Abstract
Our previous studies of microperfused single proximal tubule showed that flow-dependent Na+ and HCO3− reabsorption is due to a modulation of both NHE3 and vacuolar H+-ATPase (V-ATPase) activity. An intact actin cytoskeleton was indicated to provide a structural framework for proximal tubule cells to transmit mechanical forces and subsequently modulate cellular functions. In this study, we have used mouse proximal tubule (MPT) cells as a model to study the role of fluid shear stress (FSS) on apical NHE3 and V-ATPase and basolateral Na/K-ATPase trafficking and expression. Our hypothesis is that FSS stimulates both apical and basolateral transporter expression and trafficking, which subsequently mediates salt and volume reabsorption. We exposed MPT cells to 0.2 dynes/cm2 FSS for 3 h and performed confocal microscopy and Western blot analysis to compare the localization and expression of both apical and basolateral transporters in control cells and cells subjected to FSS. Our findings show that FSS leads to an increment in the amount of protein expression, and a translocation of apical NHE3 and V-ATPase from the intracellular compartment to the apical plasma membrane and Na/K-ATPase to the basolateral membrane. Disrupting actin by cytochalasin D blocks the FSS-induced changes in NHE3 and Na/K-ATPase, but not V-ATPase. In contrast, FSS-induced V-ATPase redistribution and expression are largely inhibited by colchicine, an agent that blocks microtubule polymerization. Our findings suggest that the actin cytoskeleton plays an important role in FSS-induced NHE3 and Na/K-ATPase trafficking, and an intact microtubule network is critical in FSS-induced modulation of V-ATPase in proximal tubule cells.
Keywords: cell mechanotransduction, ion transporter, protein trafficking, glomerular tubular balance, epithelial cells
Fluid shear stress (FSS) produced by renal tubular flow modulates proximal Na+, HCO3−, Cl−, and water reabsorption, as well as distal Na+ absorption and K+ secretion (1–5). In the kidney proximal tubule, glomerular tubular balance (GTB) allows for a rapid response to the changes of glomerular filtration rate (GFR) and provides for a nearly proportional change in Na+ and HCO3− in the fluid filtered from the glomeruli (1). The physiological importance of this regulation is to prevent loss of solute following increases in GFR, and to preserve adequate distal delivery of sodium and fluid when GFR is reduced. This highly regulated uptake of Na+ and HCO3− depends on the activity of a group of transport proteins that are located on the luminal and peritubular membrane of proximal tubule cells. Sodium reabsorption across the proximal tubule is driven by basolateral sodium pumps (Na/K-ATPase) and enters through apical NHE3. HCO3− reabsorption is regulated by apical transporters NHE3 and vacuolar H+-ATPase (V-ATPase) in tandem with the basolateral Na+/HCO3− cotransporter (NBC). We have previously investigated the role of FSS in modulating Na+ and HCO3− reabsorption in mouse proximal tubule (MPT) using an in vitro microperfusion technique. Our findings indicated that both NHE3 (3, 6) and V-ATPase (3) activities were enhanced by increases in luminal flow rate. These experiments confirmed that both water (Na+ reabsorption) and HCO3− transport scaled linearly with the bending moments (torque) on the brush border microvilli due to fluid flow and that the latter served as mechanosensors of FSS as first proposed in Guo et al. (7). Similarly, Ohno et al. (8) have shown that chronic hyperfiltration induced by unilateral nephrectomy increased cell volume and NHE3 and Na/K-ATPase activities. It is uncertain whether this ion transport is due to an increase in the intrinsic activity of the transporters and/or an increase in their number at the membrane. Indeed, NHE3 undergoes continuous recycling between the plasma membrane and the large pool of subcellular endosomes (9, 10), and redistribution between these pools was suggested to contribute to the regulation of exchange activity (11). Whether the transporter trafficking is downstream of the flow stimulation, however, remains unknown. These changes, which occur on a time scale of hours or longer, are the primary focus of the present study.
Our recent mathematical model, Weinstein et al. (12), which includes torque-dependent solute transport in a compliant tubule, has predicted that coordinated regulation of both luminal and peritubular transporters is required for a variation of overall Na+ reabsorption to occur while preserving the integrity of cell volume and composition. Specifically, if increases in luminal flow rate are to increase transepithelial Na+ and HCO3− reabsorption, they must increase basolateral Na/K-ATPase and NBC activities proportionately using torque-dependent scaling. Preisig (13) demonstrated that luminal flow indirectly modulated NBC activity by changes in pHi secondary to flow-dependent changes in apical NHE3 activity. However, the effect of FSS on Na/K-ATPase has yet to be examined.
