Abstract
Cytotoxic lymphocytes such as natural killer (NK) and CD8 T cells play important roles in immunosurveillance by killing virally infected or malignant cells. The homeostatic cytokine, IL-15, promotes the development, function, and survival of NK and CD8 T cells. IL-15 is normally presented in trans as a surface complex with IL-15 receptor-alpha-chain (IL-15Rα) by dendritic cells (DCs) and monocytes. Signaling by IL-15 occurs via the IL-2/IL-15 receptor β-chain (CD122) which is expressed primarily by NK1.1+ cells and CD8 T cells. The use of preformed complexes of IL-15 with soluble IL-15Rα complexes to boost the effector function of CD122+ cytolytic lymphocytes such as NK and CD8 T cells has recently gained considerable attention. Here we describe the impact of transient and prolonged in vivo stimulation by IL-15/IL-15Rα complexes on NK and CD8 T cells. Whereas transitory stimulation increased the number of activated NK cells and significantly enhanced their effector function, prolonged stimulation by IL-15/IL-15Rα complexes led to a marked accumulation of mature NK cells with considerably impaired activation, cytotoxicity, and proliferative activity, and an altered balance of activating and inhibitory receptors. In contrast to NK cells, CD8 T cells exhibited an activated phenotype and robust T cell receptor stimulation and effector function upon chronic stimulation with IL-15/IL-15Rα complexes. Thus, prolonged stimulation with the strong activating signal leads to a preferential accrual of mature NK cells with altered activation and diminished functional capacity. These findings point to a negative feedback mechanism to preferentially counterbalance excessive NK cell activity and may have important implications for cytokine immunotherapy.
Keywords: cytokine complexes, natural killer cell dysfunction, end-stage cells
Natural killer (NK) cells are innate effector cells that play a critical role in immunosurveillance by eliminating virally infected and transformed cells (1, 2). Target recognition and effector function by NK cells are known to be controlled by a balance of activating and inhibitory receptors including members of Ly49 (mouse)/KIR (human) family, CD94/NKG2, natural cytotoxicity receptors, and Fc receptors, as well as costimulatory receptors that are essential for tuning their response (3, 4). The repertoire of activating and inhibitory receptors expressed by NK cells determines whether these cells can recognize and kill infected cells. Activation stimuli normally endow NK cells with cytotoxic function by up-regulating the expression of granzyme B and perforin and the production of effector cytokines such as IFNγ, TNFα, and granulocyte macrophage colony-stimulating factor (GM-CSF) (1, 2). However, NK cell hyporesponsiveness has been observed upon prolonged stimulation with strong immune activating signals (5–11) and in various chronic inflammatory and autoimmune diseases including chronic hepatitis (12), tuberculosis (13), AIDS (14), diabetes (15), and systemic onset juvenile rheumatoid arthritis (16). Whether NK cells become dysfunctional through direct or indirect mechanisms is unknown.
The NK cell life cycle is influenced by IL-15. Development of NK cells from precursors in bone marrow depends on the presence of this cytokine as shown by a lack of NK cells in IL-15 and γ-chain knockout mice (17, 18). During an infection, recognition of danger signals by dendritic cells (DCs) and other myeloid cells leads to production of IL-15 and other cytokines such as IL-12 that are important for activation and proliferation of NK cells as well as cytotoxic CD8 T cells and NKT cells (19–21). Each of these effector cells expresses the IL-2/IL-15 receptor β-chain (CD122) and IL-15 is presented to them in trans as a complex with IL-15-receptor-α-chain (IL-15Rα) by DCs and monocytes (22). NK cells exhibit robust effector functions during the peak of an immune response, but lose cytotoxic and proliferative potential during the contraction phase (23). This is followed by apoptotic clearance of most NK cells, although some may be retained as long-lived “memory” NK cells (23, 24).
Recent studies raised the possibility that soluble IL-15/IL-15Rα complexes may be a promising and potent agent for tumor immunotherapy (25–28). Understanding the biological consequence of long-term cytokine therapy on the immune system is thus extremely important. Therefore, we sought to examine the impact of transient and sustained in vivo stimulation with IL-15/IL-15Rα complexes on NK and CD8 T cells. Here we show that transient stimulation increased the size of the NK cell pool and boosted their activation and functional ability compared with NK cells from untreated mice. Unexpectedly, we found that sustained stimulation led to global impairment in NK cell activation and function, accompanied by marked accumulation of mature NK cells with a KLRG1+CD11b+CD27− phenotype. Unlike NK cells, CD8 T cells exhibited robust effector functions and an activated phenotype upon both transient and prolonged stimulation by IL-15/IL-15Rα complexes. Our data reveal that NK and CD8 T cells respond differently to chronic stimulation with this strong activating signal and that NK cells become functionally hyporesponsive upon chronic stimulation with IL-15/IL-15Rα complexes, which has important implications for immunotherapy and vaccine formulation.
