Abstract
Neuregulin 1 (NRG1) is a trophic factor that acts by stimulating ErbB receptor tyrosine kinases and has been implicated in neural development and synaptic plasticity. In this study, we investigated mechanisms of its suppression of long-term potentiation (LTP) in the hippocampus. We found that NRG1 did not alter glutamatergic transmission at SC-CA1 synapses but increased the GABAA receptor-mediated synaptic currents in CA1 pyramidal cells via a presynaptic mechanism. Inhibition of GABAA receptors blocked the suppressing effect of NRG1 on LTP and prevented ecto-ErbB4 from enhancing LTP, implicating a role of GABAergic transmission. To test this hypothesis further, we generated parvalbumin (PV)-Cre;ErbB4−/− mice in which ErbB4, an NRG1 receptor in the brain, is ablated specifically in PV-positive interneurons. NRG1 was no longer able to increase inhibitory postsynaptic currents and to suppress LTP in PV-Cre;ErbB4−/− hippocampus. Accordingly, contextual fear conditioning, a hippocampus-dependent test, was impaired in PV-Cre;ErbB4−/− mice. In contrast, ablation of ErbB4 in pyramidal neurons had no effect on NRG1 regulation of hippocampal LTP or contextual fear conditioning. These results demonstrate a critical role of ErbB4 in PV-positive interneurons but not in pyramidal neurons in synaptic plasticity and support a working model that NRG1 suppresses LTP by enhancing GABA release. Considering that NRG1 and ErbB4 are susceptibility genes of schizophrenia, these observations contribute to a better understanding of how abnormal NRG1/ErbB4 signaling may be involved in the pathogenesis of schizophrenia.
Neuregulin 1 (NRG1) is a trophic factor that acts by activating ErbB receptor tyrosine kinases, including ErbB4. NRG1 signaling has been implicated in various steps in neural development, including neuron migration, axon guidance, synapse formation, and expression of neurotransmitter receptors (1). Studies of NRG1 have attracted much attention because both NRG1 and ErbB4 were identified as susceptibility genes of schizophrenia and NRG1 and ErbB4 mutant mice show schizophrenia-relevant behaviors (1–4).
Recent studies suggest that NRG1 plays a role in neurotransmission and synaptic plasticity (1). NRG1 has been shown to suppress the induction of LTP acutely at Schaffer collateral (SC)-CA1 synapses in adult rodent hippocampus (5–8), but it has no effect on basal synaptic transmission (5, 7, 9). NRG1 regulation of long-term potentiation (LTP) requires ErbB4 (8); however, underlying mechanisms remain unclear. In vitro studies suggest that NRG1 may alter functions of pyramidal neurons and glutamatergic transmission. For example, it could suppress NMDA receptor (NMDAR) currents in prefrontal cortical (PFC) neurons in culture (10). NRG1 was shown to stimulate internalization of surface AMPA receptors (AMPARs) in dissociated hippocampal neurons (11). Moreover, changes in ErbB4 levels in neonatal hippocampal slices alter dendritic spine size and AMPA synaptic currents (12). Conversely, ErbB4 expression is largely restricted to GABAergic interneurons in the brain (5, 13). NRG1 stimulates GABA release in the PFC slices in a manner dependent on ErbB4 (14) and suppresses the firing of PFC pyramidal neurons by increasing GABAergic transmission (15). These effects are inhibited when the ErbB4 gene is specifically ablated in parvalbumin (PV)-positive interneurons (15). Which of these mechanisms is involved in NRG1 regulation of LTP remains unclear.
In the current study, we carried out a series of experiments to investigate how NRG1 inhibits the induction of LTP in the hippocampus. We studied the effect of NRG1 on glutamatergic transmission in hippocampal pyramidal neurons and investigated the relationship between NRG1 regulation of hippocampal LTP and GABAergic transmission. We explored the consequences of ablating the ErbB4 gene in PV-positive interneurons and glutamatergic neurons. Our results indicate that NRG1 suppresses LTP by stimulating GABA release and identify a critical role of ErbB4 in PV-positive neurons in synaptic plasticity and contextual fear conditioning.