Cytoskeletal elements, such as microtubules, actin filaments, and their associated molecular motors, are intimately involved in mechanical signaling (14, 15). Dramatic remodeling of cytoskeleton has been reported for a variety of cells exposed to FSS for 3–5 h or more (16, 17). Our group recently compared the response of cultured endothelial and MPT cells in response to 5-h FSS. Marked differences in actin cytoskeletal and junctional protein reorganization were observed. To explain the striking difference in behavior of tall cuboidal MPT cells and flat endothelial cells to FSS on this time scale, we proposed a conceptual “junctional buttressing” model (18). In MPT, we have also reported that an intact actin network is required for the stimulation of Na+ and HCO3− transport in the presence of higher tubular flow rate (3, 6). However, whether actin filaments facilitate the FSS-induced activation or translocation of transporters was not known.
In our current study, we examine the distribution of NHE3, V-ATPase, and Na/K-ATPase in an immortalized MPT cell line by immunofluorescence and Western blot analysis, and find that in the absence of FSS, the distribution of apical transporters across the cytoplasm were diffuse, with no compartment being more prevalent in either NHE3 or V-ATPase immunostaining. Sodium pumps were seen mostly at the basolateral membrane, interacting with actin stress fibers. After subjecting the cell monolayer to FSS, both NHE3 and V-ATPase were found to translocate to the apical membrane. Membrane sodium pump expression increased twofold in comparison with control. As disruption of the actin cytoskeleton blunts flow-induced Na+ and HCO3− reabsorption, we tested whether this would also block all three transporters translocation and the increment of their expression. Our data suggest that FSS-induced NHE3 and sodium-pump trafficking are largely dependent on an intact actin cytoskeleton, whereas V-ATPase depends mainly on the microtubule network.
Results
FSS Increases Apical NHE3 Abundance and Cytochalasin D Inhibits This Effect.
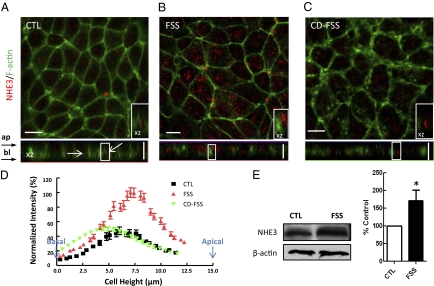
A double-label immunofluorescence study was carried out to determine the localization of NHE3 and actin microfilaments in MPT cells in response to 3-h FSS at 0.2 dynes/cm2. This is equivalent to 5nl/min in vivo, a standard reference state for mouse proximal tubule. NHE3 in control cells were found to be weakly distributed on the apical membrane and in the cytosol (Fig. 1A). XZ image showed that NHE3 was mostly present in the subapical compartment and did not colocalize with lateral F-actin (Fig. 1A, arrows and inset). This was confirmed by quantitative region scan analysis as shown by the cytosolic NHE3 distribution in Fig. 1D. Under control conditions, ≈20% of the total NHE3 was located in the apical membrane (Table S1). Following exposure to 3-h FSS, concomitant with dramatic actin cytoskeleton reorganization (18, 19), the overall expression of NHE3 was found to be significantly increased (Fig. 1B and Table S1). The total area under the intensity distribution curve in Fig. 1D was more than doubled. This result was further confirmed by Western blotting (Fig. 1E). The total amount of NHE3 protein abundance increased to 172 ± 30% (n = 3). In addition, we witnessed a greatly enhanced expression of NHE3 near the apical surface membrane of MPT cells (Fig. 1B), suggesting that apical insertion of NHE3 is one of the mechanisms of FSS-induced increase of Na+ and HCO3− reabsorption. In the presence of cytochalasin D (CD), as illustrated in Fig. 1C, the linear perijunctional actin bands were replaced by large subcortical aggregates. The FSS-induced increment of NHE3 staining was completely abrogated, and the antigen was relocated into an intracellular compartment (Fig. 1C), with a distribution that did not differ significantly from the control condition (Fig. 1D). Importantly, FSS was no longer able to stimulate NHE3 apical abundance, indicating that the FSS-induced activation of NHE3 trafficking occurred via an intact cytoskeleton.
Fig. 1.
