containing minimal PES medium, and inoculated with BC008. After 3 to 4 wk of growth in the light at room temperature, when the infestation of the chips was apparent but not massive, chips were retrieved. Surface growth outside of the chip was removed by gently brushing under sterile conditions, and incubation overnight was performed in fresh medium. Boring biomass per unit area could be assessed sacrificially by extracting lipid-soluble pigments in acetone, and subsequent spectrophotometric determination of chlorophyll a. For most microscopy-based experiments, clean chips were placed in a custom-made incubation chamber built on a microscopic slide and allowed to incubate for at least 2 h before experimentation. Confocal laser microscopy allowed to optically section the areas around the calcite chip surface without physically disturbing any gradients that had formed. To take the image in Fig. 3, after a long period of growth of several months, calcite chips were cleaved once more along the direction of boring, to expose the boring filaments lengthwise. The chip was then placed on the stage chamber with the freshly cleaved surface facing up, so that filaments and boreholes close to the new surface, but still within the solid, could be imaged optimally. Single-plane horizontal fluorescent images were stored in two separate channels: green fluorescence for calcium and red autofluorescence for chlorophyll a. Three-dimensional images or vertical optical sections were reconstructed from stored data as needed. In all cases, 1 μm calcium green-5N (Molecular Probes) was used as fluorophor. Fluorescence intensity in the green was converted into absolute Ca2+ concentrations using a two point-calibration, whereby the signal inside the solid chip corresponds to zero Ca2+ and the signal far away from the chip corresponds to the medium in equilibrium with calcite (10 mM Ca2+). Concentration versus fluorescence curves for calcium green in PES medium were run separately in a spectrofluorometer (Turner Designs). In PES medium, at pH 8.3, the dissociation constant for the dye was approximately 5 mM, and a linear approximation described the relationship as well as a hyperbolic one in the measured range. The linear relationship was used for extrapolation into the supersaturated region, as it will bias by defect (i.e., it is a conservative concentration estimator). The saturation kinetics of the dye was pH-dependent, so that potentially, pH excursions of the medium may have influenced the measurement of Ca2+. Potential pH changes (an alkalinization of the medium by either photosynthesis, or by the uptake of protons), if present, could have only resulted in an underestimation of Ca2+ concentration.
Forty gene sequences of cyanobacterial P-type ATPases from the National Center for Biotechnology Information database and Cyanobase were identified by having both Ca2+ and ATP-binding motifs. The sequence alignments at both amino acid and nucleotide level were analyzed. Group-specific sequence alignments were used to design degenerate primers matching conserved sequence regions corresponding to the phosphorylation and ATP-binding sites. These primers were used to clone putative Ca2+ P-type ATPases from BC008 through PCR amplification of its genomic DNA. PCR products were cloned into pCR4 vector through the TOPO TA cloning system (Invitrogen). Primers for the specific housekeeping genes, 16S rRNA gene and rnpB (the RNA component of RNase P), were also designed. High quality, DNA-free RNA extracts (20) from each sample were used to synthesize cDNA, primed using random hexamers (BioRad). Primers specific to each putative gene obtained in cloning efforts, as well as to the housekeeping genes, were designed and used to amplify approximately 0.15-kbp fragments from the cDNA in each extract using a real-time quantitative PCR (qPCR) procedure. All samples and controls are listed in SI Text. To evaluate differential gene expression, we used REST software, which addresses the measurement of uncertainty in expression ratios by using randomization and bootstrapping techniques (32). The quantification strategy is based on the expression ratio of a target gene versus reference gene(s). The difference in qPCR signal between control (nonboring culture) and sample is the numerator. In the denominator, one uses the difference in qPCR signal between control and sample for the reference gene. We used two reference genes: rnpB and 16S rRNA. Results using either one were consistent, but the statistics were more robust when using a combination of both. The data we present are based on the geometric mean between the two corresponding differences. We used bootstrapping with 5,000 iterations to provide 95% confidence intervals for expression ratios.
Supplementary Material
Acknowledgments
We thank Douglas Chandler and Page Baluch for microscopy training, Scott Bates for help with RT-PCR, and Ruth Potrafka for general laboratory support. This research was funded by National Science Foundation Grant 0311945 (to F.G.-P.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011884108/-/DCSupplemental.