Results
NRG1 Does Not Alter Glutamatergic Receptor-Mediated Synaptic Responses in the Hippocampus.
NRG1 suppresses the induction of LTP at the SC-CA1. This effect may be mediated by reduced glutamate release or postsynaptic response at excitatory synapses or by increased GABAergic transmission. To dissect the involved mechanism, we first measured field excitatory postsynaptic potentials (fEPSPs) at SC-CA1 synapses. As shown in Fig. S1A, their slopes at different stimulation intensities were not altered by NRG1, indicating that NRG1 at 1 nM, a concentration at which it suppresses LTP (5, 7), had no effect on basal synaptic transmission. Moreover, paired-pulse ratios (PPRs) of fEPSPs in the absence and presence of NRG1 were similar (Fig. S1B), suggesting that NRG1 had no effect on presynaptic glutamate release. These results suggest that AMPAR-mediated synaptic transmission may not be affected by NRG1. To determine whether NRG1 regulates NMDAR-mediated response, fEPSPs were measured in Mg2+-free buffer to release the NMDAR block and in the presence of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione to block AMPAR. NRG1 had no effect on the amplitudes of NMDA fEPSPs because the input/output curves without or with NRG1 completely overlapped (Fig. S1C), suggesting that NRG1 may not alter NMDAR-mediated response.
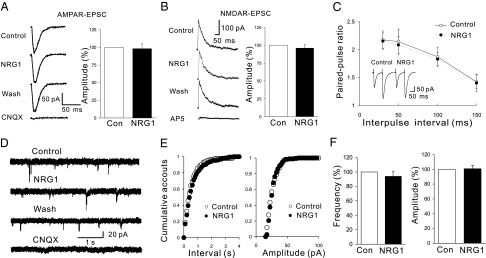
To study the effect of NRG1 on AMPA and NMDA response further, we measured AMPA- and NMDA-mediated excitatory postsynaptic currents (EPSCs) in pyramidal neurons in a whole-cell configuration. As shown in Fig. 1 A and B, AMPAR or NMDAR EPSCs did not change after NRG1 treatment, in agreement with studies of fEPSPs. Similarly, PPRs of EPSCs to two consecutive stimuli with different time intervals were not altered by NRG1 (Fig. 1C), suggesting that NRG1 has no effect on glutamate release. Finally, we tested whether NRG1 affects spontaneous synaptic transmission by measuring miniature excitatory postsynaptic currents (mEPSCs) from CA1 pyramidal neurons. Both the amplitude and frequency of mEPSCs were not altered by 1 nM NRG1 (Fig. 1 D–F). Together, these observations demonstrate that NRG1 does not alter glutamatergic transmission at hippocampal SC-CA1 synapses.
Fig. 1.
AMPAR- or NMDAR-mediated currents were not altered in NRG1-treated hippocampal slices. BMI (20 μM) was included in artificial cerebrospinal fluid (ACSF) for experiments in this figure. AMPA and NMDA currents were evoked by holding membrane potentials at −70 mV and +40 mV, respectively. NRG1 has no effect on peak amplitudes of AMPA currents (A; n = 9; P > 0.05) or NMDA currents (B; n = 6; P > 0.05). Notice that 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 100 μM D(−)-2-amino-5-phosphonovaleric acid (AP5) were included in ACSF in A and B, respectively. Con, control. (C) NRG1 did not change PPRs of evoked EPSCs at SC-CA1 synapses (n = 6; P > 0.05). (Inset) Representative recordings. (D–F) There was no effect of NRG1 on mEPSCs on CA1 pyramidal neurons. Shown are representative traces (D) and cumulative plots (E) of mEPSC frequencies and amplitudes before and after NRG1 treatment. (F) Summary of results (n = 9; P > 0.05).
NRG1 Increases GABAergic Transmission in the Hippocampus.