Effect of FSS on NHE3 trafficking and expression in confluent MPT cells. Immunofluorescence images of MPT cells under control (CTL) (A), FSS (B), and cytochalasin D (CD-FSS) conditions (C). The antibody stained for NHE3 (red) and actin filaments (green), respectively. In control, NHE3 scattered in the cytoplasm and mainly localized below the actin rings (arrows). After 3-h FSS at 0.2 dynes/cm2, the NHE3 staining was stronger and the stained areas were larger than those in control cells. (Inset) Majority of NHE3 now localize at the apical region. (D) Distributions of normalized NHE3-stained fluorescence intensity. The values were normalized such that the mean maximum value in the stimulation group was 100% in each experiment. The fluorescence intensity of the cells was shifted to the right (cell apex) after application of FSS (red). This effect was blunted with the addition of CD (green). (E) Western blot analysis showing NHE3 levels under control and FSS conditions. Expression levels of NHE3 were normalized on actin expression in the same lane. Results in bar graph are means ± SE and are percentage of control condition (*P < 0.05). Clearly, FSS induced NHE3 upregulation. (Inset) Magnified view of the boxed region on XZ images (10 × 5 μm). (Scale bars, 10 μm.)
FSS Induces Up-Regulation of Na/K-ATPase Expression and Translocation.
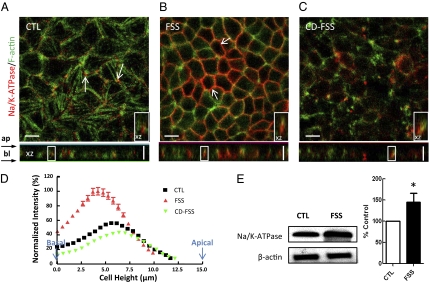
Next, we tested the distribution of Na/K-ATPase in MPT cells after FSS stimulation. Sodium-pump staining was mostly observed at the lateral membrane (Fig. 2A, arrows) in controls. Colocalization of Na/K-ATPase and F-actin was found in the XZ image, consistent with previous studies (20) on MDCK cells, indicating that Na/K-ATPase tightly interacts with the membrane cytoskeleton. Cells that are treated with FSS exhibited a greater surface expression of Na/K-ATPase (Fig. 2 B and E) on the basolateral membrane as compared with the control cells. The increase in membrane Na/K-ATPase expression occurred mostly at the cell periphery (Fig. 2B, arrows). XZ image revealed an enhanced colocalization between Na/K-ATPase and actin at the basolateral membrane (Fig. 2B Inset). Fig. 2D and Table S1 show that 67.5 ± 1.0% of total Na/K-ATPase was primarily localized at the cell basolateral membrane and FSS significantly increased the amount of Na/K-ATPase to 88.5 ± 0.9% (P < 0.001, n = 30) distributed in the basolateral membrane compared with the whole cell. Western blot analysis shows there was a 45 ± 21% (P < 0.05, n = 3) increment in the abundance of Na/K-ATPase expression in the whole cell lysates (Fig. 2E). The magnification of Fig. 2 A and B was the same. The reason that cells appear smaller in Fig. 2B is that after FSS is applied, cells experience significant changes in their basal area and there is a redistribution of the cell volume after FSS. Under control condition, cells possess poorly formed or nonexistent peripheral actin bands near their apical surface and are rounded at their tops with a significantly larger basal area due to the presence of spreading stress fibers at their basal membrane. This plays a supporting role for the tall columnar cell structure. Under the stimulation of FSS, tight junctions and adherens junctions form and basal stress fibers disappear. Cells adapt to a new shape in which they tightly bind to one another near their apical surface, causing the basal part of the cells to retract. Our previous work on the effect of FSS on actin cytoskeleton reorganization has described these findings in detail (18). In the presence of CD, the observed inhibitory effects of actin disruption resulted in a markedly reduced expression of sodium pumps at the basolateral membrane surface, which was obvious by immunostaining (Fig. 2C). These findings suggest that stimulation of Na/K-ATPase by FSS results at least in part from stimulation of exocytic insertion of Na/K-ATPase protein, which requires actin cytoskeletal integrity.
Fig. 2.
