Abstract
Priming of the organ-specific premetastatic sites is thought to be an important yet incompletely understood step during metastasis. In this study, we show that the metastatic tumors we examined overexpress granulocyte-colony stimulating factor (G-CSF), which expands and mobilizes Ly6G+Ly6C+ granulocytes and facilitates their subsequent homing at distant organs even before the arrival of tumor cells. Moreover, G-CSF–mobilized Ly6G+Ly6C+ cells produce the Bv8 protein, which has been implicated in angiogenesis and mobilization of myeloid cells. Anti–G-CSF or anti-Bv8 antibodies significantly reduced lung metastasis. Transplantation of Bv8 null fetal liver cells into lethally irradiated hosts also reduced metastasis. We identified an unexpected role for Bv8: the ability to stimulate tumor cell migration through activation of one of the Bv8 receptors, prokineticin receptor (PKR)-1. Finally, we show that administration of recombinant G-CSF is sufficient to increase the numbers of Ly6G+Ly6C+ cells in organ-specific metastatic sites and results in enhanced metastatic ability of several tumors.
Keywords: breast cancer, myeloid, CSF3, prokineticin 2
Metastasis is a major cause of death from solid tumors. To metastasize, tumor cells need to degrade and invade the extracellular matrix, intravasate, be carried through blood or lymphatic vessels, extravasate at the secondary site, and finally, establish secondary tumors (1). In addition, recent evidences suggest that, at least in some circumstances, tumors are able to modify the distant microenvironment before arrival of metastatic tumor cells to create the so-called premetastatic niche (2). This ability of tumors to affect distant tissues is expected to enable cancer cells to target specific organs in which they can initiate secondary tumor growth and supports Paget's seed and soil hypothesis. Bone marrow-derived cells (BMDCs) are thought to be major players in these processes (3, 4). Although several molecules have been implicated (3–6), the mechanisms of tumor-dependent BMDC mobilization and the precise identity and significance of these cells in metastasis are incompletely understood.
VEGFR-1, a tyrosine kinase receptor that binds VEGF-A, VEGF-B, and placenta growth factor (7, 8), has been implicated as one of the key regulators of BMDC mobilization and premetastatic priming owing to its expression in a population of hematopoietic progenitor cells and the ability of anti–VEGFR-1 antibodies to reduce metastasis (3). However, more recent studies have questioned this conclusion and reported that anti–VEGFR-1 treatment has no effect in clinically relevant models of metastasis, raising the possibility that alternative pathways mediate tissue priming for metastasis (9). Therefore, key challenges are further defining the molecular and cellular changes occurring in the premetastatic tissues and identifying the factors initiating such premetastatic environment.
In the present study, we analyzed several metastatic and nonmetastatic breast cancer models for their ability to trigger BMDC mobilization. This analysis led to identification of Ly6G+Ly6C+ myeloid cells as a major cell type that accumulates in premetastatic tissues and facilitates colonization by cancer cells and subsequent metastasis. We also identified tumor-derived granulocyte-colony stimulating factor (G-CSF) as a key initiator and regulator of these processes.
Results
Metastatic Tumors Induce Gene Expression Changes in Premetastatic Lungs.
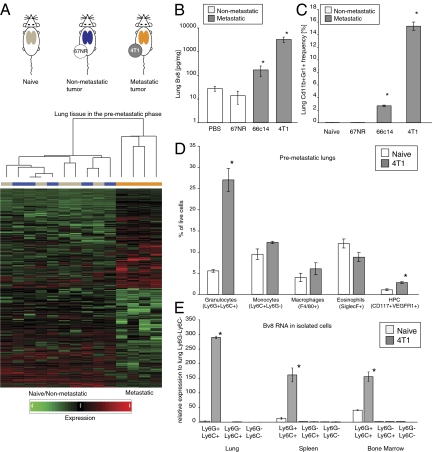
To investigate changes triggered by primary tumors in lungs before the arrival of metastatic tumor cells, we initially used the 4T1-related lines of mouse breast carcinoma as a model (10, 11). These cells provide a phenotypic spectrum ranging from nonmetastatic cells (67NR and 168FARN) to cells able to complete all steps of metastasis (4TO7, 66c14, and 4T1) (Fig. S1A). We performed cDNA microarray comparing total lungs from mice without any tumors (naïve) with those from mice bearing nonmetastatic (67NR) or metastatic (4T1) breast carcinomas in the premetastatic phase (Fig. 1A and SI Text, Defining the Premetastatic Lungs). Our analysis identified 260 genes specifically up-regulated and 274 genes down-regulated more than twofold in lungs of mice bearing 4T1 tumors relative to lungs of naïve mice or mice bearing nonmetastatic 67NR tumors (Fig. 1A). We decided to focus on Bv8, because it was one of the top up-regulated genes (Fig. S1D). Bv8 is a secreted protein that has been previously characterized as a proangiogenic factor (12), an inducer of growth and mobilization of hematopoietic cells (13), and a neuromodulator (14, 15). Quantitative (q)RT-PCR analysis of Bv8 expression in lung tissues confirmed the microarray results (Fig. S1E). Moreover, we found a strong correlation between high Bv8 expression and metastatic potential in multiple tumor models examined. Increased Bv8 levels were measured in the premetastatic lungs of mice bearing mouse 66c14, 4TO7, and 4T1 (Fig. 1B and Fig. S2A) as well as human MDA-MB-231 (Fig. S2B) breast carcinomas. We also detected elevated Bv8 levels in the lungs of mice bearing Lewis Lung Carcinoma (LLC) (Fig. S2B). Furthermore, high Bv8 expression was found in premetastatic lungs of 8-wk-old polyoma virus middle T antigen under control of mouse mammary tumor virus promoter (MMTV-PyMT) transgenic mice (Fig. S2B). Premetastatic stage in MMTV-PyMT mice was confirmed by histology (Fig. S2C).
