Abstract
Hyperphosphorylated tau plays an important role in the formation of neurofibrillary tangles in brains of patients with Alzheimer's disease (AD) and related tauopathies and is a crucial factor in the pathogenesis of these disorders. Though diverse kinases have been implicated in tau phosphorylation, protein phosphatase 2A (PP2A) seems to be the major tau phosphatase. Using murine primary neurons from wild-type and human tau transgenic mice, we show that the antidiabetic drug metformin induces PP2A activity and reduces tau phosphorylation at PP2A-dependent epitopes in vitro and in vivo. This tau dephosphorylating potency can be blocked entirely by the PP2A inhibitors okadaic acid and fostriecin, confirming that PP2A is an important mediator of the observed effects. Surprisingly, metformin effects on PP2A activity and tau phosphorylation seem to be independent of AMPK activation, because in our experiments (i) metformin induces PP2A activity before and at lower levels than AMPK activity and (ii) the AMPK activator AICAR does not influence the phosphorylation of tau at the sites analyzed. Affinity chromatography and immunoprecipitation experiments together with PP2A activity assays indicate that metformin interferes with the association of the catalytic subunit of PP2A (PP2Ac) to the so-called MID1-α4 protein complex, which regulates the degradation of PP2Ac and thereby influences PP2A activity. In summary, our data suggest a potential beneficial role of biguanides such as metformin in the prophylaxis and/or therapy of AD.
Formation of paired helical filaments (PHFs) in the brain is a characteristic hallmark in the pathogenesis of Alzheimer's disease (AD) and related tauopathies. The major protein component of PHFs is the hyperphosphorylated form of tau, which in its normophosphorylated form is a microtubule-associated protein that stimulates and stabilizes microtubule assembly (1). Whereas tau normally contains 2–3 mol of phosphates per mole, tau phosphorylation levels in AD brains are three- to fourfold higher. Upon hyperphosphorylation, tau dissociates from the microtubules and subsequently sequesters normal tau and other microtubule-associated proteins, which inhibits assembly and depolymerizes microtubules (2).
Tau in PHFs has been found to be differently phosphorylated at more than 30 serine/threonine residues compared with normal tau (3). Among the classical phosphoseryl/phosphothreonyl phosphatases, PP2A seems to be the major tau phosphatase in the brain (4–8). Indeed, reduction of both activity and expression of PP2A has been observed in AD brains repeatedly (9–13). This makes PP2A activity a valuable target for the development of a potential therapy for AD.
Though the dimethylbiguanide metformin and related guanidines have been used as antidiabetics for many years, their precise mechanisms of action remain unclear. One favored mechanism involves the activation of AMPkinase (AMPK) via the inhibition of complex I of the respiratory chain (14–16). Activated AMPK carries the signal to the mTOR pathway. mTOR signaling integrates the information on nutrient and energy supply and stimulates cell growth and proliferation. Activated AMPK, however, is a sensor for an inappropriate AMP:ATP ratio and thereby detects insufficient energy reserve. It phosphorylates the mTOR inhibitor TSC2 (17) and the mTOR interaction factor raptor (18), which both lead to a reduction of mTOR kinase activity and an activation of the major mTOR inhibitor PP2A (19).
Because of the interaction of metformin with AMPK, mTOR, and PP2A, we have hypothesized that biguanides, by activating AMPK and PP2A, would be potent compounds to dephosphorylate tau. Here we show that, indeed, treatment of primary neurons with metformin and derivatives leads to an immediate reduction of the phosphorylation of PP2A-dependent tau epitopes. Surprisingly, PP2A activation by metformin seems to be AMPK stimulation independent, because it did not lead to an increase of the phosphorylation of the AMPK target ACC and induced only a weak stimulation of the phosphorylation of AMPK itself. Furthermore, the AMPK activator AICAR did not cause similar effects on tau phosphorylation.
Results
Okadaic Acid Increases Phosphorylation of PP2A-Dependent Tau Epitopes.
Tau is phosphorylated at numerous serine and threonine residues. Several of these residues have been shown to depend on PP2A activity and be dephosphorylated by PP2A in vitro (1, 20, 21). To confirm these observations in living cells, primary cortical neurons from wild-type mice were treated with increasing concentrations of okadaic acid (OA) for 4 h. Protein lysates of treated cells were analyzed by Western blot. Phosphorylation of Serine (Ser) 202, Ser262, Ser356, and Ser396 was analyzed relative to total tau using specific antiphospho antibodies and the anti-tau5 antibody detecting total tau. As expected, phosphorylation of Ser202, Ser262, and Ser356 increased with the concentration of OA (Fig. S1), confirming that the phosphorylation of these epitopes is regulated by PP2A or a closely related phosphatase. Phosphorylation of Ser396, however, did not increase significantly in cells treated with OA, which confirms previous observations that Ser396 is not an efficient PP2A target (21).
Metformin Inhibits mTORC1 and Activates PP2A.