Results and Discussion
Chronic Stimulation with IL-15/IL-15Rα Complexes Impairs NK Cell Activation but Not Proliferation.
We evaluated the impact of transient (2 d) and sustained (14 d) in vivo stimulation with IL-15/IL-15Rα complexes on NK cell numbers in various lymphoid and nonlymphoid organs (Fig. S1A). Total splenocyte numbers increased ∼1.5-fold upon transient stimulation and ∼3.5-fold upon sustained stimulation compared with untreated mice (Fig. S1B). NK cells, identified as NK1.1+CD3ε− cells hereafter, increased significantly in a dose-dependent manner in the spleen (Fig. S1 C and D), expanding ∼2.5- and ∼14-fold upon transient and sustained stimulation, respectively. Likewise, we found that NK cells expanded to similar extents in lymph nodes, bone marrow, liver, and lung upon exposure to IL-15/IL-15Rα complexes (Fig. S1E).
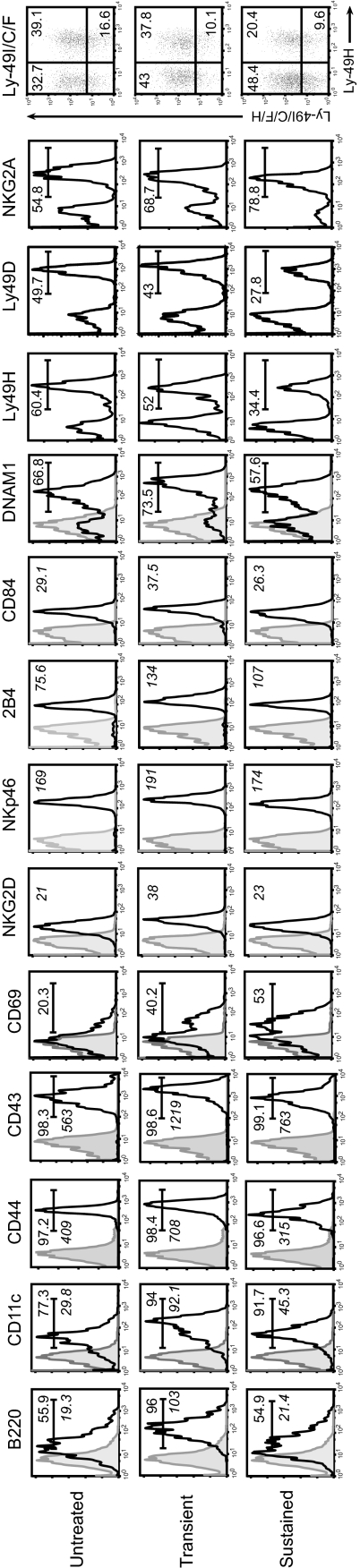
Next we evaluated NK cell activation following stimulation with IL-15/IL-15Rα complexes by flow cytometry using surface markers known to be up-regulated on NK cells upon activation (24, 29, 30). Splenic NK cells from untreated controls displayed a surface phenotype indicative of steady-state cells (B220lowCD11clowCD44+CD43+), whereas transient stimulation by IL-15/IL-15Rα complexes triggered global NK activation as indicated by their uniform B220+CD11c+CD44highCD43high surface phenotype (Fig. 1 and Table S1). Unexpectedly, we observed marked alterations in the activation status of NK cells upon sustained stimulation with IL-15/IL-15Rα complexes. In this setting, surface levels of B220, CD44, CD43, and CD11c were significantly reduced compared with transiently activated NK cells. Strikingly, the patterns of expression for these activation markers were nearly superimposable between untreated and chronically stimulated NK cells, indicating that prolonged stimulation by IL-15/IL-15Rα complexes does not maintain a prototypical activation state among NK cells. As expected, transient activation induced an up-regulation of CD69 on NK cells (Fig. 1 and Table S1). Interestingly, the percentage of CD69+ NK cells remained high upon sustained treatments. Thus, NK cells acquire an altered activation status following chronic stimulation.