Fig. 1.
Bv8 is strongly up-regulated in premetastatic lungs of mice bearing metastatic tumors. (A) Design and results of the microarray study comparing gene expression in lungs from BALB/c naïve mice and mice bearing nonmetastatic tumors 67NR and metastatic 4T1 tumors. Lungs from different experimental groups are color-coded (gray, from naïve mice; blue, from 67NR-bearing mice; orange, from 4T1-bearing mice). Note that the metastatic gene expression profile is clearly separated from both naïve and nonmetastatic profiles. Each profile column represents one individual mouse. (B) Bv8 protein concentrations in the premetastatic lungs of mice bearing various tumors (n = 3 per group). (C) FACS analysis of Cd11b+Gr1+ cells in the premetastatic lungs of BALB/c mice bearing various tumors. Asterisk indicates significant difference relative to the nonmetastatic group. (D) FACS analysis of different cell populations in premetastatic lungs from mice bearing 4T1 tumors 2 wk after tumor inoculation. (E) Bv8 expression in Ly6GLy6C cell subpopulations isolated from lungs of naïve or 4T1 tumor-bearing mice. In D and E, asterisk indicates significant difference relative to naïve group. Graphs present means ± SEM.
Bv8-Expressing Ly6G+Ly6C+ Granulocytes Are a Major Component of the Premetastatic Lung Microenvironment.
Cd11b+Gr1+ myeloids are known to be a major source of Bv8 (16, 17). Furthermore, they have been implicated in several important processes in tumor biology (18–21). Therefore, we hypothesized that metastatic tumors are able to modify the lung microenvironment, at least in part, through mobilization of Cd11b+Gr1+ cells from the bone marrow (BM) and subsequent homing in the lungs. In agreement with this hypothesis, we observed increased numbers of Cd11b+Gr1+ cells infiltrating lungs of mice bearing the metastatic tumors 4TO7, 66c14, 4T1, LLC, MDA-MB-231, and MMTV-PyMT, whereas lungs from mice bearing nonmetastatic tumors 67NR did not show any increase in Cd11b+Gr1+ cells (Fig. 1C and Fig. S2D). In addition, we detected only very low numbers of these cells in organs that are not typically a target for metastasis in the 4T1 model, such as kidney (Fig. S2E).
To further define the composition of the premetastatic microenvironment, we performed FACS analysis to characterize Cd11b+Gr1+ cells and identify subpopulations accumulating in the premetastatic lungs (Fig. 1D). Because the anti-Gr1 antibody recognize two antigens, Ly6G and Ly6C, these cells represent a heterogeneous population that includes granulocytes (expressing both Ly6G and Ly6C), monocytes (expressing Ly6C but not Ly6G), macrophages, dendritic cells, and myeloid suppressor cells (20, 22). We observed a marked enrichment (five- to sixfold) in Ly6C+Ly6G+ granulocytes and a modest accumulation of Ly6C+Ly6G- monocytes and F4/80+ macrophages in the premetastatic lungs of 4T1 tumor-bearing mice (Fig. 1D). We did not observe any significant changes in the frequency of eosinophils (SiglecF+ cells) or dendritic cells in response to the primary tumor (Fig. 1D). Moreover, we detected the previously described VEGFR1+CD117+ hematopoietic progenitor cells (HPCs) (3), although their frequency was much lower than that of Ly6G+Ly6C+ cells (2.7% for HPC vs. 27% for Ly6G+Ly6C+ cells) (Fig. 1D). We then stained sections of premetastatic lungs with anti-Ly6G antibody to visualize Ly6G+Ly6C+ cells. In agreement with the FACS data, we observed increased accumulation of these cells in lungs derived from mice bearing metastatic tumors (Fig. S2 F and G). Among Cd11b+Gr1+ cells isolated from lungs, BM, or spleen of mice bearing 4T1 tumors, only Ly6G+Ly6C+ cells strongly expressed Bv8 (Fig. 1E and Fig. S2H). However, we detected only marginal Bv8 transcripts levels in Ly6G-Ly6C+ and Ly6G-Ly6C- cells, suggesting that the primary tumor secretes factors that specifically up-regulate Bv8 expression in Ly6G+Ly6C+ granulocytes (Fig. 1E). Furthermore, we detected Bv8-positive cells only in lungs isolated from mice bearing tumors that are able to metastasize both in the premetastatic and metastatic phases (Fig. S2 I and J). Thus, Bv8-expressing Ly6G+Ly6C+ cells seem to be a major component of the premetastatic microenvironment.
Tumor-Derived G-CSF Initiates a Premetastatic Environment in Distant Organs.