References
- 1.Golubic S, Friedmann EI, Schneider J. The lithobiontic ecological niche, with special reference to microorganisms. J Sediment Res. 1981;51:475–478. [Google Scholar]
- 2.Trudgill ST. Bioerosion of intertidal limestone, Co. Clare, Eire- 3: Zonation, process and form. Mar Geol. 1987;74:111–121. [Google Scholar]
- 3.Shachak M, Jones CG, Granot Y. Herbivory in rocks and the weathering of a desert. Science. 1987;236:1098–1099. doi: 10.1126/science.236.4805.1098. [DOI] [PubMed] [Google Scholar]
- 4.Le Campion-Alsumard T, Golubic S, Hutchings P. Microbial endoliths in skeletons of live and dead corals: Porites Lobata (Moorea, French Polynesia) Mar Ecol Prog Ser. 1995;117:149–157. [Google Scholar]
- 5.Aline T. Dissolution of dead corals by euendolithic microorganisms across the northern Great Barrier Reef (Australia) Microb Ecol. 2008;55:569–580. doi: 10.1007/s00248-007-9302-6. [DOI] [PubMed] [Google Scholar]
- 6.Golubic S, Seong-Joo L, Browne KM. Cyanobacteria: Architects of Sedimentary Structures. In: Riding R, Awramik SM, editors. Microbial Sediments. New York: Springer-Verlag; 2000. pp. 57–67. [Google Scholar]
- 7.Zardi GI, Nicastro KR, McQuaid CD, Gektidis M. Effects of endolithic parasitism on invasive and indigenous mussels in a variable physical environment. PLoS ONE. 2009;4:e6560. doi: 10.1371/journal.pone.0006560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexandersson ET. Marks of unknown carbonate-decomposing organelles in cyanophyte borings. Nature. 1975;254:237–238. [Google Scholar]
- 9.Schneider J, Le Campion-Alsumard T. Construction and destruction of carbonates by marine and freshwater cyanobacteria. Eur J Phycol. 1999;34:417–426. [Google Scholar]
- 10.Garcia-Pichel F. Plausible mechanisms for the boring on carbonates by microbial phototrophs. Sediment Geol. 2006;185:205–213. [Google Scholar]
- 11.Cockell CS, Herrera A. Why are some microorganisms boring? Trends Microbiol. 2008;16:101–106. doi: 10.1016/j.tim.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Tribollet A, Atkinson MJ, Langdon C. Effects of elevated pCO2 on epilithic and endolithic metabolism of reef carbonates. Glob Change Biol. 2006;12:2200–2208. [Google Scholar]
- 13.Tribollet A. The boring microflora in modern coral reefs: a review of its roles. In: Wisshak M, Tapanila L, editors. Current Developments in Bioerosion. Heidelberg: Springer-Verlag; 2008. pp. 67–94. [Google Scholar]
- 14.Lagerheim G. Note sur le Mastigocoleus, nouveau genre des algues marines de l’ ordre des Phycochromacées. Notarisia. 1886;1:65–69. [Google Scholar]
- 15.Chacón E, Berrendero E, Garcia-Pichel F. Biogeological signatures of microboring cyanobacterial communities in marine carbonates from Cabo Rojo, Puerto Rico. Sediment Geol. 2006;185:215–228. [Google Scholar]
- 16.Walsby AE. Cyanobacterial heterocysts: Terminal pores proposed as sites of gas exchange. Trends Microbiol. 2007;15:340–349. doi: 10.1016/j.tim.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Wade BD, Garcia-Pichel F. Evaluation of DNA extraction methods for molecular analyses of microbial communities in modern microbialites. Geomicrobiol J. 2003;20:549–561. [Google Scholar]
- 18.Nagata T, Iizumi S, Satoh K, Kikuchi S. Comparative molecular biological analysis of membrane transport genes in organisms. Plant Mol Biol. 2008;66:565–585. doi: 10.1007/s11103-007-9287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kühlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 20.Soule T, Garcia-Pichel F, Stout V. Gene expression patterns associated with the biosynthesis of the sunscreen scytonemin in Nostoc punctiforme ATCC 29133 in response to UVA radiation. J Bacteriol. 2009;191:4639–4646. doi: 10.1128/JB.00134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obara K, et al. Structural role of countertransport revealed in Ca(2+) pump crystal structure in the absence of Ca(2+) Proc Natl Acad Sci USA. 2005;102:14489–14496. doi: 10.1073/pnas.0506222102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torrecilla I, Leganés F, Bonilla I, Fernández-Piñas F. A calcium signal is involved in heterocyst differentiation in the cyanobacterium Anabaena sp. PCC7120. Microbiology. 2004;150:3731–3739. doi: 10.1099/mic.0.27403-0. [DOI] [PubMed] [Google Scholar]
- 23.Margolis S, Rex RW. Endolithic algae and micrite envelope formation in bahamian oolites as revealed by scanning electron microscopy. Geol Soc Am Bull. 1971;82:843–852. [Google Scholar]
- 24.Bathurst RGC. Boring algae, micrite envelopes and lithification of molluscan biosparites. Geol J. 1966;5:15–32. [Google Scholar]
- 25.Kobluk DR, Risk MJ. Micritization and carbonate-grain binding by endolithic algae. AAPG Bull. 1977;61:1069–1082. [Google Scholar]
- 26.Friedman GM, Schultz D, Guo B, Sanders JE. Vaterite (an uncommon polymorph of CaCO3); occurrences in boreholes demonstrate unexpected longevity. J Sediment Res. 1993;63:663–664. [Google Scholar]
- 27.Macintyre IG, Prufert-Bebout L, Reid RP. The role of endolithic cyanobacteria in the formation of lithified laminae in Bahamian stromatolites. Sedimentology. 2000;47:915–921. [Google Scholar]
- 28.Orr JC, et al. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437:681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- 29.Tribollet A, Godinot C, Atkinson M, Langdon C. Effects of elevated pCO2 on dissolution of coral carbonates by microbial euendoliths. Global Biogeochem Cycles. 2009;23:GB3008. [Google Scholar]
- 30.De'ath G, Lough JM, Fabricius KE. Declining coral calcification on the Great Barrier Reef. Science. 2009;323:116–119. doi: 10.1126/science.1165283. [DOI] [PubMed] [Google Scholar]
- 31.Provasoli L. Media and prospects for the cultivation of marine algae. In: Watanabe A, Hattori A, editors. Cultures and Collections of Algae. Proceedings of the U.S.–Japan Conference. Hakone, Japan, September 1966. Tokyo: Japanese Society of Plant Physiology; 1968. pp. 63–75. [Google Scholar]
- 32.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.