Effect of FSS (3 h at 0.2 dynes/cm2) on Na/K-ATPase trafficking and expression in MPT cells. Immunofluorescence images of MPT cells under control (CTL) (A), FSS (B), and cytochalasin D (CD-FSS) conditions (C). The antibody stained for Na/K-ATPase (red) and actin filaments (green), respectively. In CTL, Na/K-ATPase weakly distributed mainly at the basolateral membrane with some intensive staining near the cell-cell contacts (arrows). After FSS, an elevation in membrane Na/K-ATPase expression was observed mainly at the cell periphery (arrows). Colocalization of Na/K-ATPase and actin was prominent (XZ image, Inset). CD significantly blunted the flow-dependent effects. (D) Distributions of normalized Na/K-ATPase-stained fluorescence intensity. The fluorescence intensity of the cells was shifted to the left (cell base) after application of FSS (red line). This effect was blunted with the addition of CD (green line). (E) Na/K-ATPase and actin control were detected by Western blot. Results in bar graph are means ± SE and are percentage of control condition (*P < 0.05). (Inset) Magnified view of the boxed region on XZ images (10 × 5 μm). (Scale bars, 10 μm.)
FSS Induces V-ATPase Trafficking, and CD Had No Effect on This Behavior.
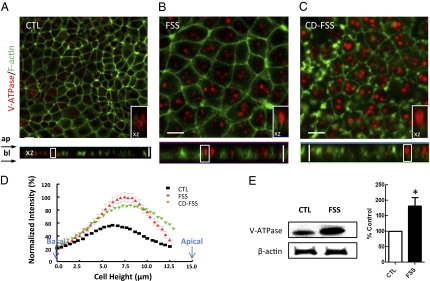
We then assessed the FSS-induced distribution of another apically localized protein, V-ATPase. Under static conditions, the low-magnification image clearly shows only 20% of the cells possessed V-ATPase staining, and they were mostly concentrated in subapically located endocytic vesicles (Fig. 3A). In FSS-treated cells, an up-regulation of V-ATPase occurred: V-ATPase was found in every single cell with greater expression at the cell surface (Fig. 3B). The area under the intensity distribution curve indicates that the total fluorescence intensity of V-ATPase was increased after FSS, consistent with the Western blot analysis. The average of FSS increased band intensity from the whole-cell lysates was 182 ± 27% (P < 0.05, n = 3) (Fig. 3E). In contrast to the result of NHE3 and Na/K-ATPase, in the presence of CD, the distribution of V-ATPase remained similar to what was observed in the FSS treatment condition without CD (Fig. 3D), and was primarily localized at the apical surface (Fig. 3C). Taken together, these data show extensive FSS-induced translocation of V-ATPase to the apical surface and up-regulation of V-ATPase expression, and this modulation apparently is actin cytoskeleton-independent.
Fig. 3.
Effect of FSS (3 h at 0.2 dynes/cm2) on V-ATPase trafficking and expression in MPT cells. Immunofluorescence images of MPT cells under control (CTL) (A), FSS (B), and cytochalasin D (CD-FSS) conditions (C). The antibody stained for V-ATPase (red) and actin filaments (green), respectively. In CTL, only 20% of the cells express V-ATPase. After FSS stimulation, V-ATPase was expressed more evenly and brightly in every cell. There is a translocation of V-ATPase witnessed in the XZ image. CD did not affect the flow-dependent up-regulation and translocation of V-ATPase. (D) Distributions of normalized V-ATPase-stained fluorescence intensity. The fluorescence intensity of the cells was shifted to the right (cell apex) after application of FSS (red). (E) V-ATPase and actin control were detected by Western blot. Results in bar graph are means ± SE and are percentage of control condition (*P < 0.05). (Inset) Magnified view of the boxed region on XZ images (10 × 5 μm). (Scale bars, 10 μm.)
Colchicine (Col) Inhibits FSS-Induced V-ATPase Trafficking.