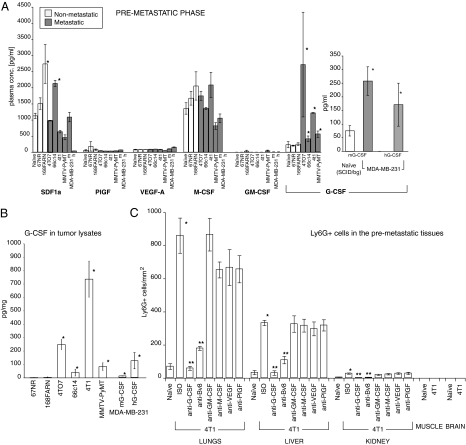
To determine which cytokine(s) secreted by tumor cells might be responsible for mobilizing Ly6G+Ly6C+ cells, we measured plasma and tumor levels of several factors previously implicated in mobilization of myeloid cells: VEGF-A, placenta growth factor (PlGF), stromal-derived factor (SDF)1α, macrophage colony stimulating factor (M-CSF), GM-CSF, and G-CSF (23) (Fig. 2 A and B). Among these, only G-CSF fully correlated with the ability of tumors to metastasize (Fig. 2 A and B). We also found that G-CSF is released by metastatic tumor cells in vitro (Fig. S3A). G-CSF is a major regulator of granulopoiesis produced by a variety of cell types (24) and plays a key role in neutrophil mobilization from the BM (25). Another attractive feature of G-CSF as a potential regulator of a process taking place at a distant site from the tumor of origin is its endocrine mode of action (24). Recently, G-CSF has been also implicated in the acute mobilization of endothelial progenitors induced by vascular disrupting agents (26).
Fig. 2.
Increased G-CSF and Bv8 levels are associated with a metastatic phenotype. (A) Plasma levels of various cytokines in the premetastatic phase in mice bearing nonmetastatic or metastatic tumors. In samples from mice with MDA-MB-231 tumors, m indicates mouse, and h is human. (B) Levels of G-CSF in whole-tumor extracts isolated from mice in the premetastatic phase. (C) Numbers of Ly6G+ cells (per millimeter squared) in various organs during the premetastatic phase in mice bearing 4T1 tumors. Asterisk indicates significant difference relative to naïve group; two asterisks indicate significant difference relative to isotype control (ISO) group (C). Data shown are means ± SEM.
Interestingly, in mice bearing human MDA-MB-231 tumors, we detected increased tumor and plasma levels of both human and mouse G-CSF, suggesting that host cells infiltrating the tumor are also a significant source of G-CSF (Fig. 2A). Administration of anti–G-CSF antibody to mice bearing mouse 4T1, 66c14, or MMTV-PyMT tumors completely abolished the accumulation of Ly6G+Ly6C+ cells in the peripheral blood and lungs (Fig. 2C and Figs. S4 A–C and S5). It also reduced lung Bv8 levels (Fig. S6 A–D). In contrast, treatment with anti–GM-CSF, anti–M-CSF, or anti-VEGF neutralizing antibodies did not have any significant effect on the number of Ly6G+Ly6C+ cells in any of the examined tissues (Fig. 2C and Figs. S4 and S5 A and B). The neutralizing activity of these antibodies was confirmed in in vitro bioassays (Fig. S7). Also, none of these treatments reduced Bv8 concentrations in the premetastatic lungs (Fig. S6 A and B). Treatment with anti-Bv8 antibody also reduced mobilization of Ly6G+Ly6C+ cells into the premetastatic tissues in mice bearing 4T1, 66c14, and MMTV-PyMT tumors (Fig. 2C and Figs. S4 and S5). To define the tissue specificity of Ly6G+Ly6C+ cells mobilization, we examined the presence of Ly6G+ cells in various tissues during the premetastatic phase (Fig. 2C and Fig. S4A). In mice bearing 4T1 tumors, we could detect significant increases in the numbers of these cells not only in the lung but also in the liver and spleen, organs in which metastasis is known to occur, although at later time points than in lungs (11, 27) (Fig. S4A). In tissues in which 4T1 tumors do not or rarely metastasize, we did not detect any Ly6G+ cells (muscle and brain) or their accumulation was minimal (kidney).
Neutralization of G-CSF, Bv8, or Ly6G+Ly6C+ Cells Reduces Metastasis.
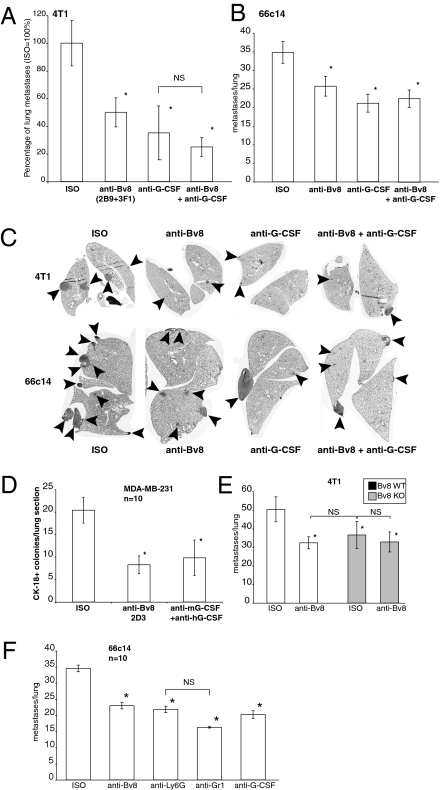
We orthotopically inoculated 66c14, 4T1, MDA-MB-231, or MMTV-PyMT tumors cells in the mammary fat pad of mice. Anti-Bv8 or anti–G-CSF treatment resulted in significant reduction of lung metastasis in all models tested (Fig. 3 A–D and Fig. S8G), whereas it had little effect on primary tumor growth (SI Text, Anti-Bv8 Anti–G-CSF Antibodies Inhibit Metastasis). Depending on the model, anti-Bv8 and anti–G-CSF treatments resulted in 30–60% and 40–70% inhibition, respectively.