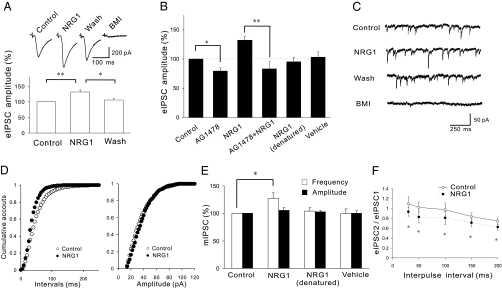
Having ruled out a possible effect of NRG1 on glutamatergic transmission, we next determined whether NRG1 regulates GABAergic transmission in the hippocampus. Evoked inhibitory postsynaptic currents (eIPSCs) were evoked by stimulating SC and measured in pyramidal neurons in the CA1 region by whole-cell recording. They were inhibited by bicuculline methiodide (BMI), a GABAA receptor antagonist. The eIPSC amplitudes were increased in NRG1-treated slices in comparison to control (Fig. 2 A and B and Fig. S2). In contrast, vehicle or denatured NRG1 had no effect (Fig. 2B). Moreover, treatment with AG1478, an inhibitor of ErbB tyrosine kinases (14), prevented NRG1 from increasing eIPSC amplitudes in the hippocampus (Fig. 2B). These results suggest that NRG1 increases depolarization-evoked GABA currents through activation of ErbB tyrosine kinases. Next, we examined whether spontaneous inhibitory synaptic transmission is affected by NRG1. Miniature IPSCs (mIPSCs) were measured at CA1 pyramidal neurons that were inhibited by BMI. Although NRG1 had no effect on their amplitudes, it increased the frequency to 127 ± 11% of control (n = 8; P < 0.05; Fig. 2 C–E). This effect could be reversed 10 min after washout. Again, denatured NRG1 or vehicle had no effect on either the amplitude or frequency of mIPSCs. These results suggest that NRG1 promotes the release of GABA from presynaptic terminals. We have also characterized the PPRs and found that they were reduced at every interval, indicating increased probability of GABA release (16) (Fig. 2F). These results suggest that NRG1 may increase GABA release in the hippocampus, providing a potential mechanism of its regulation of LTP. Notice that treatment with AG1478 in the absence of exogenous NRG1 also decreased eIPSCs (to 79 ± 5.3% of control, n = 8; P < 0.05; Fig. 2B), suggesting the involvement of endogenous NRG1 in regulating GABAergic transmission.
Fig. 2.
NRG1 increases GABAA receptor-mediated synaptic response. (A) Increased eIPSCs in NRG1-treated slices. Shown are representative eIPSC traces (Upper) and quantitative analysis (Lower) (n = 11). (B) AG1478 blocks NRG1 enhancement of eIPSCs. Slices were pretreated without or with 5 μM AG1478 for 10 min and then with 1 nM NRG1, boiled NRG1, or vehicle (n = 8 for control, AG1478, and AG1478 plus NRG1; n = 7 for denatured NRG1; n = 5 for vehicle) (C–E) NRG1 increases mIPSC frequency but not amplitudes in CA1 pyramidal cells. Shown are representative traces (C) and cumulative plots (D) of mIPSC frequencies and amplitudes. (E) Summarized data of NRG1 (n = 8), denatured NRG1 (n = 4), and vehicle (n = 6). (F) NRG1 reduced PPRs of eIPSCs in the hippocampus (n = 5). *P < 0.05; **P < 0.01.
Blockade of GABAA Receptor-Mediated Transmission Prevents NRG1 from Suppressing LTP Induction.
