Abstract
The switch between black and yellow pigment is mediated by the interaction between Melanocortin receptor 1 (Mc1r) and its antagonist Agouti, but the genetic and developmental mechanisms that modify this interaction to obtain different coat color in distinct environments are poorly understood. Here, the role of Wnt/β-catenin signaling in the regulation of pigment-type switching was studied. Loss and gain of function of β-catenin in the dermal papilla (DP) of the hair follicle results in yellow and black animals, respectively. β-Catenin activity in the DP suppresses Agouti expression and activates Corin, a negative regulator of Agouti activity. In addition, β-catenin activity in the DP regulates melanocyte activity by a mechanism that is independent of both Agouti and Corin. The coordinate and inverse regulation of Agouti and Corin renders pelage pigmentation sensitive to changes in β-catenin activity in the DP that do not alter pelage structure. As a result, the signals that specify two biologically distinct quantitative traits are partially uncoupled despite their common regulation by the β-catenin pathway in the same cells.
Keywords: pheomelanin, eumelanin
Coat-color variation and adaptation is a model system for studying the genetic basis of phenotypic diversity and evolutionary change, in part because of the knowledge of genes involved in pigmentation and their developmental interactions, and in part because strong selective pressure drives dramatic and quantifiable variation in closely related populations adapting to different environments (1). In several examples studied, this variation is driven by modulation of a receptor-ligand system that regulates pigment-type switching (2–7). Activity of Mc1r promotes the production of black pigment (eumelanin), whereas inhibition of Mc1r activity shifts the balance toward the production of yellow pigment (pheomelanin) (8). In the absence of both agonists and antagonists, basal activity of Mc1r is sufficient for signaling that supports black pigment production in mice (9, 10). Mc1r activity is augmented by agonists such as α-MSH (11–13), whereas production of yellow pigment requires the antagonistic binding of Agouti to Mc1r (14). The effect of Agouti on pigment type switching depends on two additional components, Attractin and Mahagonin, that are epistatically downstream of Agouti and upstream of Mc1R, and together with it comprise the Agouti signaling pathway (15–19).
In mouse pelage, pigment production and deposition are restricted to the hair follicle and hair shaft, respectively. During the active growth phase (anagen) of the mature hair follicle, pigment is synthesized by melanocytes resident in the hair bulb at the base of the follicle and adjacent to the dermal papilla (DP), a specialized mesenchymal component of the hair follicle that plays important roles in controlling follicle morphogenesis, stem cell activity, hair shaft formation, and pigmentation (20–22). Keratinocytes in the hair bulb that give rise to the inner layers of the hair shaft take up pigment from nearby melanocytes as part of their differentiation program, leading to the formation of pigmented hair.
Mc1r receptor is specifically expressed on the surface of melanocytes throughout the growth phase of the hair cycle. In contrast, a sharp peak of Agouti expression occurs in DP cells during the early growth phase of the hair cycle (20, 21, 23). This peak generates a narrow window in which binding of Agouti sufficient to suppress Mc1r activity occurs while the distal segment of the hair shaft is formed. The resultant provisional switch to pheomelanin deposition generates a subapical yellow band in an otherwise black hair. Despite the predominance of black pigment, the presence of lighter pigment in the hair tip creates the overall appearance of a mottled brown hair coat that provides adaptive coloration in the natural environment (1). Modest variations in the length of this apical pheomelanin band can dramatically alter coat appearance and represent one mechanism by which adaptive coloration changes occur (2, 7, 21).
The interaction between Mc1r and Agouti is modified by other genes. Pomc encodes the precursor of α-MSH, which binds to Mc1r and both directly augments its activity and competitively inhibits Agouti binding (11, 14). β-Defensin also binds to Mc1r and antagonizes Agouti activity by competitively inhibiting Agouti binding to Mc1r, but the direct interaction between β-defensin and Mc1r does not by itself change Mc1r signaling (24, 25). Corin encodes a transmembrane serine protease that is expressed specifically in the DP and modifies Agouti signaling by narrowing the period of effective Agouti activity downstream of Agouti expression (21). In the absence of Corin, Agouti activity is prolonged and the yellow band is extended leading to lighter coat color.