Fig. 3.
Neutralization of G-CSF or Bv8 inhibits metastasis. (A) Number of metastases in lungs of mice bearing 4T1 tumors and treated with indicated antibodies (n = 10) for 5.5 wk after tumor inoculation. (B) Number of metastases per lung in mice bearing 66c14 tumors 6 wk after tumor inoculation. Tumors were implanted, and treatment was performed as in A (n = 10). (C) Representative images of lung sections from mice bearing 4T1 or 66c14 tumors and treated with the indicated antibodies. Sections correspond to A (4T1 tumors) or B (66c14 tumors). Arrowheads indicate metastases. (D) Average numbers of CK-18–positive tumor colonies per lung section of SCID/bg mice bearing MDA-MB-231-X1.1 tumors and treated with indicated antibodies for 7 wk. (E) Number of lung metastases in mice transplanted with either Bv8 WT (white bars) or Bv8 KO (gray bars) fetal liver cells and treated with indicated antibodies. (F) Numbers of lung metastases in mice bearing 66c14 tumors and treated with indicated antibody. Asterisk indicates significant difference relative to ISO group. Data shown are means ± SEM.
When 4T1 or 66c14 tumor cells were orthotopically inoculated in mice lacking Bv8 in bone marrow cells (BMCs) (Fig. 3E and Fig. S8 D and E), metastasis was reduced to a degree comparable with that observed in response to anti-Bv8 treatment, providing a genetic validation of the findings obtained with antibodies.
To assess the role of Ly6G+Ly6C+ cells in the development of metastasis, mice bearing orthotopic 66c14 tumors were treated with anti-Ly6G antibody (clone 1A8 specifically depleting neutrophils) (28) or anti-Gr1 antibody (clone RB6-8C5 depleting both monocytes and neutrophils) (Fig. 3F). Treatment with each antibody significantly reduced the number of lung metastases to a degree comparable with anti-Bv8 or anti–G-CSF antibody. The effects of anti-Ly6G treatment were almost equivalent to those of the anti-Gr1 antibody, indicating that Ly6G+Ly6C+ neutrophils are largely responsible for the metastasis in the tested models.
Pretreatment with Recombinant G-CSF Induces Metastasis.
We asked whether G-CSF administration is sufficient to initiate a premetastatic microenvironment. We analyzed the frequency of Ly6G+Ly6C+ and Cd11b+Gr1+ cells in mice treated daily with recombinant (r)G-CSF for 5 consecutive d (Fig. 4 A and B). As expected, delivery of rG-CSF induced mobilization of Cd11b+Gr1+ and Ly6G+Ly6C+ cells, because we detected marked increases in these cells in BM, peripheral blood, and spleen. We also measured a significant increase in the frequency of Cd11b+Gr1+ and Ly6G+Ly6C+ cells in the lungs (Fig. 4 A and B), coincident with increased expression of Bv8 (Fig. 4C). In addition, we observed an increased frequency of Cd11b+Gr1+ cells (and enhanced Bv8 expression) in the liver but the kidneys (Fig. 4C). To determine whether these effects may result in enhanced metastatic potential, mice were treated with rG-CSF for 5 consecutive d before and after injection of tumor cells through the tail vein. Despite the fact that this route bypasses several of the steps occurring during metastasis, it enabled us to focus on the potential role of G-CSF and the G-CSF–initiated premetastatic microenvironment at the final stages of metastasis, such as extravasation, survival, and tumor growth at the distant organs. We injected mice with the metastatic cell lines 66c14, 4T1, and B16F10 or nonmetastatic cell line 67NR. rG-CSF administration increased the numbers of lung metastases in mice injected with B16F10, 4T1, or 6614c cells (Fig. 4D). This was followed by increases in lung mass, which were in agreement with the number of visible metastases (Fig. S9A). Remarkably, the nonmetastatic cell line 67NR exhibited metastatic behavior in the lungs after pretreatment with G-CSF (Fig. 4E). Authenticity of the tumors in mice pretreated with G-CSF was confirmed by histological analysis of H&E-stained sections (Fig. S9 B–D).
Fig. 4.