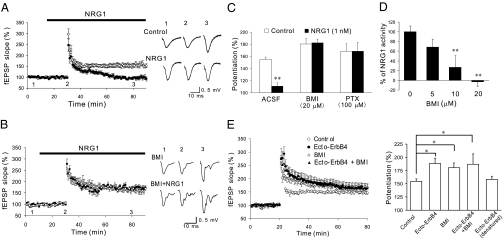
LTP induction at excitatory synapses is known to be inhibited by increased GABA-mediated transmission (17, 18). Because NRG1 has no effect on AMPAR- or NMDAR-mediated synaptic response, we examined whether increased GABAergic transmission by NRG1 plays a role in suppressing LTP induction. As shown in Fig. 3A, NRG1 suppressed LTP induced by tetanus stimulation. Treatment with 20 μM BMI increased LTP significantly (Fig. 3B), consistent with previous observations (17, 18). Intriguingly, BMI prevented NRG1 from suppressing LTP induction in a dose-dependent manner (Fig. 3 B–D). At 20 μM, BMI completely blocks the action of NRG1. This result suggests that NRG1 suppression of LTP requires GABAA receptor-dependent transmission. To eliminate a possible off-target effect of BMI, hippocampal slices were treated with picrotoxin (PTX), another GABAA receptor inhibitor that acts by inhibiting the channel (unlike BMI that binds to the receptor) (19). As shown in Fig. 3C, NRG1 was no longer able to suppress LTP in hippocampal slices in the presence of 100 μM PTX. Finally, application of diazepam, a benzodiazepine agonist known to enhance the activation of GABAA receptor, prevented LTP induction at 1 μM, a concentration that produced comparable enhancement (∼25%) of eIPSCs as NRG1 (Fig. S3). Together, these results indicate a critical role of GABAA receptor-dependent neurotransmission in NRG1-induced suppression of LTP induction.
Fig. 3.
NRG1-induced suppression of LTP is blocked by GABAA antagonists. (A) NRG1 suppresses LTP at SC-CA1 synapses in the hippocampus. Normalized fEPSP slopes were plotted every 1 min for control (○) or slices treated with 1 nM NRG1 (●), which was applied during the period indicated by the bar. (Right) Averaged traces taken before (1), 20 min after starting NRG1 application (2), and 50 min after tetanus stimuluation (3). (B) BMI prevented NRG1 from inhibiting LTP. Normalized fEPSP slopes were plotted as in A. BMI (20 μM) was bath-applied 20 min before the application of NRG1. (Right) Representative traces. (C) PTX prevented NRG1 from inhibiting LTP. (D) Dose-dependent inhibition by BMI. (E) Ecto-ErbB4 increases LTP, and this effect is blocked by BMI. Brain slices were treated with ecto-ErbB4 30 min prior to LTP induction. (Left) Normalized fEPSP slopes of control or slices treated with 1 μg/mL ecto-ErbB4, 20 μM BMI, ecto-ErbB4 plus BMI, or denatured ecto-ErbB4. (Right) Quantitative analysis of data (n = 5–8 slices). *P < 0.05; **P < 0.01.
Neutralizing Endogenous NRG1 Potentiates LTP.
NRG1 is detectable in the hippocampus of adult brain (14). To determine whether endogenous NRG1 regulates LTP, hippocampal slices were treated with ecto-ErbB4, a neutralizing peptide that specifically blocks NRG1 activation of ErbB kinases and Erk and facilitation of GABA release (14) (Fig. S4). As shown in Fig. 3E, treatment with 1 μg/mL ecto-ErbB4 alone increased LTP. This result is in agreement with the finding that ErbB4 inhibition reduces eIPSCs (Fig. 2B) and supports the notion that endogenous NRG1 may suppress LTP by increasing depolarization-evoked GABA currents through activation of ErbB tyrosine kinases. Considering that both BMI and ecto-ErbB4 increased LTP, we then determined whether they act via the same mechanism—attenuating GABAergic transmission. If so, ecto-ErbB4 should not be able to increase LTP in the presence of BMI. To test this hypothesis, hippocampal slices were treated with 1 μg/mL ecto-ErbB4 and 20 μM BMI. LTP in the presence of both ecto-ErbB4 and BMI was not different from that in the presence of only ecto-ErbB4 or BMI. These results provide further support to the idea that NRG1 suppresses LTP via enhancing GABAA receptor-mediated transmission.
ErbB4 in PV-Positive Interneurons Is Critical for Synaptic Plasticity and Contextual Fear Conditioning.