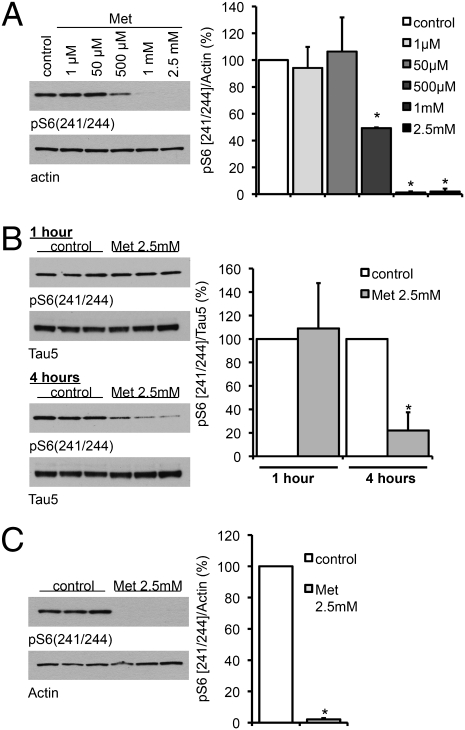
The anti-type II diabetes drug metformin has been shown to inhibit mTOR activity (22, 23). Because PP2A is regulated by mTOR (24), we speculated that metformin would be able to (i) increase PP2A activity and (ii) thereby reduce tau phosphorylation at PP2A-dependent epitopes. To confirm that metformin could inhibit mTORC1 signaling in primary neurons from wild-type mice, we measured the phosphorylation of S241/244 of ribosomal protein S6 following incubation with increasing concentrations of metformin (Fig. 1). A dose-dependent decrease in S6 phosphorylation was detected (Fig. 1A), and significant dephosphorylation was present within 4 h of exposure to 2.5 mM metformin (Fig. 1B). This ability of metformin to dephosphorylate S6 was not specific to neurons, but clearly apparent in the rat hepatoma cell line H4IIE, which were incubated with metformin for 16 h (Fig. 1C). Results were confirmed by measuring the phosphorylation status of p70S6kinase, another target of mTORC1/PP2A after metformin treatment (Fig. S2) in primary neurons. Furthermore, phenformin, a metformin derivative, also promoted dephosphorylation of p70S6kinase with time (Fig. S3).
Fig. 1.
Metformin decreases the phosphorylation of S6 ribosomal protein. (A) Primary cortical neurons of wild-type mice were treated with metformin (Met) at the indicated concentrations for 16 h. (B) Primary cortical neurons of wild-type mice were treated with or without 2.5 mM metformin for 1 h and 4 h. (C) H4 cells were treated with or without 2.5 mM metformin for 16 h. Cell lysates were analyzed by Western blotting using an antibody to phosphorylated S6 (241/244). Band intensities were normalized to either actin or total tau (Tau5). In each case a representative blot is shown along with the quantification of three independent experiments (n = 3). *P < 0.01.
Metformin Induces Dephosphorylation of Tau in Cortical Neurons.
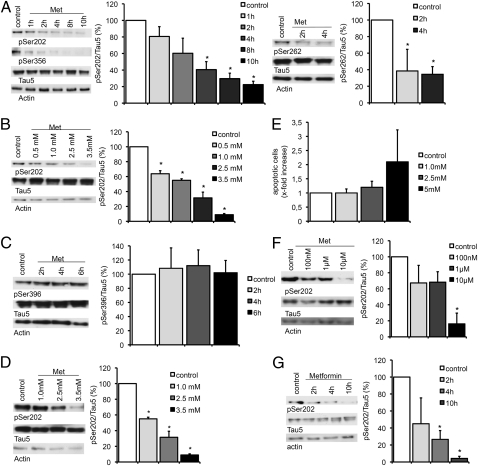
Because activation of PP2A should result in a dephosphorylation of PP2A-dependent tau epitopes, primary cortical neurons from wild-type mice were treated with 2.5 mM metformin for different time intervals, and the phosphorylation of the PP2A-dependent tau epitopes Ser202, Ser356, and Ser262 was analyzed by Western blot. A significant decrease of the phosphorylation of all three epitopes was observed after 2–4 h. Furthermore, dephosphorylation of these epitopes increased with time (Fig. 2A) and dosage (Fig. 2B). By contrast, phosphorylation of the PP2A-insensitive epitope Ser396 did not change after metformin treatment (Fig. 2C), indicating that metformin only affects PP2A-dependent sites. Similar analyses of primary cortical neurons from mice that express the human tau protein instead of the murine showed that human tau epitopes reacted in the same way to metformin treatment (Fig. 2D). Comparable results were obtained when treating cells with the metformin derivative phenformin (Fig. S4A) and with the mTOR inhibitor/PP2A activator rapamycin (Fig. S4B), which adds further proof that indeed, the enzyme couple mTOR/PP2A influences phosphorylation of tau at the sites analyzed. To make sure that the observed effects are not due to an increase in cellular apoptosis, TUNEL assays with increasing amounts of metformin on primary cortical neurons were performed showing that metformin did not cause a significant increase of programmed cell death in these cells at concentrations sufficient to induce tau dephosphorylation (Fig. 2E).
Fig. 2.