G-CSF promotes premetastatic priming and enhances the metastatic potential of several tumors. (A) FACS analysis of Cd11b+Gr1+ cells in tissues isolated from mice pretreated with vehicle or rG-CSF. (B) Representative images of lung sections collected from mice pretreated with vehicle or G-CSF as in A and stained with anti-Ly6G antibody. (Scale bar: 50 μm.) (C) Bv8 levels measured by ELISA in tissues matching those in A. (D) Number of metastases in lungs of mice pretreated with vehicle or rG-CSF and injected i.v. with indicated tumor cells. Representative H&E lung sections (66c14 and 4T1) or images of whole lungs (B16F10) from each group are shown below the graphs. (E) Number of tumors in lungs of BALB/c mice i.v. injected with nonmetastatic 67NR cells and pretreated with human rG-CSF as in C. Frequency denotes numbers of mice with detectable tumors in lungs. (F) Numbers of metastases in lungs of mice injected i.v. with 66c14 cells and treated daily with vehicle or mouse rG-CSF and indicated antibody. Asterisk indicates significant difference compared with Vehicle (A–E) or Vehicle/ISO (F) groups. Two asterisks indicate significant difference compared with G-CSF/ISO group. Data shown are means ± SEM.
G-CSF–Induced Metastasis Depends on Bv8-Expressing Ly6G+Ly6C+ Cells.
To define the role of Bv8-expressing Ly6G+Ly6C+ cells in metastasis, mice were treated with rG-CSF together with anti-Bv8, anti-Ly6G, or anti-Gr1 neutralizing antibody. Anti-Bv8 treatment reduced the increase in Cd11b+Gr1+ cells in the lungs (Fig. S9E). However, anti-Gr1 or anti-Ly6G antibodies completely abolished the G-CSF effect on Cd11b+Gr1+ cells. As expected, anti–G-CSF antibody treatment prevented the G-CSF effects (Fig. S9E). We next injected MDA-MB-231, 66c14, or 4T1 cells through the tail vein in mice that had been pretreated with G-CSF in the presence or absence of anti-Bv8 antibody. Anti-Bv8 antibody treatment significantly reduced G-CSF–induced lung metastases of 66c14 (Fig. 4F and Fig. S9F), 4T1 cells (Fig. S9G), or MDA-MB-231 (Fig. S9H). Treatment with an anti-Gr1 or anti-Ly6G antibody also markedly inhibited the prometastatic effects of G-CSF (Fig. 4F), further supporting the hypothesis that Bv8-expressing Ly6G+Ly6C+ cells are predominantly responsible for the G-CSF–induced premetastatic priming and metastasis.
G-CSF Does Not Enhance Metastasis in Mice Treated with Cytotoxic Chemotherapy.
We treated mice bearing orthopic 66c14 or 4T1 tumors with taxotere in the presence or absence of G-CSF as described in SI Materials and Methods. Taxotere treatment effectively reduced primary tumor growth (Fig. S10 A and B) and numbers of lung metastases (Fig. S10C). Acute administration of recombinant G-CSF did not have any effect on the primary tumor and metastasis nor did it antagonize taxotere effects, whereas it reduced taxotere-induced neutropenia (Fig. S10 D and E). Similarly, taxotere reduced metastasis in mice pretreated with G-CSF and injected with tumor cells through the tail vein (Fig. S10F). Interestingly, taxotere did not abolish the G-CS–induced accumulation of myeloid cells, suggesting that most of its antimetastatic activity was caused by its direct antisurvival effect on the tumor cells (Fig. S10G).
G-CSF Induces Expression of Several Prometastatic Molecules.
Microarray analysis (Fig. 1A) indicated that, in addition to Bv8, the premetastatic lungs are enriched in a number of factors that have been previously shown to promote metastasis, including MMP-9, S100A8, and S100A9 (Fig. S1D). Although MMP-9 has been reported to promote invasion (29) and survival (30) of tumor cells in the lung environment, S100A8 and S100A9 mediate recruitment of myeloid and tumor cells in the lungs (4). To investigate whether the G-CSF/Bv8 axis promotes metastasis, at least in part, through regulation of these molecules, we measured their expression levels in lungs of mice pretreated with rG-CSF. We found that all three molecules are significantly up-regulated (Fig. S11A) after G-CSF treatment. Anti-Bv8 significantly reduced the G-CSF–induced expression of MMP-9, S100A8, and S100A9 (Fig. S11A). We then asked whether increased expression of these factors is directly linked to the Ly6G+Ly6C+ cells accumulating in the premetastatic lungs. We isolated several cell subpopulations from naïve or premetastatic lungs and subsequently measured the expression of Bv8, MMP-9, S100A8, and S100A9 (Fig. S11 B and D). Interestingly, only Ly6G+Ly6C+ and Cd11b+Gr1+ cells sorted from lungs of mice pretreated with G-CSF or mice bearing 4T1 tumors expressed significant levels of MMP-9, S100A8, and S100A9 along with Bv8 (Fig. S11 B–D). We did not detect significant levels of these molecules in Ly6G-Ly6C+ or Ly6G-Ly6C- cells or in Cd11b+Gr1- and Cd11b-Gr1- cells. Also, expression of both S100 proteins was markedly induced in Ly6G+Ly6C+ cells isolated from mice pretreated with G-CSF or mice bearing 4T1 tumors (Fig. S11 B and D). These findings suggest that G-CSF can increase local concentrations of MMP-9, S100A8, and S100A9 through mobilization of Ly6G+Ly6C+ cells into the lungs and by inducing expression of these molecules in the mobilized cells.
Bv8 Directly Stimulates Tumor Cell Migration and Metastasis.