Among ErbB proteins, ErbB4 is highly expressed in adult brains, and its ablation in the entire brain increases LTP and prevented NRG1 from suppressing LTP (8). In light of its expression in PV-positive neurons (13, 14, 20–22), we generated PV-Cre;ErbB4−/− mice in which ErbB4 was specifically knocked out in PV-positive neurons. Expression of the Cre recombinase in PV-Cre mice is under the control of a minimal promoter of the PV gene that is active at postnatal day 13 in rodents (23–25). Western blot analysis indicated that ErbB4 was reduced but not abolished in the hippocampus of PV-Cre;ErbB4−/− mice (Fig. S5). This was not unexpected, because ErbB4 expression in other cells was not altered in the mutant mice. Immunostaining evidence indicates that ErbB4 immunoreactivity was abolished in PV-positive neurons in PV-Cre;ErbB4−/− slices (Fig. S5), demonstrating loss of ErbB4 specifically in PV-positive neurons. Ablation of ErbB4 in PV-positive interneurons seemed to have little effect on basal GABAergic transmission. As shown in Fig. S6 A–C, hippocampal pyramidal neurons of PV-Cre;ErbB4−/− and control littermates produced similar mIPSCs. Nevertheless, the eIPSC PPRs in PV-Cre;ErbB4−/− hippocampus were increased (Fig. S6D), consistent with pharmacological inhibition of ErbB4 (Fig. 2B) and in further support of the involvement of endogenous NRG1 in regulating GABA release.
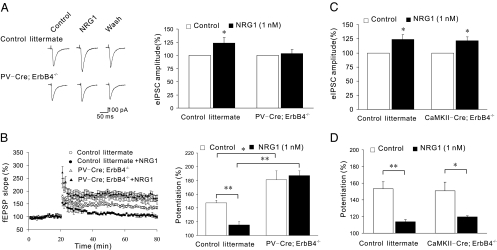
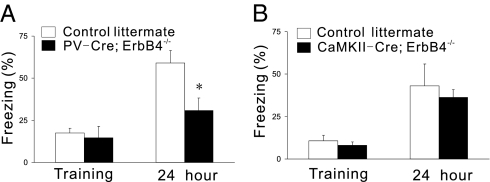
As shown in Fig. 4A, the potentiation effect of NRG1 on eIPSCs was abolished in hippocampal slices from PV-Cre;ErbB4−/− mice, indicating a critical role for NRG1/ErbB4 signaling in PV-positive neurons to regulate GABAergic transmission. Hippocampal LTP was enhanced in PV-Cre;ErbB4−/− slices in comparison to those of control littermates (Fig. 4B). This result agrees with an earlier report of ErbB4 null mutation (8) and underscores PV-positive neurons as a possible cellular target. Importantly, NRG1 was unable to suppress LTP in PV-Cre;ErbB4−/− hippocampal slices (Fig. 4B), suggesting that NRG1 suppression of LTP requires ErbB4 in PV-positive neurons. Together, these pieces of in vivo evidence support a working model in which NRG1 activates ErbB4 in PV-positive neurons to facilitate GABAergic transmission, and thus suppresses LTP. To determine whether NRG1 malfunction in the hippocampus could cause relevant cognitive deficits, we measured contextual fear conditioning, a behavior implicated in hippocampal function, in PV-Cre;ErbB4−/− mice. As shown in Fig. 5A, the mutant mice were impaired in this behavior, suggesting problems in associating learning and memory when ErbB4 is ablated in PV-positive neurons.
Fig. 4.
Ablation of ErbB4 in PV-positive neurons prevents NRG1 from increasing GABAergic transmission and suppressing LTP. (A) Representative eIPSC traces from control and PV-Cre;ErbB4−/− mice. (Right) Quantitative analysis of data with eIPSCs before application of 100% NRG1 (n = 9 for control; n = 8 for PV-Cre;ErbB4−/− mice). (B) Normalized fEPSP slopes from control and NRG1-treated hippocampal slices of indicated mice. (Right) Quantitative data (n = 4–6 slices). (C) NRG1 regulation of eIPSCs in CaMKII-Cre;ErbB4−/− mice. eIPSCs were recorded from CaMKII-Cre;ErbB4−/− mice (n = 9) or control littermates (n = 5). (D) NRG1 suppression of LTP induction in CaMKII-Cre;ErbB4−/− mice. Shown was % potentiation of fEPSP slopes that were recorded as Fig. 1 (n = 4–5 slices). *P < 0.05; **P < 0.01.
Fig. 5.