Metformin induces dephosphorylation of tau at PP2A-sensitive sites. Representative Western blots and quantifications of three or four independent experiments are shown in A–D, F, and G. Band intensities were normalized to actin and compared with total tau (Tau 5). (A) Primary cortical neurons of wild-type mice were treated with 2.5 mM metformin (Met) over increasing time intervals. Cell lysates were analyzed by Western blot using antibodies detecting phosphorylation at specific PP2A-sensitive tau sites (Ser202, Ser262, Ser356; n = 3). (B) Primary cortical neurons from wild-type mice were treated with increasing concentration of metformin over 4 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at Ser202 (n = 4). (C) Primary cortical neurons of wild-type mice were treated with 2.5 mM metformin over increasing time intervals. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at the PP2A-insensitive tau site Ser396 (n = 3). (D) Primary cortical neurons of transgenic mice expressing human tau instead of murine tau were treated with increasing concentration of metformin over 4 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at Ser202 (n = 3). (E) TUNEL assay of primary cortical neurons of wild-type mice treated with increasing concentrations of metformin. Percentages of apoptotic cells are shown. (F) Primary cortical neurons of wild-type mice were treated with low concentrations of metformin over 24 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at Ser202 (n = 3). (G) Primary cortical neurons of wild-type mice were treated with 2.5 mM metformin over increasing time intervals in medium without insulin. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at Ser202 (n = 3). *P < 0.05.
Metformin has been in clinical use for patients with type II diabetes for many years. Peak plasma concentrations at dosages that are being applied to patients, however, are around 12 μM. These are much lower than what we have used in the above experiments. Because tau dephosphorylation increases with time after metformin treatment, in the next series of experiments, metformin concentrations were reduced significantly using longer treatment periods (>24 h) instead. Again, primary cortical neurons of wild-type mice were treated with the respective amounts of metformin and subsequently analyzed for phosphorylation at the PP2A-dependent tau site Ser202. Already at 100 nM metformin, phosphorylation of Ser202 decreased slightly, whereas at 10 μM, phosphorylation of Ser202 is significantly reduced (Fig. 2F).
Metformin effects have been shown to be insulin sensitive. To rule out an insulin-dependent effect on tau phosphorylation, we incubated primary neurons with 2.5 mM metformin, this time using medium without insulin. As in the previous experiments, metformin had a strong dephosphorylation effect on tau (Fig. 2G), suggesting that metformin acts in an insulin-independent way.
Metformin Functions Through PP2A Activity.
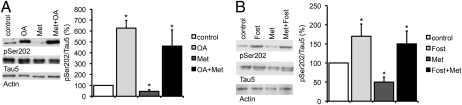
To show that metformin requires PP2A activity to regulate tau dephosphorylation, primary cortical neurons of wild-type mice were incubated with or without 10 nM OA before metformin treatment. In confirmation of our previous findings, phosphorylation of Ser202 increased after OA treatment and decreased significantly after treatment with 2.5 mM metformin over 4 h. By contrast, metformin had no effect on the Ser202 phosphorylation pattern of OA-treated cells (Fig. 3A), indicating that the metformin effect on the phosphorylation of tau depends on PP2A activity. Similar data were generated with the metformin derivative phenformin (Fig. S5). In confirmation of the OA data, primary neurons were also treated with the more specific PP2A inhibitor fostriecin. Again, whereas phosphorylation at Ser202 increased after treatment with 1 μM fostriecin over 4 h and decreased after treatment with 2.5 mM metformin over the same period, metformin did not have any effect on fostriecin-pretreated cells (Fig. 3B).
Fig. 3.
Inhibition of PP2A blocks the metformin effect on the phosphorylation of tau. Representative Western blots and quantifications of three or four independent experiments are shown in the subsequent experiments. Band intensities were normalized to actin and compared with total tau (Tau 5). (A) Primary cortical neurons of wild-type mice were treated with either OA (10 nM), only metformin (Met, 2.5 mM), or with OA (10 nM) before metformin (2.5 mM) for 4 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at a specific PP2A-sensitive tau site (n = 4). *P < 0.05. (B) Primary cortical neurons of wild-type mice were treated with either fostriecin (Fost, 1 μM), only metformin (Met, 2.5 mM), or with fostriecin (1 μM) before metformin (2.5 mM) over 4 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at a specific PP2A-sensitive tau site (n = 3). *P < 0.05.