Fig. 1.
Lowered activation status and altered balance of activating and inhibitory receptors on NK cells upon sustained in vivo stimulation with IL-15/IL-15Rα complexes. The first five histograms show representative histograms for expression of activation markers (B220, CD11c, CD44, CD43, and CD69) on splenic NK cells. Other histograms show expression of activating and inhibitory receptors (NKG2D, NKp46, 2B4, CD84, DNAM1, Ly-49H, Ly-49D, and NKG2A) on splenic NK cells. Dot plot shows expression of Ly-49I/C/F/H versus Ly-49H to determine percentage of Ly-49I/C/F+ NK cells. Numbers indicate percentage; italicized numbers indicate mean fluorescence intensity (MFI).
NK cell expression of various activating and inhibitory receptors (3, 31) was then characterized. Transient stimulation with IL-15/IL-15Rα complexes caused significant increases in surface levels of various activating receptors including NKG2D, CD84, 2B4, NKp46, and DNAM1; however and rather unexpectedly, we found that sustained stimulation led to their down-regulation (Fig. 1 and Table S1). In parallel, the percentage of NK cells expressing the activating receptors, Ly49H and Ly49D, decreased. On the other hand, we found that the proportions of NK cells expressing inhibitory receptors, NKG2A and Ly49I/C/F, were significantly increased upon chronic stimulation. Thus, prolonged stimulation with IL-15/IL-15Rα complexes leads to an altered expression pattern of activating and inhibitory receptors on NK cells.
KLRG1-Expressing Mature NK Cells Accumulate upon Chronic Stimulation with IL-15/IL-15Rα Complexes.
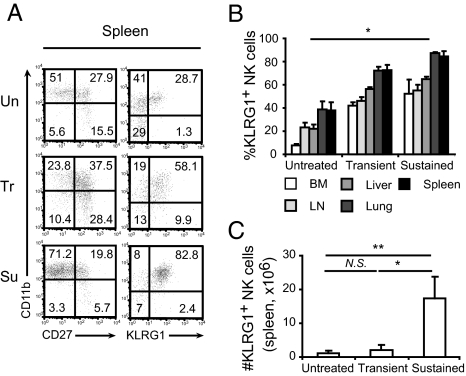
NK cells can be classified into four main developmental stages on the basis of surface CD11b and CD27 levels (32, 33). NK cells are thought to progress from the most immature CD11b−CD27− stage to CD11b−CD27+ through CD11b+CD27+ to CD11b+CD27−. The CD11b+CD27+ and CD11b+CD27− NK cell subsets are both classified as “mature”; however, cells belonging to the CD11b+CD27− subset are considered the most mature, or terminally differentiated, of the two populations. CD11b+CD27− NK cells exhibit reduced effector function and proliferative potential compared with the CD11b+CD27+ subset and represent the predominant KLRG1-expressing NK cell subset (23, 32–35). We sought to determine the developmental status of NK cells upon transient and prolonged treatment with IL-15/IL-15Rα complexes using these markers. In untreated controls, mature NK cells comprised 50% of splenic NK cells (Fig. 2A and Fig. S2A) and ∼15% of bone marrow NK cells (Fig. S2B). Upon transient stimulation with IL-15/IL-15Rα complexes, CD27+ NK cells increased in the spleen (Fig. 2A and Fig. S2A) and bone marrow (Fig. S2B), suggesting activation and/or selective expansion and mobilization of immature cells. However, sustained stimulation led to a significant increase in the proportion of mature NK cells, comprising ∼75% in the spleen (Fig. 2A and Fig. S2A) and 35% in bone marrow (Fig. S2B). Because mature NK cells also express KLRG1+ (23, 34), we next analyzed CD11b vs. KLRG1 expression on NK cells. Splenic CD11b+KRLG1+ NK cells increased from ∼30% in untreated mice to ∼80% (Fig. 2A and Fig. S2A) upon sustained stimulation. A sharp increase in the frequency of mature NK cells (CD11b+KLRG1+) was also observed in the bone marrow (Fig. S2B) and similar trends were observed for KLRG1+ NK cells in various lymphoid and nonlymphoid organs including lymph node, liver, lung, and spleen (Fig. 2B). Interestingly, the absolute number of splenic KLRG1+ NK cells, which increased only modestly from 1 × 106 in controls to 2 × 106 upon transient stimulation, soared to ∼17 × 106 upon sustained stimulation with IL-15/IL-15Rα complexes (Fig. 2C).