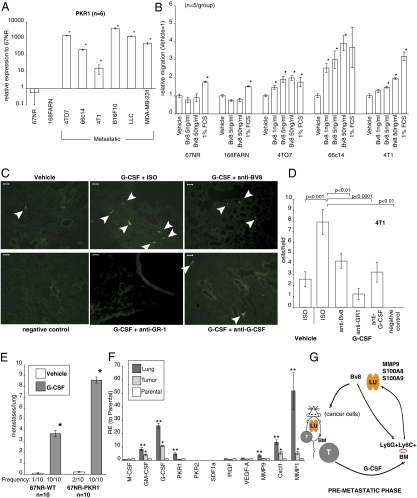
Anti-Bv8 treatment largely prevented the effects of G-CSF on the development of lung metastasis (Fig. 4F and Fig. S9 F–H). However, the same treatment only partially inhibited G-CSF–induced mobilization and homing of Cd11b+Gr1+ cells into the premetastatic lungs (Fig. S9E). This finding suggests that additional mechanisms are involved in the prometastatic activity of Bv8. We tested the hypothesis that Bv8 might have direct effects on tumor cells. We measured the expression levels of Bv8 receptors (PKR-1/GPR73 and PKR-2/GPR73L1) in both nonmetastatic and metastatic tumor cells. We detected significant expression of PKR-1 only in metastatic tumor cell lines (4TO7, 66c14, 4T1, B16F10, and LLC as well as MDA-MB-231), whereas the nonmetastatic cell lines (67NR and 168FARN) exhibited much lower or undetectable levels of PKR-1 (Fig. 5A). We were unable to detect PKR-2 in any of the cell lines tested, except in LLC (Fig. S12A). Also, G-CSFR was undetectable in these cells (Fig. S12B), arguing against the possibility that G-CSF may have direct effects on cancer cells. To determine whether PKR-1 is functional, we stimulated tumor cells with Bv8 and then measured levels of phosphorylated ERK1/2 (Fig. S12C). We detected significant ERK1/2 activation in response to Bv8 only in the metastatic cell lines (Fig. S12C). Bv8 has been shown to promote migration of myeloid cells (31). Therefore, we considered the possibility that Bv8 might also promote migration of metastatic tumor cells. Indeed, Bv8 induced migration of such cells in a dose-dependent manner (Fig. 5B). In contrast, nonmetastatic cell lines did not exhibit enhanced migration in response to Bv8. To confirm that the G-CSF–induced premetastatic microenvironment facilitates migration of tumor cells in vivo, we injected fluorescently labeled (with CellTracker) 4T1 or 66c14 cells into BALB/c nude mice pretreated with G-CSF in the presence or absence of anti-Bv8, anti-Gr1, or anti–G-CSF antibodies, and we assessed the numbers of tumor cells that were able to extravasate and seed the lung tissue. CellTracker was stable in cells for more than 48 h after the labeling (Fig. S12D). We detected significant increases in the number of tumor cells in the lungs of mice pretreated with G-CSF compared with controls (Fig. 5 C and D and Fig. S12E). This effect was significantly reduced by administration of anti-Bv8, anti-Gr1, or anti–G-CSF antibodies (Fig. 5 C and D and Fig. S12E).
Fig. 5.
Bv8 mediates G-CSF induced metastasis through enhancement of tumor cell migration. (A) qRT-PCR analysis of PKR1 expression by cancer cells in vitro. (B) In vitro transwell migration of cancer cells in response to Bv8. (C) In vivo extravasation of 4T1 cells in BALB/c nude mice pretreated with rG-CSF and indicated antibodies. White arrowheads indicate 4T1 tumor cells in lungs. (Scale bar: 50 μm.) (D) Quantification of the in vivo extravasation assay from C. (E) Number of tumors in lungs of BALB/c mice pretreated with rG-CSF and i.v. injected with 67NR or 67NR-PKR1 cells. Frequency denotes numbers of mice with detectable tumors in lungs. In A–E, asterisk indicates significant difference compared with 67NR cells (A), untreated group (B), or vehicle (E). (F) Gene expression analysis of MDA-MB-231 cells isolated either from lung metastases (Lung) or primary tumors (Tumor). Asterisk indicates significant difference compared with parental cell line; double asterisks indicate significant difference compared with Tumor or parental cell lines. (G) Schematic model of the role of G-CSF and Bv8 in metastasis. LU, lung; T, primary tumor; BM, bone marrow. Data are means ± SEM.
As noted (Fig. 4E), nonmetastatic 67NR cells exhibit metastatic properties when i.v. injected in mice pretreated with G-CSF, likely because of the substantially increased expression of MMP9 and other prometastatic molecules in the G-CSF–primed lungs. We speculated that metastasis of 67NR cells might be enhanced by overexpression of PKR-1. Indeed, overexpression of PKR-1 in 67NR cells, to a level comparable with the expression in 66c14 cells (Fig. S12F), resulted in significant enhancement of 67NR metastasis in the G-CSF pretreated mice (Fig. 5E and Fig. S12 G and H). Conversely, PKR-1–deficient 66c14 cells (Fig. S12I) had significantly reduced numbers of G-CSF–induced metastasis compared with cells expressing control shRNA (Fig. S12J).
Tumor Cells Colonizing the Lungs Express Elevated Levels of G-CSF and PKR-1.