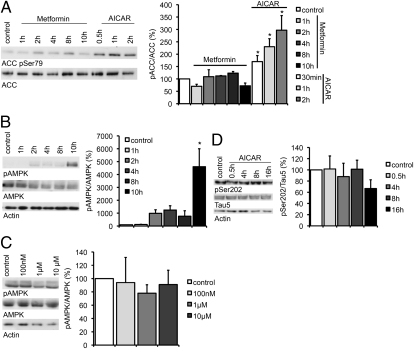
Because metformin had been shown in other systems to activate AMPK, an antagonist of mTOR, we assumed that metformin would activate PP2A via AMPK activation. To examine this hypothesis, primary cortical neurons were treated with 2.5 mM of metformin and analyzed by Western blot for the phosphorylation of the AMPK target ACC using an antibody detecting phosphorylated Ser79. The AMPK agonist AICAR was used as a positive control. To our surprise, though ACC phosphorylation significantly increased after AICAR treatment, neither metformin (Fig. 4A) nor phenformin (Fig. S6) had an effect on the phosphorylation pattern of ACC. Analysis of the phosphorylation pattern of AMPK itself showed that metformin does induce AMPK phosphorylation significantly after incubation with 2.5 mM metformin for 10 h (Fig. 4B). However, no significant effect was seen after shorter intervals of incubation with 2.5 mM metformin or when using smaller amounts of metformin for a time period of 24 h (Fig. 4C), indicating that metformin does not induce AMPK activity in primary cortical neurons in the concentration and the time intervals that are necessary for PP2A activation and tau dephosphorylation. Furthermore, we did not find a significant effect of the AMPK agonist AICAR on the phosphorylation pattern of tau during the first 16 h of treatment (Fig. 4D), which further indicates that AMPK activation alone is not sufficient to mimic the effect of metformin on tau and that metformin could mediate tau dephosphorylation independently of AMPK.
Fig. 4.
Metformin effects on the phosphorylation of tau are AMPK independent. Representative Western blots and quantifications of three to five independent experiments are shown in the subsequent experiments. (A) Primary cortical neurons of wild-type mice were treated with either 2.5 mM of metformin (Met) or with 5 mM of AICAR over increasing time intervals. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation of the AMPK target ACC. Band intensities were compared with total ACC (n = 4). (B) Primary cortical neurons of wild-type mice were treated with 2.5 mM metformin over increasing time intervals. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylated AMPK (pThr 172). Band intensities were compared with total AMPK (n = 3). (C) Primary cortical neurons of wild-type mice were treated with increasing concentrations of metformin over 24 h. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation of AMPK (pThr 172). Band intensities were normalized to AMPK total (n = 3). (D) Primary cortical neurons of wild-type mice were treated with 5 mM of AICAR over increasing time intervals. Cell lysates were analyzed by Western blot using an antibody detecting phosphorylation at a specific PP2A-sensitive tau site. Band intensities were normalized to actin and compared with total tau (Tau 5; n = 5). *P < 0.05.
Metformin Interferes with the Composition of the PP2A Protein Complex.
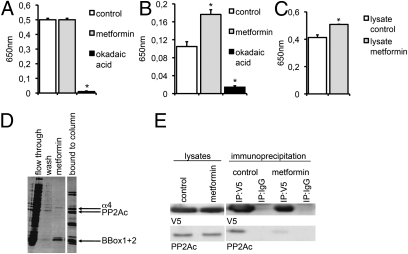
To analyze if metformin has direct effects on PP2A activity, either purified PP2Ac (catalytic subunit of PP2A) or PP2A holoenzymes, which were immunopurified from HeLa cell lysates using a PP2A-A subunit-specific antibody, were incubated with or without 10 mM metformin or 10 nM OA. Subsequently, PP2A activity was measured with a commercial PP2A activity assay. Though no influence of metformin on purified PP2Ac was measured (Fig. 5A), activity of the immunopurified PP2A protein complex was significantly increased after metformin incubation (Fig. 5B), indicating that the metformin effect on PP2A activity is mediated by a component of the PP2A protein complex. The PP2A inhibitor OA reduced the activity of purified PP2Ac and the PP2A immunocomplex significantly. Furthermore, we have treated primary neurons with metformin and analyzed PP2A activity in the cell lysates. Again, metformin treatment led to a significantly increased phosphatase activity (Fig. 5C).
Fig. 5.
Metformin increases PP2A activity in vitro. Three independent experiments were measured in A–C. (A) Purified PP2Ac was incubated with or without metformin (10 mM) or OA (10 nM). PP2A activity was determined in a malachite green assay. (B) Immunoprecipitated PP2A holoenzymes were incubated with or without metformin (10 mM) or OA (10 nM). PP2A activity was determined in a malachite green assay. (C) Cells were treated with or without metformin (2.5 mM) and lysed. PP2A activity was determined in a malachite green assay (n = 3). *P < 0.05. (D) Assembly of the PP2Ac- MID1-α4 protein complex is influenced by metformin. Affinity chromatography analysis shows the binding of immobilized PP2Ac to the MID1 B-box domains and α4. The flow-through, the washing fraction, the fraction obtained with a buffer containing 100 μM metformin, and the final elution fraction were collected and analyzed on SDS gels. Gels were stained with Coomassie. (E) Association between PP2Ac and α4 is influenced by metformin. Coimmunoprecipitations of PP2Ac and V5-tagged α4 in the presence or absence of metformin were performed and analyzed on Western blots using PP2Ac- or V5-specific antibodies. This experiment has been repeated three times.