Fig. 2.
Accumulation of mature NK cells upon sustained in vivo stimulation with IL-15/IL-15Rα complexes. (A) Representative dot plots showing CD11b versus CD27 (Left) and KLRG1 (Right) expression on NK cells from spleens (Un, untreated; Tr, transient; Su, sustained). Numbers indicate percentage. (B) Percentage of KLRG1+ NK cells in spleen (black, n = 9), lung (dark gray, n = 3), liver (gray, n = 3), lymph nodes (light gray, n = 3), and bone marrow (white, n = 3). (C) Number of KLRG1+ NK cells in the spleen of untreated mice or upon transient and sustained treatments (n = 6). Significance: *P < 0.05, **P < 0.01; NS, not significant. Data are means ± SD.
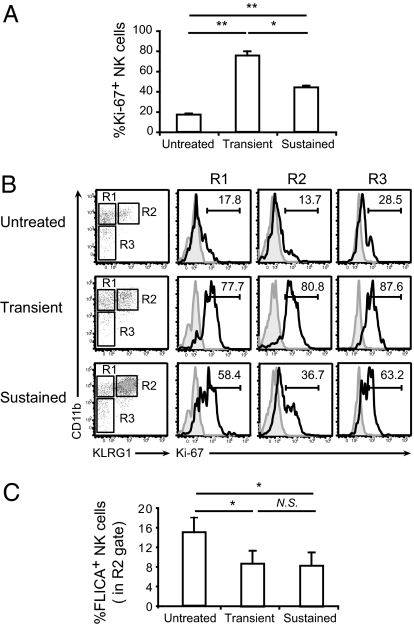
KLRG1+ NK cells have been reported to exhibit properties of end-stage or senescent cells during the contraction phase of immune responses including decreased proliferative capacity, increased apoptosis, and reduced effector function (23, 35). Having shown that the NK cell compartment following sustained stimulation with IL-15/IL-15Rα complexes is dominated by a large number of KLRG1+ mature NK cells, we sought to determine whether they might also exhibit hallmarks of senescence. Using intracellular cytofluorimetric analysis of the Ki-67 antigen, a well-established measure of proliferative capacity, we found that Ki-67 levels were reduced in total NK cells upon chronic stimulation compared with transient stimulation (Fig. 3A). Likewise, Ki-67 levels in the KLRG1+ mature NK cell subset, which comprised ∼90% of the total NK cell compartment, were reduced by ∼50% upon chronic stimulation compared with transient stimulation (Fig. 3B).
Fig. 3.
Reduced proliferative capacity of NK cells upon sustained in vivo stimulation with IL-15/IL-15Rα complexes. (A) Percentage of proliferating NK cells in spleens of untreated mice or upon transient and sustained treatments determined by intracellular Ki-67 staining (n = 6). (B) Representative histograms showing Ki-67 staining in NK cells at different maturation stages, on the basis of CD11b and KLRG1 expression. Gray filled, isotype control; black line, KLRG1. Numbers indicate percentage. R1, CD11b+KLRG1−; R2, CD11b+KLRG1+; and R3, CD11b−KLRG1−. (C) Percentage of apoptotic KLRG1+ NK cells (gate R2) analyzed by FLICA assay (n = 3). Significance: *P < 0.05, **P < 0.01; NS, not significant. Data are means ± SD.
Next we evaluated expression of activated effector caspases by the KLRG1+ NK cells in our experimental system as a measure of apoptosis. Using the FLICA assay, we found that the percentage of KLRG1+ mature NK cells with activated caspase 3/7 was reduced upon transient and sustained stimulation with IL-15/IL-15Rα complexes compared with controls (Fig. 3C). Thus, stimulation with IL-15/IL-15Rα complexes leads to a reduction in apoptosis among mature NK cells.
Sustained Stimulation with IL-15/IL-15Rα Complexes Generates Functionally Impaired NK Cells.