Studies on other polarized epithelial cells have suggested that microtubules are involved in the targeting of intracellular vesicles to the apical pole of epithelial cells (21, 22). In Col-treated rats, Brown and coworkers (23) found a reduction in apical V-ATPase content. We therefore assessed the effect of Col on FSS-induced V-ATPase trafficking in cultured MPTs by double-staining V-ATPase and acetylated α-tubulin. In control cells, the immunofluorescence for the α-tubulin antibodies exhibited radial or reticular patterns in the cytoplasm, and intense fluorescence was observed in the perinuclear region (Fig. S1A), presumably where the microtubule organizing center is located. Interestingly, a tail-like structure was often found connected to the cell periphery (Fig. S1A, asterisk). From the XZ image, microtubules were found mainly arranged in bundles of filaments running along the apical-basal axis of the cells. V-ATPase was found mainly beneath the apical tubulin “cap.” After FSS stimulation, the distribution of microtubules seemed to be accumulated in smaller and more condensed cytoplasmic areas (Fig. S1B). The tails were extended to long string-like structures (Fig. S1B, arrows). V-ATPase staining was similar to what we found in the last section, and Col resulted in a strong loss of microtubules in MPT cells (Fig. S1C, green). We observed only residual labeling located in the subapical region, which may belong to stable microtubules located in the terminal web. Importantly, treatment with Col led to a marked decrease in the surface expression of V-ATPase (Fig. S1C, red). A normally bright vesicular V-ATPase staining under FSS was replaced by a scattered distribution of small particles throughout the cell cytoplasm. Moreover, we witnessed a basal V-ATPase staining in the XZ image (Fig. S1C, arrow). It is not known whether Col perfusion-induced V-ATPase internalization is dependent on membrane endocytosis or on the dissociation of V-ATPase from the plasma membrane followed by internalization due to interactions with the microtubule network. Nevertheless, Col-induced depolymerization of microtubules blunted the effect of FSS on V-ATPase redistribution and up-regulation. In Fig. S2, the intensity distribution of V-ATPase under control, FSS, and Col-FSS conditions were compared.
Discussion
The present study, carried out on immortalized MPT cells, was designed to define whether membrane transporter protein trafficking is involved in FSS-induced regulation of the protein activity. The main points are the following: (i) FSS-induced stimulation of apical NHE3 and V-ATPase activity was associated with increased membrane protein abundance. (ii) FSS-induced transporter trafficking was not only limited to the apical domain of the plasma membrane, where microvilli and primary cilia mechanotransducers make direct contact with the flow, but also involved basolateral membrane protein activities, such as Na/K-ATPase. (iii) Actin cytoskeleton disruption blocked the FSS-stimulating effect on up-regulation and trafficking of NHE3 and Na/K-ATPase. (iv) FSS-induced increase in apical V-ATPase abundance relies on an intact microtubule network, but not actin filaments.
Laminar FSS stimulates Na+ and HCO3− absorption in the renal proximal tubule mainly by activation of the apical NHE3 and V-ATPase. This effect was repeatedly documented by groups using different experimental methods in rats (13, 24). Our recent isolated single-tubule perfusion study demonstrated that the flow-dependent Na+ reabsorption was diminished in NHE3-null mice (3). However, the fact that flow impacts more than just NHE3 is supported by observations that flow dependence is seen in the presence of EIPA (NHE3 inhibitor) or in NHE3 knockout mice (3). How the flow modulates luminal transporter activity is less well understood. Girardi et al. (25) reported that 4 h after uninephrectomy an increment in GFR is accompanied by an activation of NHE3 and an up-regulation of NHE3 expression (both protein and mRNA content). Other studies have indicated that rapid regulation of NHE3 activity in epithelial cells usually involves, at least in part, changes in the amount of NHE3 present in the plasma membrane regulated by changes in exocytosis and endocytosis (10, 26). Our confocal and Western results not only indicate that FSS induces transporter protein synthesis, but more importantly, luminal protein trafficking to the apical plasma membrane. Previous microperfusion studies from our laboratory demonstrated that both NHE3 and V-ATPase activity were stimulated by both acute and chronically increased tubule flow rates in proximal tubules (3, 8). This finding suggests that an increase in flow rate stimulates recruitment in transporters to their specific membrane domains, which might be one of the mechanisms by which chronic hyperfiltration leads to increased Na+ and HCO3− absorption.
Another aim of the current study was to provide evidence that peritubular transporters can be regulated by luminal flow. According to the predictions of the torque-dependent solute transport model of Weinstein et al. (12), the peritubular transporters must be modulated by luminal flow to allow coordinated transport of Na+, HCO3− at the basolateral membrane and entry into the interstitium and peritubular capillaries from the cell when apical transporter activity is increased. The authors calculated two key parameters: sodium flux and cell volume in the absence or presence of peritubular transporters. It was found that increases in luminal transporter density alone produced at most a 30% increase in Na+ flux compared with a nearly linear torque-dependent increase over a broad range of physiological flow rates, when both luminal and peritubular transporter density was torque dependent. In addition, a lethal increase in cell volume could occur if there is no coordinated scaling of luminal and peritubular transporter activity. Our findings show an alteration of the amount of membrane Na/K-ATPase stimulated by FSS (Table S1). This increase is observed in the presence of a mild increment of the total cellular pool of Na/K-ATPase, suggesting that translocation of intracellular Na-pumps to the basolateral membrane occurs in response to increased luminal FSS. Whether Na/K-ATPase turnover rate is regulated with application of luminal flow has not been answered.