PP2A activity can be regulated by association of PP2Ac with the α4 protein. α4 binds to the B-box domains of the MID1 protein and thereby triggers the binding of the microtubule-associated pool of PP2Ac to the ubiquitin ligase MID1. Via this interaction, MID1 can ubiquitinate PP2Ac and induce its proteasomal degradation, thereby regulating the activity of microtubule-associated PP2Ac (25). To test if metformin might induce the activity of PP2A by interfering with its negative regulators MID1 and α4, we analyzed the binding of the protein complex in an in vitro assay. All three proteins (α4, the B-box domains of MID1, and PP2Ac) were expressed in Escherichia coli and mixed to allow protein complex assembly. Afterward the complexes were bound to a column via the His-tagged PP2Ac. Interestingly, both, the B-boxes and α4 could be eluted from the column by adding metformin to the washing buffer (Fig. 5D). To confirm that metformin inhibits the assembly of the MID1-α4-PP2Ac complex in cells, we performed coimmunoprecipitation experiments using overexpressed V5-tagged α4 protein and analyzed the amount of PP2Ac that is associated with α4 in the presence or absence of 2.5 mM metformin. Clearly, addition of 2.5 mM metformin to lysates of α4-V5-expressing HeLa cells led to a dissociation of α4 from PP2Ac (Fig. 5E). Taken together, these data suggest that metformin activates PP2A activity by inhibiting the binding of PP2Ac to its negative regulators MID1 and α4.
Metformin Induces Dephosphorylation of Tau in Vivo.
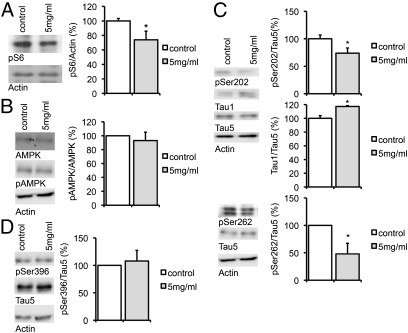
Metformin had previously been shown to be active in the brain after oral administration (26, 27). Chen et al. (27) have found that a dosage of only 2 mg/mL in the drinking water fed to mice for 6 d would cause equivalent levels of 1 μM of metformin in the brain. To see if metformin would activate PP2A and dephosphorylate tau in vivo, we fed pairs of mice with or without 5 mg/mL of metformin in the drinking water for 16–24 d. Brains were lysed and analyzed via Western blot for phosphorylation of S6 and AMPK. Though no induction of AMPK phosphorylation was seen, S6 was significantly dephosphorylated in the metformin-treated samples (Fig. 6 A and B and Fig. S7A). Phosphorylation of tau was tested at Ser202 using both an antibody detecting the phosphorylated form and the tau-1 antibody that detects tau dephosphorylated at the same site. In addition, epitopes Ser262 and the PP2A-independent epitope Ser396 were analyzed. A significant decrease of Ser202 and Ser262 phosphorylation and an increase of the dephosphorylated form of tau at position Ser202 were observed in the metformin-treated mice (Fig. 6C and Fig. S7B), indicating that metformin has an in vivo effect on the phosphorylation of tau. In confirmation with the data in the primary neurons, no effect of metformin on Ser396 was detected (Fig. 6D).
Fig. 6.
Metformin decreases tau phosphorylation in vivo. Pairs of wild-type mice were fed with or without 5 mg/mL of metformin in the drinking water for 16–24 d. Representative Western blots and quantification of three technical replicates are shown in the subsequent experiments. (A) Brains were lysed and analyzed via Western blots for phosphorylation of S6. Band intensities were compared with actin. (B) Brains were lysed and analyzed via Western blots for phosphorylation of AMPK (pThr 172). Band intensities were compared with AMPK total. (C) Brains were lysed and analyzed via Western blots for phosphorylation of Tau. pSer202 was detected using a phospho-specific pSer202 antibody and a tau1 antibody, which detects dephosphorylated Ser202. Phosphorylation at position Ser262 was detected using a phospho-specific Ser262 antibody. Band intensities were normalized to actin and compared with total tau (Tau5). (D) Brains were lysed and analyzed via Western blots for phosphorylation of the PP2A-insensitive site pSer396. Band intensities were normalized to actin and compared with total tau (Tau5). *P < 0.05.
Discussion
Hyperphosphorylation of the microtubule-associated tau protein is a major component in the pathogenesis of AD and related tauopathies. We show here that, in confirmation with the literature, several of the sites that are hyperphosphorylated in AD respond to OA treatment, an inhibitor of PP2A activity. Furthermore, we show that the anti-type II diabetes medication metformin and its derivative phenformin activate PP2A and efficiently dephosphorylate tau in vitro and in vivo in a PP2A-dependent manner.
PP2A is regulated by the kinase mTOR (19), and both enzymes affect the phosphorylation status of p70S6kinase and the ribosomal protein S6. As we show here, the phosphorylation status of p70S6kinase and S6 is reduced after metformin treatment. However, it is difficult to distinguish if metformin activates PP2A or, as had been postulated before in cancer cells, primarily inhibits mTOR activity (22, 28). Here we found that metformin has a direct effect on the association between PP2Ac, the regulatory subunit of PP2A—α4—and the ubiquitin ligase MID1, which targets microtubule-associated PP2A for degradation via the proteasome. Our data suggest that metformin interferes with PP2A activity directly by inhibiting its proteasomal degradation rather than via mTOR inhibition.