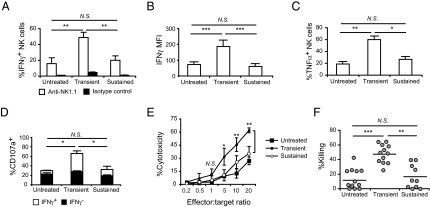
To evaluate the impact of sustained stimulation with IL-15/IL-15Rα complexes on the function of NK cells, we first determined cytokine production by intracellular flow cytometry. Using anti-NK1.1 stimulation, we observed an approximately threefold increase in the percentage of splenic IFNγ+ NK cells after transient stimulation (∼50%) compared with untreated mice (∼17%), whereas only a small fraction of NK cells (∼20%) was IFNγ+ upon sustained stimulation (Fig. 4A). With paramethoxyamphetamine (PMA)/ionomycin stimulation, the majority of NK cells produced IFNγ with no major differences between conditions (Fig. S3A). However, the amount of IFNγ per NK cell was significantly reduced upon sustained stimulation compared with transient stimulation as indicated by MFIs (60 vs. 186, P < 0.001) (Fig. 4B). IFNγ production was also reduced in NK cells from lymph nodes, bone marrow, and liver upon prolonged stimulation (Fig. S3B). A similar reduction in TNFα was observed in splenic NK cells (Fig. 4C). Next, IFNγ expression was analyzed in KLRG1− and KLRG1+ NK cells upon stimulation with PMA/ionomycin (Fig. S3C, Left) and anti-NK1.1 (Fig. S3C, Right). Following transient or sustained stimulations, level of IFNγ expressed by NK cells did not change, on the basis of KLRG1 expression; therefore, NK cell function is diminished regardless of the maturation stage.
Fig. 4.
Impaired effector function of NK cells upon sustained in vivo stimulation with IL-15/IL-15Rα complexes. (A) Percentage of IFNγ+ NK cells in spleen (gated on CD49b+CD3ε− cells) upon 5-h anti-NK1.1 stimulation (n = 5). White bars, anti-NK1.1 (PK136); black bars, isotype control. (B) IFNγ production by NK cells upon 5-h PMA/ionomycin stimulation. MFI of IFNγ+ NK cells shown for spleen (n = 11). (C) TNFα production by NK cells upon 5-h PMA/ionomycin stimulation. Percentage of TNFα+ NK cells shown for spleen (n = 3). (D) Degranulation of NK cells indicated by CD107a staining. Splenocytes were stimulated on anti-NK1.1–coated plates and intracellularly stained with anti-IFNγ. Percentage of CD107a+ IFNγ− (black) and IFNγ+ (white) NK cells shown (n = 6–7). (E) Cytotoxicity of NK cells analyzed in vitro. NK cell-enriched splenocytes were cultured with a constant number (1 × 104) of YAC1 cells at different effector:target ratios as indicated for 4 h and cytotoxicity was measured by lactate dehydrogenase release assay. Filled square, untreated; filled circle, transient; open circle, sustained (n = 3). (F) Cytotoxicity of NK cells analyzed in vivo by CFSE-based assay. Percentage of in vivo killing in untreated (n = 12) mice or upon transient (n = 12) and sustained (n = 10) treatments calculated as described in Materials and Methods. Each circle represents an individual mouse. Lines indicate the average value. Significance: *P < 0.05, **P < 0.01, ***P < 0.001; NS, not significant. Data are means ± SD.
We also evaluated degranulation of secretory lysosomes, an indication of target lysis, by quantifying surface deposition of LAMP-1/CD107a. The percentage of CD107a+ NK cells was significantly higher upon transient stimulation (65.9%) compared with controls (25.7%) when stimulated on anti-NK1.1–coated plates, whereas the percentage of CD107a+ NK cells upon sustained stimulation was similar to untreated controls (Fig. 4D) and correlated with the percentage of IFNγ+ cells (Fig. 4D, white bars). Stimulation with PMA/ionomycin, however, gave a different picture with the majority of NK cells expressing surface CD107a in all conditions (Fig. S3D). We evaluated the impact of sustained stimulation with IL-15/IL-15Rα complexes on NK cell cytotoxicity in vitro and in vivo. Cytotoxicity of NK cells against NK cell-sensitive YAC1 cells was tested in vitro using a lactate dehydrogenase release assay. Whereas there was no difference at low effector:target ratios, at high effector:target ratios, NK cells exhibited increased cytotoxicity after transient treatment compared with both sustained treatment and untreated controls (Fig. 4E). There was only moderate increase in cytotoxicity upon sustained treatment compared with controls. Next, using a carboxyfluorescein succinimidyl ester (CFSE)-based in vivo killing assay in which allogeneic splenocytes serve as targets, the ratio between CFSEhigh (allogeneic, target) and CFSElow (syngeneic, control) populations in spleen from each experimental condition was compared with the ratio in NK1.1-antibody–depleted mice 3 h after i.v. transfer of target cells (Fig. S3E). Depletion of NK cells with anti-NK1.1 antibody was confirmed by anti-CD49b staining (Fig. S3F). The cytotoxicity of NK cells was greater than four times higher upon transient stimulation compared with untreated controls, whereas NK cells exhibited poor killing activity upon sustained stimulation (16.5%) (Fig. 4F). Finally, we tested whether an additional proinflammatory stimulus known to activate NK cells could recover their function upon sustained stimulation by IL-15/IL-15Rα complexes. Injection of a TLR3 ligand, PolyI:C (36), in combination with the last dose of sustained treatments did not have any effect on IFNγ production by NK cells (Fig. S3G). Taken together, these results reveal that prolonged in vivo stimulation with IL-15/IL-15Rα complexes generates NK cells with reduced functional activity.