The current study strongly indicates that an intact actin and microtubule cytoskeleton is essential for FSS-induced membrane protein trafficking in MPT cells. The cytoskeleton is a dynamic structure that maintains cell shape, often protects the cell, and enables cellular motion. In fact, our recent “junctional buttressing” model has illustrated a dramatic cytoskeletal reorganization of junctional membrane proteins and actin filaments after onset of FSS (18), a response that was surprisingly opposite that observed for confluent endothelial cell (EC) monolayers in Thi et al. (27). In our tall cuboidal cells we witnessed a formation of tight and adherens junctions, a disappearance of basal stress fibers, and an accumulation of focal adhesion proteins in the basement membrane after 3–5 h of FSS (18). In marked contrast, a 10-fold larger FSS applied for the same time produced a disruption of junctions, a creation of stress fibers, and a dispersion of focal adhesion proteins in much flatter ECs (27). This could be explained by the large difference in cell geometry, and the comparable rotational moment on the cell produced by the FSS in each case. The mechanosensing structure in the case of the EC appears likely to be its surface glycocalyx (28, 29), whereas for MPT cells this appears to be either its microvilli (7, 18) or possibly its primary cilia. This difference might affect which cytoskeletal structure is altered in the transmission of the FSS, because microvilli are formed from an actin filament bundle, whereas primary cilia are formed from microtubules. It must be acknowledged that the ultrastructural changes reported here were observed only on the time scale of hours; whether they occur as rapidly as changes found in GTB remains to be investigated.
How the cytoskeletal changes regulate transporter activities in our tall columnar epithelial cells is unclear, but it could reflect a dependence on localization, assembly into higher-order complexes, or a possible direct mechanical effect of cytoskeletal elements on transport proteins. Previous studies suggest that NHE3 is associated indirectly with cytoskeletal elements through a coordinated binding of NHE3 regulatory factor 1 (NHERF1/EBP50), a bridging molecule that also associates with ezrin, which, in turn, binds directly to actin (30). Hayashi et al. (31) illustrated that disruption of the apical actin network of epithelial cells using TxB or Y-27632 resulted in massive internalization of NHE3, which is accompanied by a drastic drop in the ability of the cells to reabsorb Na+ and the accompanying osmotically driven water. When incubated with endothelin (32), the increase of the exocytic insertion of NHE3 protein was also inhibited by CD in OK cells. With respect to Na/K-ATPase, it has been shown that Na/K-ATPase is tightly connected with ankyrin/fodrin-actin membrane cytoskeleton. In epithelial cells, ankyrin links fodrin to the Na/K-ATPase, and ankyrin binding coordinates assembly of the E-cadherin and Na/K-ATPase at the basolateral membrane. This suggests that E-cadherin may play a role in the assembly and stabilization of the membrane cytoskeleton and the Na/K-ATPase at the basolateral cell surface of MDCK cells (33). From the above, it is likely that dramatic cytoskeletal reorganization in response to flow might also favor protein activation and targeting. There is currently no consensus on how these events are linked.
In this study, V-ATPase trafficking in response to FSS was found to be dependent upon intact microtubules, but not on actin filaments. This is comparable to the findings in other cell types (34). There is the possibility that vesicles containing the V-ATPase do not require actin filaments to target their destination, but instead a local depolymerization in the actin network might allow vesicles to traverse the dense subapical network of actin filaments and thereby gain access to the plasma membrane. In our single-tubule perfusion study, when luminal perfusion was increased from 4 to 20 nL/min, the increment of volume and HCO3– reabsorption was almost twofold (3). After adding the CD into the luminal perfusate, the increment of Jv due to enhanced flow rate was diminished by 83% in comparison with a 67% inhibition of flow-stimulated JHCO3 increase. This 16% difference may be due to the fact that CD was not able to inhibit flow-dependent V-ATPase trafficking.
In summary, we have described a unique effect of FSS that may contribute to the understanding of the mechanism of flow-induced changes on ion transport activity and have, in the process, revealed a role for the cytoskeletal network in the FSS-induced trafficking of the transporters to their functional membrane locations. Our finding indicates the intact actin cytoskeleton is not only important for regulation of flow-dependent Na+ and HCO3− absorption, but also for the flow-induced NHE3 and Na/K-ATPase trafficking, whereas the intact microtubule network is critical for the flow-induced V-ATPase trafficking. Such modulation may occur in both acute and chronic hyperfiltration by higher GFR in regulation of proximal tubule transport in the kidney.