In a recent paper, Meske and coworkers (29) demonstrated that PP2A activity and GSK3β, which is the PP2A counteracting kinase on many tau phosphorylation sites, are tightly regulated in neurons. Consistent with their data, we did not see an effect of the AMPK agonist AICAR on the phosphorylation of tau (Ser202) in the first 8 h of treatment, although AMPK is activated within that time. Furthermore, we found that metformin reduces the phosphorylation of GSK3β at position Ser9, thereby activating the enzyme (Fig. S8). Taken together, these data and our observations suggest that (i) in primary neurons, metformin has an acute and prolonged effect on the activity of PP2A, which is likely to overcome GSK3β counterregulation of tau phosphorylation, and (ii) AMPK activation alone is not sufficient to mimic the action of metformin on the phosphorylation of tau.
It is perhaps surprising that we found that metformin and phenformin effects on tau phosphorylation in primary cortical neurons seem to be largely independent of AMPK. It is generally accepted that metformin, by inhibiting complex I of the respiratory chain, leads to a rise of cellular AMP:ATP ratios. AMP binding to AMPK increases AMPK phosphorylation by LKB1, which then results in an increase of AMPK activity (30). However, there is growing evidence that metformin also functions in an AMPK-independent manner in several cell systems. Thus, metformin has been shown to suppress the overexpressed oncoprotein HER2 in breast cancer cells (22) and to decrease prostate tumor cell growth by influencing cyclin D1 levels (31). In both cases, metformin effects are not abolished by AMPK inhibition. HER2 suppression, however, can sufficiently be blocked by knockdown of the PP2A and mTOR target p70S6kinase, pointing at a PP2A/mTOR-dependent mechanism underlying metformin function. Also, some of the metabolic effects seen in isolated working rat hearts and cultures of heart-derived cells after metformin treatment clearly seem AMPK independent (32). In accordance with these data, we show here that metformin has an immediate effect on the phosphorylation of the PP2A/mTOR targets p70S6 kinase, S6, and tau, but does not show a significant influence on the phosphorylation of the AMPK target ACC, and only a comparably small effect on AMPK itself in primary cortical neurons at the concentrations used here. Its effects are efficiently blocked by the PP2A inhibitors OA and fostriecin. Furthermore, whereas metformin (and its derivative phenformin) reduce tau phosphorylation acutely, the AMPK agonist AICAR does not seem to have a comparable effect. This points to an AMPK-independent mode of action of metformin on PP2A activity, or at least to one requiring more than simply AMPK activation.
Rapamycin-sensitive association of the catalytic PP2A subunit with the yeast protein TAP42 or its mammalian homolog α4 plays an important role in the regulation of PP2A activity and is probably the most important link between mTOR and PP2A. Importantly, inhibition of mTOR by rapamycin leads to the dissociation of PP2A and α4. The interaction between PP2A and α4 is an unconventional association that involves the catalytic subunit but not the structural A subunit and seems to influence PP2A activity in a tissue-dependent way (33–35). We show here that metformin influences the association between PP2A and α4, thereby possibly inducing PP2A activity.
Recently growing evidence for beneficial effects of metformin on diseases other than diabetes have been presented—particularly, metformin long-term use is associated with lower risk of certain cancers (36, 37). In contrary to such beneficial effects, metformin has been found to induce BACE1 transcription and to increase Aβ production in neuronal cell lines and primary neurons in the absence of insulin (27). Aβ forms the core of amyloid plaques, which are the second pathogenic hallmark in brains of AD patients. However, these effects can be inverted by the addition of insulin, leaving the question open of what effects metformin would cause on BACE1 expression and Aβ production in an individual with normal insulin levels. Our data show a significant insulin-independent influence of metformin and its derivative phenformin on the phosphorylation pattern of the AD-related tau protein, both after acute and chronic treatment and in vitro and in vivo. Our data therefore suggest a potential beneficial effect of long-term metformin treatment and raise the hope that metformin would have a neuroprotective and prophylactic effect in patients with the predisposition for AD. In a next step, this would have to be shown in AD mouse models. However, most of the available AD mouse models express a mutant tau variant, which has been found in the rare familial forms of AD with early onset. Ideally, metformin regulation of tau phosphorylation and cognition has to be examined in models of sporadic AD.
Methods
Primary Cultures.
Primary cortical neurons were isolated from brain of WT or transgenic [Mapttm1(EGFP)klt Tg(MAPT)8cPdav/J; Jackson Laboratory] embryos at day 14.5. Cortices were collected in DMEM (Lonza), and cells were dissociated by incubation with trypsin/EDTA for 6.5 min at 37 °C. Cells were then diluted in neurobasal medium (Gibco-BRL) containing B27 supplement (Gibco-BRL). Cells were plated at a density of 4.0 × 105 onto 0.2 mg/mL poly-D-lysine (Sigma) and 2 μg/mL laminin-coated (Sigma) 12-well plates. Neurons were incubated at 37 °C with 8% CO2. One hour after seeding, a complete change of medium was performed. Later, each 96 h, half-volumes of the medium were replaced by fresh medium. After 7 d in culture, neurons were treated.