Although the mechanism of altered activation status and reduced functional activity in NK cells upon sustained stimulation with IL-15/IL-15Rα complexes remains unclear, our results suggest that NK cell-extrinsic factors are unlikely to be culprits. First, depletion of CD8 or CD4 T cells (Fig. S4A), absence of B cells (Fig. S4B), and absence of T and B cells (Fig. S4C) had no effect on IFNγ production by NK cells. Although there was a significant increase in number of myeloid cells (Fig. S4D), these cells were not suppressive (Fig. S4E). We also determined that various regulatory molecules known to inhibit NK cell function (11, 37, 38), such as IFNγ, nitric oxide, and IL-12p40 (Fig. S4F), were not involved. We also evaluated various surface receptors and signaling molecules expressed by NK cells. The levels of CD122 and common γ-chain, which would be important for NK cell responsiveness to the IL-15 superagonist, did not change upon transient or chronic stimulation (Fig. S4G). However, we did observe a significant reduction in the level of CD3ζ-chain (Fig. S4H) upon sustained stimulation with IL-15/IL-15Rα complexes, suggesting that NK cell-intrinsic mechanisms involving signaling molecules downstream of IL-15 signaling may be involved. Further studies are needed to identify potential signaling defects that would account for NK cell hyporesponsiveness or exhaustion upon chronic stimulation.
Sustained in Vivo Stimulation with IL-15/IL-15Rα Complexes Generates a Large Number of CD8 T Cells with an Activated Phenotype and Effector Function.
We also evaluated the activation status and functional capacity of CD8 T cells to determine whether chronic stimulation by IL-15/IL-15Rα complexes might down-regulate the activity of all CD122+ cytotoxic lymphocytes. As expected, CD8 T cells, identified as CD8+CD3ε+ cells hereafter, expanded in a dose-dependent manner in the spleen (Fig. S5A). The relative abundance (Fig. S5A, Left) of CD8 T cells increased ∼1.3-fold and ∼3.2-fold upon transient and sustained stimulation, respectively, compared with untreated controls. Likewise, the absolute numbers (Fig. S5A, Right) of CD8 T cells increased ∼2-fold upon transient and ∼11-fold upon sustained stimulation.
A CD44high activated phenotype was observed among ∼90% of CD8 T cells following sustained stimulation with IL-15/IL-15Rα complexes (Fig. S5B). We also examined surface levels of the early activation marker, CD69, and found that, whereas transient stimulation induced its up-regulation, CD8 T cells were largely CD69− following prolonged stimulation (Fig. S5C). Notably, PD1 and KLRG1, two molecules associated with chronic stimulation in T cells (39), were low to negative on CD8 T cells in all conditions (Fig. S5C). We then evaluated functional parameters of CD8 T cells following sustained stimulation. Upon PMA/ionomycin stimulation, the percentage of IFNγ+ CD8 T cells (Fig. S5D, Left) and the amount of cytokine produced on a per-cell basis (Fig. S5D, Right) increased significantly upon transient and sustained stimulation compared with controls. Similar results were obtained following polyclonal T cell stimulation with plate-bound anti-CD3ε/anti-CD28 antibodies (Fig. S5E, white bars) and ex vivo stimulation of transferred OT-I cells with chicken ovalbumin SIINFEKL peptide (Fig. S5F). In addition, we observed increased levels of surface CD107a on CD8 T cells upon sustained stimulation following activation with anti-CD3ε/CD28 antibodies (Fig. S5E). These results show that in contrast to our findings with NK cells, sustained in vivo stimulation with IL-15/IL-15Rα complexes generates a substantial CD8 T cell compartment with robust effector function. Therefore, the accrual of functionally impaired effector cells following chronic IL-15 stimulation appears to be specific to NK cells.