Materials and Methods
All methods used in this article have been reported previously (18) and are described in SI Materials and Methods. Primary antibodies were obtained from either commercial sources or from independent investigators. The polyclonal antibody against NHE3 used for immunostaining was provided by M. Knepper (National Institutes of Health, Bethesda, MD). The anti-NHE3 mouse monoclonal (3H3) used for Western blotting was a gift of D. Biemesderfer (Yale University, New Haven, CT). The rabbit polyclonal antibody against V-ATPase subunit E was purchased from Santa Cruz Biotechnology. The Na/K-ATPase antibody (α5) was a gift from M. Caplan (Yale University).
Supplementary Material
Acknowledgments
We thank Nanami Gotoh, Qingshang Yan, Zhaopeng Du, Sue Ann Mentone, and Deren Shao (Yale University, New Haven, CT) for technical assistance and for valuable discussion and advice. This work is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01DK62289 (to T.W. and S.W.) and R01DK29857 (to A.M.W.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015751107/-/DCSupplemental.
References
- 1.Schnermann J, Wahl M, Liebau G, Fischbach H. Balance between tubular flow rate and net fluid reabsorption in the proximal convolution of the rat kidney. I. Dependency of reabsorptive net fluid flux upon proximal tubular surface area at spontaneous variations of filtration rate. Pflugers Arch. 1968;304:90–103. doi: 10.1007/BF00586722. [DOI] [PubMed] [Google Scholar]
- 2.Giebisch G, Windhager EE. Characterization of renal tubular transport of sodium chloride and water as studied in single nephrons. Am J Med. 1963;34:1–6. doi: 10.1016/0002-9343(63)90034-2. [DOI] [PubMed] [Google Scholar]
- 3.Du Z, et al. Axial flow modulates proximal tubule NHE3 and H-ATPase activities by changing microvillus bending moments. Am J Physiol Renal Physiol. 2006;290:F289–F296. doi: 10.1152/ajprenal.00255.2005. [DOI] [PubMed] [Google Scholar]
- 4.Malnic G, Berliner RW, Giebisch G. Flow dependence of K+ secretion in cortical distal tubules of the rat. Am J Physiol. 1989;256:F932–F941. doi: 10.1152/ajprenal.1989.256.5.F932. [DOI] [PubMed] [Google Scholar]
- 5.Satlin LM, Sheng S, Woda CB, Kleyman TR. Epithelial Na(+) channels are regulated by flow. Am J Physiol Renal Physiol. 2001;280:F1010–F1018. doi: 10.1152/ajprenal.2001.280.6.F1010. [DOI] [PubMed] [Google Scholar]
- 6.Du Z, et al. Mechanosensory function of microvilli of the kidney proximal tubule. Proc Natl Acad Sci USA. 2004;101:13068–13073. doi: 10.1073/pnas.0405179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo P, Weinstein AM, Weinbaum S. A hydrodynamic mechanosensory hypothesis for brush border microvilli. Am J Physiol Renal Physiol. 2000;279:F698–F712. doi: 10.1152/ajprenal.2000.279.4.F698. [DOI] [PubMed] [Google Scholar]
- 8.Ohno A, Beck FX, Pfaller W, Giebisch G, Wang T. Effects of chronic hyperfiltration on proximal tubule bicarbonate transport and cell electrolytes. Kidney Int. 1995;48:712–721. doi: 10.1038/ki.1995.342. [DOI] [PubMed] [Google Scholar]
- 9.Biemesderfer D, et al. Monoclonal antibodies for high-resolution localization of NHE3 in adult and neonatal rat kidney. Am J Physiol. 1997;273:F289–F299. doi: 10.1152/ajprenal.1997.273.2.F289. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza S, et al. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. J Biol Chem. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- 11.Hu MC, et al. Dopamine acutely stimulates Na+/H+ exchanger (NHE3) endocytosis via clathrin-coated vesicles: Dependence on protein kinase A-mediated NHE3 phosphorylation. J Biol Chem. 2001;276:26906–26915. doi: 10.1074/jbc.M011338200. [DOI] [PubMed] [Google Scholar]
- 12.Weinstein AM, et al. Flow-dependent transport in a mathematical model of rat proximal tubule. Am J Physiol Renal Physiol. 2007;292:F1164–F1181. doi: 10.1152/ajprenal.00392.2006. [DOI] [PubMed] [Google Scholar]
- 13.Preisig PA. Luminal flow rate regulates proximal tubule H-HCO3 transporters. Am J Physiol. 1992;262:F47–F54. doi: 10.1152/ajprenal.1992.262.1.F47. [DOI] [PubMed] [Google Scholar]