Treatments.
Cells were incubated with the respective substances as follows: metformin (Sigma) at final concentrations up to 2.5 mM for 1 to >24 h; OA (Sigma) at final concentrations from 1 nM to 10 nM for 4 h; fostricien (Sigma) at final concentration of 1 μM for 4 h; and AICAR (Cell Signaling) at final concentration of 5 mM for 0.5–16 h. For cell treatment without insulin, medium containing B27 and insulin supplement was replaced with medium containing B27 supplement without insulin 24 h before treatment. Three groups of two mice were treated with or without 5 mg/L metformin in the drinking water.
Tissue Preparation.
Mice were decapitated and brains were rapidly dissected in nitrogen. Brains were homogenized in ice-cold lysis buffer containing 10 mmol/L Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Nonidet P-40, protease inhibitors (Complete Mini; Roche), and phosphatase inhibitors (PhosStop; Roche). Lysates were sonicated and centrifuged at 25,000 × g for 10 min at 4 °C.
Western Blot and Antibodies.
Cell pellets were homogenized in Magic-Mix [48% urea, 15 mM Tris-HCl (pH 7.5), 8.7% glycerin, 1% SDS, 0.004% bromphenol blue, 143 mM mercaptoethanol], sonicated, and boiled for 5 min at 95 °C. Proteins were resolved on 8% or 10% SDS gels and blotted onto PVDF membranes (Roche). Antibodies used in this study were purchased from the following companies: Tau-5 (Biosource), anti-human PHF pSer202 (Thermo Scientific), Tau pSer356 (Biosource), Tau pSer262 (Biosource), Tau pSer396 (Sigma), Actin (Sigma), ACC (Cell Signaling), ACC pSer79 (Cell Signaling), phospho-S6 ribosomal protein pSer241/244 (Cell Signaling), p70S6 kinase (Cell Signaling), p70S6K pThr421/pSer424 (Cell Signaling), mTOR (Cell Signaling), AMPK (Cell Signaling), AMPK pThr172 (Cell Signaling), HRP anti-rabbit (Amersham), and HRP anti-mouse (Dianova). The resulting bands were quantified using Imagequant5.2. Statistical analyses were performed using SigmaStat software package (v 3.0; SPSS). For time course or dose–response studies, data were analyses by one-way ANOVA with post hoc Dunnett's or Bonferonni test to accommodate for multiple comparisons. A Student's t test was used for two-group comparisons, as appropriate.
TUNEL Assay.
Primary neurons were grown on coverslips with a density of 2 × 105 per 12-well. TUNEL assays were performed using TUNEL enzyme (Roche) and TUNEL label (Roche) following the manufacturer's instructions.
PP2A Activity Assay.
Purified PP2Ac (Upstate), cell lysates, or PP2A-holoenzymes, which were purified by immunoprecipitation using a PP2A-A subunit antibody (Millipore), were incubated with or without 10 mM metformin or 10 nM OA for 1 h. Afterward, PP2A phosphatase activity was determined using a Malachite Green Assay Kit (Upstate) following the manufacturer's instructions.
Affinity Chromatography.
The MID1-B-box domains, α4, or His-tagged PP2Ac were expressed in E. coli BL21. Cells were lysed by French press in buffer A [50 mM sodium phosphate, 300 mM NaCl, 20 mM imidazole (pH 8.0)]. Lysates were mixed, and PP2A protein complexes were immobilized on Ni-NTA columns (Qiagen) and washed extensively with buffer A containing 10% glycerol and 0.25% Tween 20. Afterward, columns were eluted with buffer A containing 100 μM metformin. Final elution of all proteins bound to the column was done using buffer A with 500 mM imidazole. All fractions were analyzed on SDS gels followed by Coomassie staining.
Supplementary Material
Acknowledgments
This work was funded by the Volkswagen Stiftung Lichtenberg Fellowship (to S.S.), Tenovus (T07/38), the Tyrolian Future Foundation, and the Integrated Center of Research and Therapy of the Medical University of Innsbruck.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.0912793107/-/DCSupplemental.