During the initial steps of immune activation, NK cells are exposed to IL-15 transpresented via IL-15Rα on DCs, an encounter that promotes their expansion and survival throughout the immune response (19). During the contraction phase, NK cells undergo apoptosis to restore their numbers to baseline, whereas some are retained as memory cells (23, 24, 35). So-called “end-stage NK cells” are characterized by reduced proliferative capacity, diminished functional potential, and elevated apoptosis (23, 35). Consistent with physiological transpresentation, we found that brief stimulation with soluble IL-15/IL-15Rα complexes led to an expansion of NK cells with markedly enhanced cytotoxic potential relative to NK cells from untreated mice. In contrast, sustained stimulation generated a large NK cell compartment dominated by CD11b+CD27−KLRG1+ NK cells with hallmarks of end-stage or senescent cells. Furthermore, these cells had a blunted activation profile that resembled unstimulated NK cells and a receptor repertoire with a higher frequency of inhibitory than activating receptors. In contrast to a normal contraction phase, however we observed a reduction in apoptotic mature NK cells. Collectively these results suggest that sustained stimulation with IL-15/IL15Rα complexes caused a massive expansion of NK cells that differentiated into developmentally mature cells. Normally, this stage of NK cell development, which is typified by a relative impairment in effector functions, would be followed by programmed cell death (23). However, continual stimulation by IL-15, which is a well-known survival signal for NK cells, prevented the natural turnover of developmentally mature NK cells (40). This outcome highlights the importance of strict regulation of IL-15 expression and transpresentation under physiological conditions (41). Thus, rather than acquiring an end-stage phenotype and then undergoing programmed cell death, these chronically stimulated NK cells, with an exhausted phenotype, accumulate in substantial numbers. The use of IL-15/IL-15Rα complexes is a promising approach against cancer (25–28). On the basis of our observations, however, therapies that aim to functionally invigorate cytotoxic lymphocytes through sustained cytokine or adjuvant treatment should be carefully designed to avoid the adverse consequence of NK cell exhaustion.
Materials and Methods
Mice.
C57BL/6 and OTI mice (The Jackson Laboratory) were maintained and/or bred under barrier conditions in the Dana-Farber Cancer Institute Animal Facility in accordance with institutional and National Institutes of Health guidelines. Dana-Farber Cancer Institute is accredited by the American Association for the Accreditation of Laboratory Animal Care.
IL-15/IL-15Rα Treatments.
Human IL-15 was a generous gift from Amgen (Thousand Oaks, CA); mouse and human IL-15 were purchased from eBiosciences. Initially, we tested different sources of mouse and human IL-15, precomplexed at different ratios with murine IL-15Rα-human IgG1-Fc fusion protein (IL-15Rα) and observed similar effects on cell function and expansion as described in this study (Fig. S6). We chose one of these combinations in this study: for each dose of IL-15/IL-15Rα complexes, 0.5 μg of IL-15 and 3 μg of recombinant murine IL-15Rα-human-IgG1-Fc (IL-15Rα, R&D) were mixed, incubated for 30 min at 37 °C, and injected intraperitoneally in 200 μL PBS. Injections were given every 2–3 d for 2 wk (five doses), whereas transient stimulation consisted of a single dose 2 d before analysis. Lymphocytes were analyzed 2 d after the last injection by flow cytometry, intracellular cytokine staining, degranulation assays, and in vivo killing assays, as described in SI Materials and Methods. In all experiments, NK cells were identified as NK1.1+CD3ε− or CD49b+CD3ε−, and CD8 T cells were identified as CD8α+CD3ε+ cells.
Statistics.
Statistical analysis was performed using Student's t test and Kruskal-Wallis test with ANOVA and GraphPad Prism software. P values <0.05 were considered significant (*P < 0.05, **P < 0.01,*** P < 0.001; NS, not significant). All data are presented as means ± SD.