The DP-specific expression of Agouti and Corin illustrates the important role the DP plays in controlling melanocyte behavior and pigmentation. In contrast with the sustained gene expression changes in the DP during the growth phase, the transient peak of Agouti expression reveals an additional level of transcriptional regulation within that phase that contributes to the regulation of pigment type switching. Several signaling pathways such as BMP, TGF, Notch, SHH, and Wnt/β-catenin are known to operate in the hair bulb and may modulate the activity of the DP to regulate melanocyte behavior (26). Here, the role of Wnt/β-catenin activity in the DP in the regulation of signals that direct pigment type switching in melanocytes was studied.
Results
Ablation of β-Catenin in the DP Results in Yellow Coat Color.
We have reported that when the β-catenin gene was specifically deleted in the DP during the midanagen phase by using a DP-specific cre line (Cor-cre), dramatic reductions in hair growth are observed (22). In these experiments, performed in a functional absence of Agouti (a/a), subtle effects on hair color (Fig. S1) are partially masked by defects in hair coat structure and cycling. However, when the same experiments are performed in the presence of Agouti (A/a), the mottled brown coat is converted to a yellow color (Fig. 1). In this Cor-cre line, cre recombinase activity is first detected at postnatal day (P)3 (22). Therefore, hair follicle development, including the recruitment of melanocytes to the developing follicle, occurs in the presence of an intact β-catenin gene in mice of the genotype Cor-cre/+;Ctnnb1Del/Flox. Deletion of the floxed β-catenin allele occurs during the early to midanagen phase (P3–P8) of the hair cycle (22) as Agouti expression declines from its peak at P3 to basal levels.
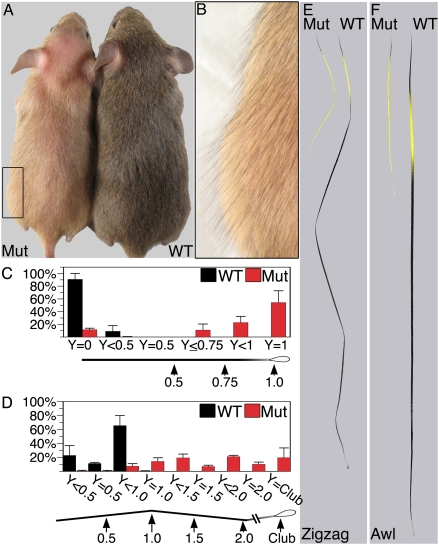
Fig. 1.
β-catenin ablation in the DP results in yellow coat color. (A and B) Three-week-old wild-type (WT) and mutant (Mut) mice are shown after the first hair cycle. In B, higher magnification of the frame in A is shown to reveal the yellow undercoat and black guard hairs. (C) Distribution of awl hairs according to the basal extension of the pheomelanin band (mean ± SD). Y indicates the position of the proximal boarder of the pheomelanin band along the distal-proximal axis of the hair. Y = 0 denotes completely black hairs, and Y = 1 represents pheomelanin extension all of the way toward the base of the hair. Note that ≈90% of awls in wild type are completely black. (D) Distribution of zigzag hairs according to the basal extension of the pheomelanin band (mean ± SD). Y is defined as in C with 1 unit representing 1 segment. (E) Examples of wild-type and mutant zigzag hairs with Y < 1 and Y = club, respectively. (F) Examples of wild-type and mutant awls with Y < 0.5 and Y = 1, respectively.