References
- 1.Gong CX, Liu F, Grundke-Iqbal I, Iqbal K. Post-translational modifications of tau protein in Alzheimer's disease. J Neural Transm. 2005;112:813–838. doi: 10.1007/s00702-004-0221-0. [DOI] [PubMed] [Google Scholar]
- 2.Alonso AD, Grundke-Iqbal I, Barra HS, Iqbal K. Abnormal phosphorylation of tau and the mechanism of Alzheimer neurofibrillary degeneration: Sequestration of microtubule-associated proteins 1 and 2 and the disassembly of microtubules by the abnormal tau. Proc Natl Acad Sci USA. 1997;94:298–303. doi: 10.1073/pnas.94.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanger DP, Betts JC, Loviny TL, Blackstock WP, Anderton BH. New phosphorylation sites identified in hyperphosphorylated tau (paired helical filament-tau) from Alzheimer's disease brain using nanoelectrospray mass spectrometry. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–2807. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 5.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron. 1996;17:1201–1207. doi: 10.1016/s0896-6273(00)80250-0. [DOI] [PubMed] [Google Scholar]
- 6.Gong CX, et al. Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res. 2000;853:299–309. doi: 10.1016/s0006-8993(99)02294-5. [DOI] [PubMed] [Google Scholar]
- 7.Bennecib M, Gong CX, Grundke-Iqbal I, Iqbal K. Role of protein phosphatase-2A and -1 in the regulation of GSK-3, cdk5 and cdc2 and the phosphorylation of tau in rat forebrain. FEBS Lett. 2000;485:87–93. doi: 10.1016/s0014-5793(00)02203-1. [DOI] [PubMed] [Google Scholar]
- 8.Kins S, et al. Reduced protein phosphatase 2A activity induces hyperphosphorylation and altered compartmentalization of tau in transgenic mice. J Biol Chem. 2001;276:38193–38200. doi: 10.1074/jbc.M102621200. [DOI] [PubMed] [Google Scholar]
- 9.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 10.Gong CX, et al. Phosphatase activity toward abnormally phosphorylated tau: Decrease in Alzheimer disease brain. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]
- 11.Vogelsberg-Ragaglia V, Schuck T, Trojanowski JQ, Lee VM. PP2A mRNA expression is quantitatively decreased in Alzheimer's disease hippocampus. Exp Neurol. 2001;168:402–412. doi: 10.1006/exnr.2001.7630. [DOI] [PubMed] [Google Scholar]
- 12.Loring JF, Wen X, Lee JM, Seilhamer J, Somogyi R. A gene expression profile of Alzheimer's disease. DNA Cell Biol. 2001;20:683–695. doi: 10.1089/10445490152717541. [DOI] [PubMed] [Google Scholar]
- 13.Sontag E, et al. Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 14.El-Mir MY, et al. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 15.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–614. [PMC free article] [PubMed] [Google Scholar]
- 16.Lander ES, et al. International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 17.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 18.Gwinn DM, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens V, Goris J. Protein phosphatase 2A: A highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trojanowski JQ, Lee VM. Phosphorylation of paired helical filament tau in Alzheimer's disease neurofibrillary lesions: Focusing on phosphatases. FASEB J. 1995;9:1570–1576. doi: 10.1096/fasebj.9.15.8529836. [DOI] [PubMed] [Google Scholar]
- 21.Wang JZ, Grundke-Iqbal I, Iqbal K. Kinases and phosphatases and tau sites involved in Alzheimer neurofibrillary degeneration. Eur J Neurosci. 2007;25:59–68. doi: 10.1111/j.1460-9568.2006.05226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 23.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67:10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg Y. Protein phosphatase 2A: Who shall regulate the regulator? Biochem Pharmacol. 1999;57:321–328. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- 25.Trockenbacher A, et al. MID1, mutated in Opitz syndrome, encodes an ubiquitin ligase that targets phosphatase 2A for degradation. Nat Genet. 2001;29:287–294. doi: 10.1038/ng762. [DOI] [PubMed] [Google Scholar]
- 26.Ma TC, et al. Metformin therapy in a transgenic mouse model of Huntington's disease. Neurosci Lett. 2007;411:98–103. doi: 10.1016/j.neulet.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer's amyloid peptides via up-regulating BACE1 transcription. Proc Natl Acad Sci USA. 2009;106:3907–3912. doi: 10.1073/pnas.0807991106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erdemoglu E, Güney M, Giray SG, Take G, Mungan T. Effects of metformin on mammalian target of rapamycin in a mouse model of endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol. 2009;145:195–199. doi: 10.1016/j.ejogrb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 29.Meske V, Albert F, Ohm TG. Coupling of mammalian target of rapamycin with phosphoinositide 3-kinase signaling pathway regulates protein phosphatase 2A- and glycogen synthase kinase-3 -dependent phosphorylation of Tau. J Biol Chem. 2008;283:100–109. doi: 10.1074/jbc.M704292200. [DOI] [PubMed] [Google Scholar]
- 30.Hardie DG. Minireview: the AMP-activated protein kinase cascade: The key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- 31.Ben Sahra I, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 32.Saeedi R, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:H2497–H2506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 33.Inui S, et al. Ig receptor binding protein 1 (alpha4) is associated with a rapamycin-sensitive signal transduction in lymphocytes through direct binding to the catalytic subunit of protein phosphatase 2A. Blood. 1998;92:539–546. [PubMed] [Google Scholar]
- 34.Nanahoshi M, et al. Regulation of protein phosphatase 2A catalytic activity by alpha4 protein and its yeast homolog Tap42. Biochem Biophys Res Commun. 1998;251:520–526. doi: 10.1006/bbrc.1998.9493. [DOI] [PubMed] [Google Scholar]
- 35.Kong M, Ditsworth D, Lindsten T, Thompson CB. Alpha4 is an essential regulator of PP2A phosphatase activity. Mol Cell. 2009;36:51–60. doi: 10.1016/j.molcel.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.