Supplementary Material
Acknowledgments
We thank Dr. Jerry Ritz for critically reading this manuscript. This study was supported in part by research funding from an American Association for Cancer Research Centennial Postdoctoral Fellowship in Cancer Research (to K.G.E.), an American Cancer Society Research Scholar Award (to S.J.T.), National Institutes of Health Grant AI067545 (to A.W.G.), and a Cancer Research Institute Scholar Award (to A.W.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012128107/-/DCSupplemental.
References
- 1.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nat Rev Immunol. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama WM. Natural killer cell immune responses. Immunol Res. 2005;32:317–325. doi: 10.1385/IR:32:1-3:317. [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 4.Vivier E, Nunès JA, Vély F. Natural killer cell signaling pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 5.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oppenheim DE, et al. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 7.Saito T, Ruffman R, Welker RD, Herberman RB, Chirigos MA. Development of hyporesponsiveness of natural killer cells to augmentation of activity after multiple treatments with biological response modifiers. Cancer Immunol Immunother. 1985;19:130–135. doi: 10.1007/BF00199721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talmadge JE, et al. Hyporesponsiveness to augmentation of murine natural killer cell activity in different anatomical compartments by multiple injections of various immunomodulators including recombinant interferons and interleukin 2. J Immunol. 1985;135:2483–2491. [PubMed] [Google Scholar]
- 10.Tripathy SK, et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J Exp Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaknin I, et al. A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood. 2008;111:1437–1447. doi: 10.1182/blood-2007-07-100404. [DOI] [PubMed] [Google Scholar]
- 12.Oliviero B, et al. Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology. 2009;137(3):1151–1160. doi: 10.1053/j.gastro.2009.05.047. 1160 e1151–1157. [DOI] [PubMed] [Google Scholar]
- 13.Bozzano F, et al. Functionally relevant decreases in activatory receptor expression on NK cells are associated with pulmonary tuberculosis in vivo and persist after successful treatment. Int Immunol. 2009;21:779–791. doi: 10.1093/intimm/dxp046. [DOI] [PubMed] [Google Scholar]
- 14.Iannello A, Debbeche O, Samarani S, Ahmad A. Antiviral NK cell responses in HIV infection: II. viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008;84:27–49. doi: 10.1189/jlb.0907649. [DOI] [PubMed] [Google Scholar]
- 15.Brauner H, et al. Distinct phenotype and function of NK cells in the pancreas of nonobese diabetic mice. J Immunol. 2010;184:2272–2280. doi: 10.4049/jimmunol.0804358. [DOI] [PubMed] [Google Scholar]
- 16.Villanueva J, et al. Natural killer cell dysfunction is a distinguishing feature of systemic onset juvenile rheumatoid arthritis and macrophage activation syndrome. Arthritis Res Ther. 2005;7:R30–R37. doi: 10.1186/ar1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vosshenrich CA, et al. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174:1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 19.Castillo EF, Stonier SW, Frasca L, Schluns KS. Dendritic cells support the in vivo development and maintenance of NK cells via IL-15 trans-presentation. J Immunol. 2009;183:4948–4956. doi: 10.4049/jimmunol.0900719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force.”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 22.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 23.Robbins SH, Tessmer MS, Mikayama T, Brossay L. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol. 2004;173:259–266. doi: 10.4049/jimmunol.173.1.259. [DOI] [PubMed] [Google Scholar]
- 24.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 26.Epardaud M, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 27.Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci USA. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sague SL, Tato C, Puré E, Hunter CA. The regulation and activation of CD44 by natural killer (NK) cells and its role in the production of IFN-gamma. J Interferon Cytokine Res. 2004;24:301–309. doi: 10.1089/107999004323065093. [DOI] [PubMed] [Google Scholar]
- 30.Vosshenrich CA, et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma CS, Nichols KE, Tangye SG. Regulation of cellular and humoral immune responses by the SLAM and SAP families of molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- 32.Chiossone L, et al. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113:5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–1524. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]
- 34.Huntington ND, et al. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 35.Robbins SH, et al. Cutting edge: Inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 36.Miyake T, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009;183:2522–2528. doi: 10.4049/jimmunol.0901500. [DOI] [PubMed] [Google Scholar]
- 37.Koblish HK, Hunter CA, Wysocka M, Trinchieri G, Lee WM. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: Inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J Exp Med. 1998;188:1603–1610. doi: 10.1084/jem.188.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Movahedi K, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 39.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Huntington ND, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: A guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.