As in wild-type mice, the longer guard hairs are black in the mutant (Fig. 1 A and B), but the undercoat is dramatically lightened. The undercoat is composed of three hair types. Within the awl population, 90% of the hairs are completely black in wild-type mice (Fig. 1C; Y = 0). In contrast, only 10% in the mutant are black and the majority exhibit a yellow band that extends to the base of the hair shaft (Fig. 1 C and F; Y = 1). Their apical tips remain black as in wild-type mice, consistent with the timing of cre activity during midanagen (Fig. 1F). It is noteworthy that a small population of mutant awls have a broad yellow band but nevertheless switch back to black pigment at the base of the hair (Fig. 1C; 0.5 ≤ Y <1). Whether the result of mosaic excision in the DP of this subset of follicles, or a differential requirement for β-catenin signaling, these follicles demonstrate that changes in the strength of β-catenin signaling may shift the balance toward pheomelanin production but need not result in an absolute block to eumelanin production.
All wild-type and mutant zigzag hairs start with a black tip followed by a subapical yellow band (Fig. 1E). However, the pheomelanin band is extended in mutant zigzag hairs, both in absolute terms and as a fraction of total hair length. In most mutant zigzags, the pheomelanin band extends past the first oblique bend, indicating a prolonged period of pheomelanin production (Fig. 1D). In contrast with awls, only a small proportion of mutant zigzag hairs exhibit yellow pigment deposition all of the way to the basal end (Fig. 1 D and E; Y = Club).
β-Catenin Activity in the DP Suppresses Agouti Expression.
Real-time PCR analysis of wild-type and mutant whole-skin preparations from P1–10 revealed the pattern and levels of Agouti expression are altered in the mutant (Fig. 2A). Both wild-type and mutant show a similar bell-shaped curve of Agouti levels peaking at P4, before alteration in β-catenin activity in the DP. However, the drop of Agouti expression in wild type is sharper and settles at basal levels 10-fold lower than that of the mutant. This change in expression was confirmed by in situ hybridization (Fig. 2 B and C). Agouti transcripts are readily observed at P4 in the DP of both wild-type and mutant mice (Fig. 2B). In contrast, Agouti transcripts are detected at P8 in mutant DP only when the detection reaction was prolonged (Fig. 2C, Left), whereas the basal level of Agouti expression in wild-type P8 mice was not detected under these conditions (Fig. 2C, Right). Regardless of genotype, no Agouti transcripts are detected in some follicles at P4, consistent with the presence of completely black guard and awl hairs in both wild-type and mutant mice.
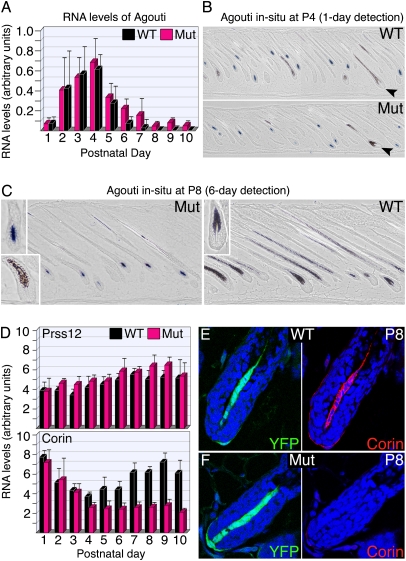
Fig. 2.
β-catenin in the DP regulates Agouti and Corin expression inversely. (A) Real-time PCR analysis of whole-skin preparations from P1–10 compares the RNA levels of Agouti between wild type (WT) and mutant (Mut) (mean ± SD). In both genotypes, Agouti expression declines dramatically after the peak to stable levels, but these levels are 10-fold higher in the mutant. (B) In situ hybridization readily detects Agouti transcripts (blue) in the DP of wild-type and mutant P4 skins. Saturated signals are obtained after 1 d of detection. In both genotypes, follicles with black pigment and no detectable Agouti transcript can be identified (arrowheads). (C) In situ hybridization for Agouti in wild-type and mutant P8 skins. After 6 d of detection, Agouti transcripts are observed only in the mutant. Insets in the upper left corners show higher magnification of hair bulbs to illustrate the presence and absence of Agouti transcript in the DP of mutant and wild type, respectively. Rare follicles with black pigment deposition and lack of Agouti expression are observed in the mutant (Lower Left Inset). (D) Real-time PCR analysis monitors the RNA levels of Corin and Prss12 from P1–10 in wild-type and mutant mice (mean ± SD). (E and F) Immunostaining of Corin in P8 wild-type (E) and mutant (F) mice. The same follicle is shown in the left and right images with YFP (green) marking the DP at Left and Corin staining (red) at Right. Blue labels nuclei.