- 14.Davies PF, et al. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–549. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- 15.Janmey PA. The cytoskeleton and cell signaling: Component localization and mechanical coupling. Physiol Rev. 1998;78:763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- 16.Malek AM, Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci. 1996;109:713–726. doi: 10.1242/jcs.109.4.713. [DOI] [PubMed] [Google Scholar]
- 17.Pavalko FM, et al. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275:C1591–C1601. [PubMed] [Google Scholar]
- 18.Duan Y, et al. Shear-induced reorganization of renal proximal tubule cell actin cytoskeleton and apical junctional complexes. Proc Natl Acad Sci USA. 2008;105:11418–11423. doi: 10.1073/pnas.0804954105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Essig M, Terzi F, Burtin M, Friedlander G. Mechanical strains induced by tubular flow affect the phenotype of proximal tubular cells. Am J Physiol Renal Physiol. 2001;281:F751–F762. doi: 10.1152/ajprenal.2001.281.4.F751. [DOI] [PubMed] [Google Scholar]
- 20.Nelson WJ, Hammerton RW. A membrane-cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Achler C, Filmer D, Merte C, Drenckhahn D. Role of microtubules in polarized delivery of apical membrane proteins to the brush border of the intestinal epithelium. J Cell Biol. 1989;109:179–189. doi: 10.1083/jcb.109.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutmann EJ, Niles JL, McCluskey RT, Brown D. Colchicine-induced redistribution of an apical membrane glycoprotein (gp330) in proximal tubules. Am J Physiol. 1989;257:C397–C407. doi: 10.1152/ajpcell.1989.257.2.C397. [DOI] [PubMed] [Google Scholar]
- 23.Brown D, Sabolic I, Gluck S. Polarized targeting of V-ATPase in kidney epithelial cells. J Exp Biol. 1992;172:231–243. doi: 10.1242/jeb.172.1.231. [DOI] [PubMed] [Google Scholar]
- 24.Maddox DA, Fortin SM, Tartini A, Barnes WD, Gennari FJ. Effect of acute changes in glomerular filtration rate on Na+/H+ exchange in rat renal cortex. J Clin Invest. 1992;89:1296–1303. doi: 10.1172/JCI115715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girardi AC, Rocha RO, Britto LR, Rebouças NA. Upregulation of NHE3 is associated with compensatory cell growth response in young uninephrectomized rats. Am J Physiol Renal Physiol. 2002;283:F1296–F1303. doi: 10.1152/ajprenal.00010.2002. [DOI] [PubMed] [Google Scholar]
- 26.Yip KP, Tse CM, McDonough AA, Marsh DJ. Redistribution of Na+/H+ exchanger isoform NHE3 in proximal tubules induced by acute and chronic hypertension. Am J Physiol. 1998;275:F565–F575. doi: 10.1152/ajprenal.1998.275.4.F565. [DOI] [PubMed] [Google Scholar]
- 27.Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper-car” model. Proc Natl Acad Sci USA. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinbaum S, Zhang X, Han Y, Vink H, Cowin SC. Mechanotransduction and flow across the endothelial glycocalyx. Proc Natl Acad Sci USA. 2003;100:7988–7995. doi: 10.1073/pnas.1332808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 30.Weinman EJ, Cunningham R, Shenolikar S. NHERF and regulation of the renalsodium-hydrogen exchanger NHE3. Pflugers Arch. 2005;450:137–144. doi: 10.1007/s00424-005-1384-8. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi H, et al. Inhibition and redistribution of NHE3, the apical Na+/H+ exchanger, by Clostridium difficile toxin B. J Gen Physiol. 2004;123:491–504. doi: 10.1085/jgp.200308979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Y, et al. ETB receptor activation leads to activation and phosphorylation of NHE3. Am J Physiol. 1999;276:C938–C945. doi: 10.1152/ajpcell.1999.276.4.C938. [DOI] [PubMed] [Google Scholar]
- 33.Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toyomura T, Oka T, Yamaguchi C, Wada Y, Futai M. Three subunit a isoforms of mouse vacuolar H(+)-ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem. 2000;275:8760–8765. doi: 10.1074/jbc.275.12.8760. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.