Impaired contextual fear conditioning in PV-Cre;ErbB4−/− but not CaMKII-Cre;ErbB4−/− mice. (A) Memory for contextual training measured 24 h after training was reduced in PV-Cre;ErbB4−/− mice (n = 6) compared with control littermates (n = 7). (B) Memory for contextual training measured 24 h after training was not changed in CaMKII-Cre;ErbB4−/− mice (n = 6) compared with control littermates (n = 6). *P < 0.05.
Ablation of ErbB4 in Pyramidal Neurons Had No Effect on NRG1 Regulation of LTP or Contextual Fear Conditioning.
In light of reported effects of NRG1 on pyramidal neurons (7, 10–12, 26, 27), we determined whether ErbB4 in pyramidal neurons is involved in NRG1 suppression of LTP. To this end, we generated mutant mice, CaMKII-Cre;ErbB4−/−, that lack ErbB4 in pyramidal neurons. Western blot analysis indicated similar levels of ErbB4 in the CA1 regions between the mutant mice and control littermates (Fig. S5), suggesting that ErbB4 may not be expressed at high levels in adult pyramidal neurons. Moreover, the mutation seemed to have little effect on NRG1 enhancement of eIPSCs (Fig. 4C), suggesting that ErbB4 in pyramidal neurons have a limited role in regulating GABAergic transmission. Intriguingly, we found that NRG1 remained able to suppress LTP in CaMKII-Cre;ErbB4−/− hippocampus. There was no difference in the NRG1 inhibitory effect between CaMKII-Cre;ErbB4−/− mice and control littermates (Fig. 4D). These results suggest that ErbB4 in pyramidal cells may not be critical for NRG1 regulation of LTP. This notion was supported by analysis of contextual fear conditioning in CaMKII-Cre;ErbB4−/− mice. They showed a similar freezing response as control littermates (Fig. 5B), suggesting that ErbB4 in pyramidal cells was not involved in hippocampus-dependent contextual conditioning.
Discussion
This paper provides evidence that NRG1 suppresses LTP at the SC-CA1 synapses in the hippocampus in a manner that requires GABAergic transmission. Pharmacologically, the NRG1 effect was blocked by GABAA receptor antagonists. Genetically, ablation of ErbB4 in PV-positive interneurons prevented NRG1 from enhancing GABAergic transmission and suppressing LTP, indicating a critical role of ErbB4 in PV-positive interneurons. Moreover, this paper provides evidence that ErbB4 in excitatory pyramidal neurons may not be necessary for NRG1 regulation of LTP. Finally, neutralizing endogenous NRG1 by ecto-ErbB4 alters LTP in the hippocampus, suggesting a role of endogenous NRG1 in regulating brain activity. Together, these results identify a unique in vivo function of NRG1/ErbB4 in regulating synaptic plasticity in the hippocampus.
There are three types of ErbB kinases that serve as NRG1 receptors, all of which are expressed in the brain, albeit at different levels. In this study, we showed that ErbB4 in PV-positive neurons is critical for NRG1 regulation of LTP. NRG1 was no longer able to suppress LTP when ErbB4 was ablated specifically in PV-positive neurons, suggesting that ErbB4 in other neurons may not be as important (Fig. 4B). This notion is supported by studies of CaMKII-ErbB4−/− mice in which ErbB4 is knocked out in excitatory neurons but NRG1 remains able to suppress LTP (Fig. 4D). Consistently, using two different methods, we demonstrated that NRG1 has no effect on AMPAR- or NMDAR-mediated currents in excitatory neurons in adult hippocampal slices (Fig. 1 and Fig. S1), in agreement with reports that NRG1 has no effect on basal synaptic transmission at hippocampal CA1 synapses (5, 7, 9). Previous studies indicate that NRG1 suppresses NMDAR currents in PFC excitatory neurons (10) or stimulates the internalization of surface GluR1-containing AMPARs after chemically induced LTP (11). The latter two effects cannot be mediated by ErbB4 because it is not expressed in excitatory neurons (13) and NRG1 regulation of LTP is not altered in CaMKII-ErbB4−/− hippocampus (Fig. 4D). Whether they are mediated by other ErbB kinases awaits further study. In addition, we speculate that different NRG1 effects reported in the literature may be attributable to variation in brain regions (hippocampus vs. PFC) or sample preparations (slices vs. dissociated neurons and slice preparation). For example, we were unable to observe the “depotentiation” effect of NRG1 on LTP, regardless of how soon NRG1 was added after LTP induction. Finally, the ErbB4 gene mutation in excitatory neurons is dependent on Cre expression that is controlled by the CaMKII promoter. In the mouse line that we used, Cre expression in the CA1 pyramidal neurons occurs at the age of 3 wk in the hippocampus (28–30). Thus, our study was unable to determine whether ErbB4 in pyramidal neurons is critical for excitatory synapse formation. It could suggest that ErbB4 is not critical for the function of excitatory synapses in pyramidal neurons.