β-Catenin Activity in the DP Activates Corin Expression.
Pheomelanin production in the presence of the low levels of Agouti expression observed in the mutant at later stages is unexpected. Corin normally inhibits Agouti activity when Agouti transcript levels are low (21). Real-time PCR analysis of RNA prepared from whole skin from P1–10 was performed for Corin and Prss12 (Fig. 2D). Prss12 is a DP-specific gene whose expression remains unaltered in DP cells lacking β-catenin sorted from P9 mice (22) (see also Fig. S2). As expected, no change in Prss12 expression between wild type and mutant was observed from P1–10. In contrast, Corin expression in the mutant is reduced from midanagen onwards. This decrease was also confirmed by real-time PCR analysis of purified DP cells FACS-sorted from P9 mice (Fig. S2) and by immunostaining for Corin in P8 mice (Fig. 2 E and F). Thus, β-catenin signaling in the DP promotes darker coat color both by suppressing Agouti and enhancing Corin expression. Furthermore, as Agouti expression remains unaltered in Corin mutants (21), β-catenin suppression of Agouti is not mediated by Corin.
β-Defensin and Pomc also inhibit Agouti activity and changes in the expression of these genes might also underlie the efficient inhibition of Mc1r activity in the mutant. Real-time PCR of whole-skin preparations revealed both β-defensin and Pomc expression levels remain unaltered in mice lacking β-catenin in the DP throughout the early to midanagen phase (Fig. S3) and, thus, suggests these genes are not involved in the observed phenotype.
β-Catenin Activity in the DP Promotes Black Pigment Production by an Additional Mechanism That Is Independent of both Agouti and Corin.
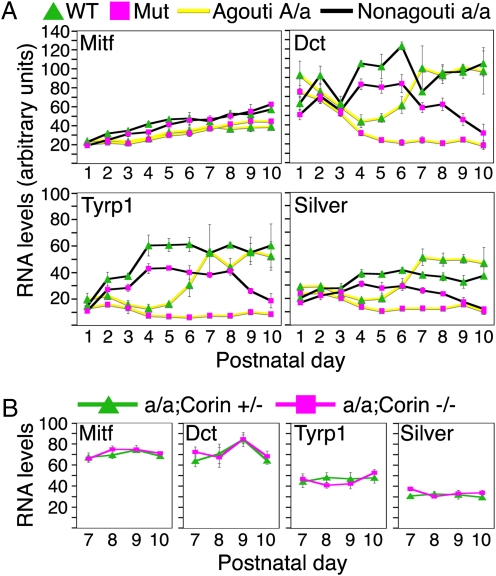
The expression of genes required in the melanocyte for eumelanin production such as Dct, Tyrp1, and Silver is regulated in part by Mc1r activity, and their levels provide a more direct assessment of changes in activity of the Agouti/Mc1r pathway. In wild-type mice with a functional allele of Agouti (A/a), the expression levels of Dct, Tyrp1, and Silver are repressed during early anagen when Agouti is high, whereas their transcript levels increase during the progression through anagen when Agouti levels decline (Fig. 3A, yellow lines), consistent with their role in eumelanogenesis and their transcriptional activation by Mc1r signaling. In contrast, expression levels of these genes remain repressed in late anagen in the mutant, in line with the changes in Agouti and Corin levels. On a nonagouti (a/a) background, the suppression in eumelanogenic gene expression observed during early anagen in A/a mice is absent (Fig. 3A, black lines), confirming that expression of these genes is inhibited during this period by Agouti activity. However, deletion of β-catenin in the DP during midanagen results in significant reduction in the RNA levels of these genes in the absence of Agouti (Fig. 3A and Fig. S4).
Fig. 3.