We next asked whether cells capable of colonizing the lungs have a high expression of G-CSF or PKR-1. To accomplish this, we performed in vivo clonal selection for MDA-MB-231 cells that either reside at the primary tumor or are able to generate metastasis in the lungs (Fig. S12K). A similar methodology has been previously used to identify gene sets that mediate breast cancer metastasis to the lung and other organs (32, 33). Gene expression profiling revealed that MDA-MB-231 cells isolated from lungs express much higher levels of both G-CSF and PKR-1 along with GM-CSF, MMP-9, and the previously identified Cxcl1 and MMP-1 (32) compared with cells isolated from the primary tumor or to parental cells (Fig. 5F). The up-regulation of GM-CSF in MDA-MB-231 cells metastasizing to the lungs is unlike the other models that we tested, suggesting that the role of GM-CSF is model-dependent. We did not detect increased expression of PKR-2, M-CSF, SDF1α, VEGF-A, or PlGF, suggesting that, to metastasize, cancer cells selectively increase expression of a narrow group of genes, which include G-CSF and PKR-1.
Discussion
Cd11b+Gr1+ and other myeloid cell types have been shown to facilitate tumor growth in a number of studies (18–20, 34). Importantly, their human counterparts have been found to be overproduced in cancer patients (35, 36). Cd11b+Gr1+ cells represent a heterogeneous cell population comprised of neutrophils, macrophages, and dendritic cells. They have been shown to promote invasion and metastasis through increased production of matrix metalloproteinases (MMPs) and TGF-β1 (37, 38) and have been also implicated in suppression of T cell-mediated responses, hence the denomination of myeloid-derived suppressor cells (MDSC) (22, 23). However, we have no evidence that immunosuppression plays a role in the effects that we described here, since inhibiting mobilization or function of myeloid cells had similar effects in immuno-competent and immuno-deficient mice.
Our data indicate that tumor-secreted G-CSF expands and mobilizes a subset of Cd11b+Gr1+ cells, Ly6G+Ly6C+ granulocytes, from BM and also induces Bv8 expression (Fig. 5G). Bv8, in turn, functions as a chemoattractant that enhances mobilization of BM-derived Ly6G+Ly6C+ granulocytes and facilitates their homing into the lung before arrival of tumor cells. After they are in the lungs, G-CSF–mobilized Ly6G+Ly6C+ cells may serve as a major source of Bv8, MMP9, S100A8, and S100A9. MMP-9 has been shown to enhance invasion and metastasis in lungs (29, 30). S100A8 and S100A9 proteins have been shown to be key components of the premetastatic niche and to mediate metastasis through mobilization of myeloid cells and cancer cells to lungs (4, 39, 40). Therefore, Ly6G+Ly6C+ cells mobilized by G-CSF create a protumorigenic microenvironment that supports extravasation, survival, and growth of secondary tumors at distant organs. Interestingly, TNFα, VEGF, and TGFβ1 have also been implicated in the regulation of S100A8 and S100A9 expression in the premetastatic lungs (4). Further studies are needed to clarify any links between G-CSF and these factors in initiation of the premetastatic environment. Moreover, an unexpected finding is the ability of Bv8 to promote metastasis through PKR-1–mediated stimulation of tumor cell migration, thus expanding the prometastatic roles of Bv8 beyond regulating mobilization and homing of Ly6G+Ly6C+ cells into the premetastatic organs.
Pretreatment with recombinant G-CSF was sufficient to mimic the premetastatic environment initiated by primary tumors. G-CSF not only was able to enhance the metastatic properties of several tumors but particularly interestingly, it was also able to promote invasive behavior in nonmetastatic tumors. Treatment with anti-Ly6G antibody significantly reduced the numbers of G-CSF–induced metastases, emphasizing the important role of Ly6G+Ly6C+ cells homing in the lung in metastasis. Furthermore, we detected increased accumulation of Ly6G+Ly6C+ cells and Bv8 expression in the liver of mice bearing metastatic tumors or mice pretreated with G-CSF, suggesting that the prometastatic effects of G-CSF and Bv8 are not restricted to the lung. Further studies are required to test this hypothesis.
Our findings raise the possibility that G-CSF up-regulation is part of a prooncogenic program that confers growth and survival advantages on tumor cells. However, the questions remain of how early in the tumorigenic process do tumor cells start to overexpress G-CSF and whether it correlates with the timing of acquisition of a more metastatic phenotype. Elucidating the signaling pathways resulting in G-CSF up-regulation should allow for answering some of these questions and may also yield additional therapeutic targets.
G-CSF is widely used in cancer therapy, because its use has substantially reduced the risks of chemotherapy-associated neutropenia (41). A key question is whether G-CSF administration may have protumor or prometastatic effects in patients. However, our data suggest that short-term administration of G-CSF, when done in conjunction with cytotoxic chemotherapy, does not increase the risk of metastasis. In contrast, prolonged exposure to high levels of G-CSF, such as those constitutively released by tumors, might result in enhanced metastasis. Indeed, G-CSF overexpression by a variety of tumors has been correlated with a poor prognosis (42–45). Interestingly, patients with all solid tumors may exhibit leukemoid reactions characterized by extreme leukocytosis (46). In numerous cases, the leukocytosis was secondary to a paraneoplastic syndrome linked to high G-CSF production by the tumor, and although the mechanisms remained unclear, it was associated with a particularly poor prognosis (46, 47). This further emphasizes the deleterious effects of G-CSF overproduction by tumors.