NRG1 suppression of LTP induction at the SC-CA1 synapses in the hippocampus has the following characteristics. First, NRG1 inhibits the induction by both tetanus- and theta-burst stimuli (5–9). Second, the effect is acute and occurs within 20 min of NRG1 treatment (5–9) (Fig. 3). Third, the NRG1 effect could be completely blocked by acute pharmacological inhibition of ErbB4 (8, 11) or ablation of the ErbB4 gene (8) (this study), suggesting a necessary role of ErbB4. Finally, both in vitro and in vivo evidence indicates that the effect requires ErbB4-dependent enhancement of GABA release by NRG1. At the cellular level, our findings demonstrate a critical role of ErbB4 in PV-positive interneurons in LTP. Recent evidence suggests a role of NRG1 in promoting the assembly and function of excitatory synapses on interneurons and inhibitory synapses on pyramidal neurons (14, 22, 47). However, underlying mechanisms await future studies. It is worthy pointing out that Cre expression in PV-Cre;ErbB4−/− mice occurs on postnatal day 13. The late-onset mutation may not be able to cause developmental phenotypes that require early gene deletion. For example, mIPSCs were not changed in PV-Cre;ErbB4−/− hippocampus. When ErbB4 is mutated by Dlx5/6-Cre, which begins to be expressed at embryo day 13.5, mice were impaired in the frequency of mIPSCs in pyramidal neurons (22). Future studies would be benefited by using mutant mice whose levels or activity of NRG1 and ErbB4 can be controlled in space and time.
Schizophrenia is a debilitating mental disorder that affects 1% of the general population (31, 32). NRG1 and ErbB4 are susceptibility genes of schizophrenia. Some patients show abnormal levels of expression or activity of NRG1 and ErbB4 isoforms in various brain regions, including the hippocampus (33–37). Schizophrenic patients are impaired in cognitive function, including memory (38). Our earlier work has shown that PV-ErbB4−/− mice were hyperactive and impaired in working memory (15). In light of the role of NRG1/ErbB4 in regulating hippocampal LTP, we measured contextual fear conditioning, a hippocampus-dependent task (39, 40). Our results suggest that the mutant mice have problems in associative learning and memory when ErbB4 is ablated in PV-positive neurons (Fig. 5). PV-positive interneurons are important in modulating cognitive processes, and disturbance in GABAergic neurotransmission could be a pathogenic mechanism of schizophrenia (41, 42). Moreover, hypofunction of the glutamatergic pathway is thought to be a mechanism of schizophrenic pathology (43). It is conceivable that the findings of this paper could shed light on the pathogenic mechanisms of schizophrenia, particularly its cognitive deficits.