β-catenin activity in the DP positively regulates a novel pathway that promotes eumelanogenesis by an Agouti- and Corin-independent mechanism. (A) Real-time PCR analysis compares eumelanogenic gene expression between mice lacking β-catenin in the DP (Mut) to littermate controls (WT) on an Agouti (A/a; yellow lines) or nonagouti (a/a; black lines) background (mean ± SEM). For statistical analysis, see Fig. S4. (B) Eumelanogenic gene expression in nonagouti mice homozygous or heterozygous for a mutant allele of Corin between P7–10, a period when robust changes in eumelanogenic gene expression are observed in nonagouti mice lacking β-catenin in the DP.
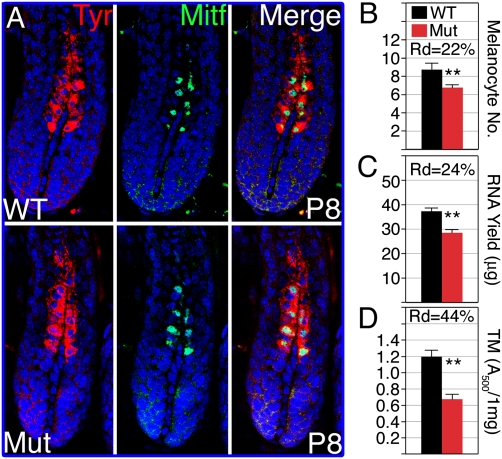
The changes in RNA levels observed by real-time PCR analysis on whole skin from nonagouti mice could reflect a change in the number of melanocytes. Double immunostaining for Mitf and Tyrosinase was used to identify melanocytes and to score their numbers at P8 (Fig. 4 A and B). A reduction of 22% in the number of melanocytes in the mutant was observed, suggesting that β-catenin activity in the DP controls melanocyte number by an Agouti-independent mechanism. However, follicular keratinocytes represent a significant fraction of cells in whole skin, and this fraction is reduced in mice lacking β-catenin in the DP as a result of decrease in proliferation of matrix keratinocytes (22). Consequently, the RNA yield from mutant skin is reduced 24% relative to that from an identical area of wild-type skin (Fig. 4C). The consequent enrichment in the fraction of RNA derived from melanocytes by RNA normalization in the real-time PCR analysis roughly compensates for the reduction in melanocyte number. This compensation likely explains the lack of change in Mitf RNA levels between wild-type and mutant skin (Fig. 3A) and suggests that alterations in the RNA levels derived from eumelanogenic genes reflect changes in gene expression per melanocyte that are independent of Agouti.
Fig. 4.
β-Catenin ablation in the DP results in reduced melanocyte activity. (A) Confocal images of P8 wild-type (Upper) and mutant (Lower) follicle immunostained for Tyrosinase (Left) and Mitf (Center). (B) Melanocyte number (mean ± SD) was scored by counting Mitf+ cells per follicle per section in P8 mice. Three hundred follicles from 3 mice per genotype were analyzed. Rd, reduction in perentage relative to wild type. Two-tailed unpaired Student's t test was used (**P < 0.0001). (C) Three dorsal skin biopsies of 12.6 mm2 along the anterior-posterior axis from 11 wild-type and 8 mutant P8 mice were obtained by using skin-biopsy punches of 2-mm radius to prepare and measure total RNA yield (mean ± SD). Two-tailed unpaired Student's t test was used (**P < 0.0001). (D) Absorbance at 500 nm (A500) was measured for total melanin extracted from 1 mg of hair (mean ± SD). Hair coat was harvested at P20 after the first hair cycle from 9 mice per genotype. Two-tailed unpaired Student's t test was used (**P < 0.0001).