In conclusion, our data suggest that metastatic progression can be inhibited by agents targeting mobilization and homing of Cd11b+Gr1+ or Ly6G+Ly6C+ cells. Although, in our experiments, depletion of Gr1+ or Ly6G+ cells elicited the strongest antimetastatic effect, a similar treatment in cancer patients might result in unacceptable neutropenia. Thus, inhibition of G-CSF or Bv8 and, potentially, PKR-1 might have advantages.
Materials and Methods
Mice.
Transgenic mice expressing MMTV-PyMT (48) were obtained from McMaster University, ON, Canada. Female BALB/c, BALB/c Nude, CB6F1 (a cross between female BALB/c and male C57BL/6), and SCID/bg were from Charles River Laboratory. Female FVB and C57BL/6 mice were obtained from Jackson Laboratory. Maintenance of animals and experimental protocols were conducted following federal regulations and approved by the Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We acknowledge F. Miller (Karmanos Cancer Institute, Detroit), E. Filvaroff (Genentech Inc., South San Francisco, CA), and K. Gurney (Genentech Inc., South San Francisco, CA) for providing reagents. We thank P. Yue and D. Dornan for data analysis. We are grateful to I. Kasman, G. Zhuang, C. Zhong, Z. Jiang, N. Lewin-Koh, A. Lima, Y. Crawford, C. Ho, F. Shojaei, J. Zavala-Solorio, L. DeGuzman, the Flow Cytometry laboratory, and the Animal Facility for assistance with experiments. We also thank L. Johnson for critical reading of the manuscript and helpful comments.
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2006.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015855107/-/DCSupplemental.
References
- 1.Nguyen DX, Bos PD, Massague J. Metastasis: From dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 2.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan RN, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erler JT, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis. 2006;9:225–231. doi: 10.1007/s10456-006-9055-8. [DOI] [PubMed] [Google Scholar]
- 9.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4–E5. doi: 10.1038/nature08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dexter DL, et al. Heterogeneity of tumor cells from a single mouse mammary tumor. Cancer Res. 1978;38:3174–3181. [PubMed] [Google Scholar]
- 11.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 12.LeCouter J, et al. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci USA. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeCouter J, Zlot C, Tejada M, Peale F, Ferrara N. Bv8 and endocrine gland-derived vascular endothelial growth factor stimulate hematopoiesis and hematopoietic cell mobilization. Proc Natl Acad Sci USA. 2004;101:16813–16818. doi: 10.1073/pnas.0407697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng MY, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 15.Giannini E, et al. The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain. Proc Natl Acad Sci USA. 2009;106:14646–14651. doi: 10.1073/pnas.0903720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaei F, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 17.Shojaei F, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci USA. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 19.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 21.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 24.Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- 25.Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Shaked Y, et al. Contribution of granulocyte colony-stimulating factor to the acute mobilization of endothelial precursor cells by vascular disrupting agents. Cancer Res. 2009;69:7524–7528. doi: 10.1158/0008-5472.CAN-09-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228. doi: 10.1186/1471-2407-8-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 29.Hiratsuka S, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 30.Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM. Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res. 2006;66:259–266. doi: 10.1158/0008-5472.CAN-05-2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong C, Qu X, Tan M, Meng YG, Ferrara N. Characterization and regulation of bv8 in human blood cells. Clin Cancer Res. 2009;15:2675–2684. doi: 10.1158/1078-0432.CCR-08-1954. [DOI] [PubMed] [Google Scholar]
- 32.Minn AJ, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bos PD, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Almand B, et al. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 36.Young MR, Lathers DM. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int J Immunopharmacol. 1999;21:241–252. doi: 10.1016/s0192-0561(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, et al. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan HH, et al. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryckman C, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48:2310–2320. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 40.Vandal K, et al. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171:2602–2609. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 41.Crawford J, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–170. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 42.Hirasawa K, Kitamura T, Oka T, Matsushita H. Bladder tumor producing granulocyte colony-stimulating factor and parathyroid hormone related protein. J Urol. 2002;167:2130. [PubMed] [Google Scholar]
- 43.Hasegawa S, Suda T, Negi K, Hattori Y. Lung large cell carcinoma producing granulocyte-colony-stimulating factor. Ann Thorac Surg. 2007;83:308–310. doi: 10.1016/j.athoracsur.2006.04.049. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto S, et al. Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol. 1999;34:640–644. doi: 10.1007/s005350050387. [DOI] [PubMed] [Google Scholar]
- 45.Mabuchi S, Matsumoto Y, Morii E, Morishige K, Kimura T. The first 2 cases of granulocyte colony-stimulating factor producing adenocarcinoma of the uterine cervix. Int J Gynecol Pathol. 2010;29:483–487. doi: 10.1097/PGP.0b013e3181d29729. [DOI] [PubMed] [Google Scholar]
- 46.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: A retrospective, single-institution study. Cancer. 2009;115:3919–3923. doi: 10.1002/cncr.24480. [DOI] [PubMed] [Google Scholar]
- 47.Perez FA, Fligner CL, Yu EY. Rapid clinical deterioration and leukemoid reaction after treatment of urothelial carcinoma of the bladder: Possible effect of granulocyte colony-stimulating factor. J Clin Oncol. 2009;27:e215–e217. doi: 10.1200/JCO.2009.22.4931. [DOI] [PubMed] [Google Scholar]
- 48.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.