Materials and Methods
ErbB4 conditional KO mice were generated by a loxP/Cre strategy. LoxP-flanked ErbB4, PV-Cre, and CaMKII-Cre mice were described previously (28, 44–46). PV-Cre mice were crossed with LoxP-flanked ErbB4 mice to generate PV-Cre;ErbB4−/− (PV-Cre;ErbB4loxP/loxP) mice, with PV-Cre;ErbB4+/+ mice as a control. CaMKII-Cre mice were crossed with loxP-flanked ErbB4 mice to generate CaMKII-Cre;ErbB4−/− (CaMKII-Cre;ErbB4loxP/loxP) mice, with CaMKII-Cre−/−;ErbB4loxP/loxP mice as a control. All mice had ad libitum access to water and food and were housed under a 12-h light/dark cycle. All experimental procedures in this study were performed according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Hippocampal slice preparation and electrophysiological recording were performed as described in SI Materials and Methods. Immunofluorescence staining and Western blot analysis were performed as described (15). Behavior analysis was carried out with 8- to 12-wk-old mice by investigators unaware of their genotype. Detailed procedures are described in SI Materials and Methods. Data were analyzed by paired or unpaired t tests and one-way ANOVA, followed by Dunnett's tests, using SPSS software (SPSS, Inc.). Unless otherwise indicated, data were expressed as the mean ± SEM, the number of experiments is indicated by “n,” and statistical significance was considered when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Silvia Arber (University of Basel, Basel, Switzerland) for PV-Cre mice, Dr. Joe Tsien (Medical College of Georgia, Augusta, GA) for CaMKII-Cre mice, and Dr. Mark Sliwkowski (Genentech, South San Francisco, CA) for NRG1. This work was supported, in part, by the National Natural Science Foundation of China (Grants U0632007 and 81030022 to T.-M.G. and Grant 30928007 to L.M.), the National Basic Research Program of China (Grant 2006CB504100), the Program for Changjiang Scholars and Innovative Research Team (Grant IRT0731), the Key Project of Guangdong Province (Grant 9351051501000003; 06Z007 to T.-M.G.); and the National Institutes of Health (L.M. and W.-C.X.). L.M. is a Distinguished Investigator of the National Alliance for Research of Schizophrenia and Depression (NARSAD).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010669107/-/DCSupplemental.
References
- 1.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law AJ, et al. Neuregulin 1 (NRG1) transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPS associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JZ, et al. Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [DOI] [PubMed] [Google Scholar]
- 5.Huang YZ, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, et al. Ligand-dependent recruitment of the ErbB4 signaling complex into neuronal lipid rafts. J Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjarnadottir M, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: Differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. NeuroReport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar SS, Mott DD. Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Res. 2008;1208:67–73. doi: 10.1016/j.brainres.2008.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Gu ZL, Jiang Q, Fu AKY, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signaling in prefrontal cortex. J Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vullhorst D, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Wen L, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci USA. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron-principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wigström H, Gustafsson B. Facilitated induction of hippocampal long-lasting potentiation during blockade of inhibition. Nature. 1983;301:603–604. doi: 10.1038/301603a0. [DOI] [PubMed] [Google Scholar]
- 18.Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: Role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akaike N, Hattori K, Oomura Y, Carpenter DO. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl-conductance by different mechanisms. Experientia. 1985;41:70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- 20.Yau HJ, Wang HF, Lai CR, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: Preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 21.Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2010;20:724–744. doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–1380. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 23.Finlay BL, Darlington RB. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- 24.del Río JA, de Lecea L, Ferrer I, Soriano E. The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res Dev Brain Res. 1994;81:247–259. doi: 10.1016/0165-3806(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 25.Hof PR, et al. Cellular distribution of the calcium-binding proteins parvalbumin, calbindin, and calretinin in the neocortex of mammals: Phylogenetic and developmental patterns. J Chem Neuroanat. 1999;16:77–116. doi: 10.1016/s0891-0618(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen YJJ, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros CS, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci USA. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsien JZ, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 30.Zakharenko SS, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 5. [DOI] [PubMed] [Google Scholar]
- 32.Frankland PW, Sakaguchi M, Arruda-Carvalho M. Starting at the endophenotype: A role for alpha-CaMKII in schizophrenia? Mol Brain. 2008;1:5. doi: 10.1186/1756-6606-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 34.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: Association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 35.Hahn CG, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 36.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chong VZ, et al. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100:270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis DA, Sweet RA. Schizophrenia from a neural circuitry perspective: Advancing toward rational pharmacological therapies. J Clin Invest. 2009;119:706–716. doi: 10.1172/JCI37335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 40.Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- 41.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 42.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 43.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 44.García-Rivello H, et al. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 45.Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- 46.Hippenmeyer S, et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ting A, et al. Neuregulin1 promotes excitatory synapse development specifically in GABAergic interneurons. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.2538-10.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.