Mutant mice on a nonagouti background continue to produce eumelanin, but their coat color is distinct from wild type (Fig. S1). Because structural changes and lack of regeneration may contribute to the duller appearance of the mutant hair coat, pigment content was analyzed directly in hair from the first hair cycle of nonagouti wild-type and mutant mice (27). Mutant hair samples contain only 56% of the total melanin found in an equal weight of wild-type hair (Fig. 4D). Note that the size of mutant hairs is at most half of that of wild type (Fig. 1 E and F; see also ref. 22) and, thus, 1 mg of mutant hairs corresponds to hair produced by twice the number of follicles that produce 1 mg of wild-type hairs. Therefore, this analysis assays the melanin production from a higher number of melanocytes in the mutant, even after taking into the account the reduction in melanocyte number per follicle. It clearly illustrates that a phenotypic consequence of the Agouti-independent reduction in eumelanogenic gene expression is reduced accumulation of melanin in the hair shaft.
Although the effect of Corin ablation on pigmentation is only observed in the presence of Agouti (21), the possibility of similar cryptic changes in the expression of the eumelanogenic genes in nonagouti mice and a consequent Agouti-independent mechanism of Corin action had not been evaluated. To address this question, eumelanogenic gene expression was compared between nonagouti mice homozygous or heterozygous for a mutant Corin allele (Fig. 3B). No change in gene expression was detected in mice lacking Corin, suggesting that Corin action promotes eumelanogenesis by interfering with Agouti activity and not by circumventing the pathway by some Agouti-independent mechanism such as augmenting Mc1r or Pomc activities. Furthermore, this result demonstrates that alterations in expression of Dct, Tyrp1, and Silver observed in the absence of β-catenin in the DP of a/a mice are both Agouti- and Corin-independent. These observations also reveal a heretofore unanticipated third signaling component from the DP that depends on β-catenin activity in these cells and acts on melanocytes to increase the expression levels of eumelanogenic genes.
Constitutively Activated β-Catenin in the DP Results in Black Mice.
In wild-type mice, Corin levels are relatively constant over the anagen phase, whereas Agouti transcript levels peak dramatically at P4. The loss of function experiments establish a role for β-catenin activity in suppressing Agouti and sustaining Corin expression after the normal peak of Agouti, but the role of β-catenin signaling in Agouti regulation during the peak remains unclear. The levels of Agouti transcripts in the absence of β-catenin at late anagen are dramatically lower than those during the peak, implying that an independent regulator drives peak expression. Nevertheless, β-catenin regulation may limit the height and width of this peak and, thereby, contribute to specifying pheomelanin bandwidth. This hypothesis could not be tested directly in this experimental model because deletion of the β-catenin gene during the first hair cycle occurs after the peak in Agouti expression, whereas follicle regeneration is sufficiently defective during the second hair cycle to preclude analysis (22). However, this hypothesis predicts that increased levels of activated β-catenin in the DP would suppress Agouti during its peak expression and result in darker hairs. It is also consistent with the lack of pheomelanin band in most awls of wild-type mice, because it has been suggested that β-catenin signaling is higher in the DP of awls than that of zigzag hairs (28).
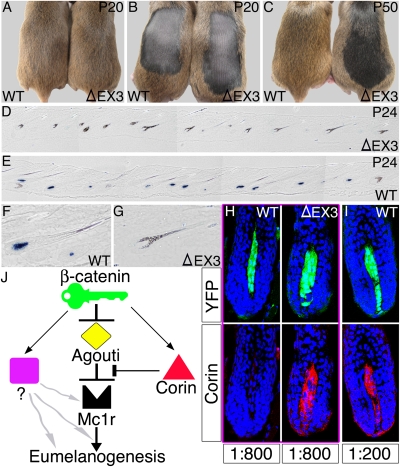
To explore the effect of higher levels of activated β-catenin on Agouti expression, the Cor-cre line was used in conjunction with a conditional allele of β-catenin in which exon3 is flanked by loxP sites (Ctnnb1Flox3) (29). Exon3 encodes a domain that marks β-catenin for targeted degradation upon phosphorylation, and deletion of this exon results in production of a constitutively activated β-catenin protein. No gross change in hair structure was observed in mice of the genotype Cor-cre/+;Ctnnb1Flox3/+. No pigmentation phenotype was observed at the end of the first hair cycle (Fig. 5A), consistent with prevalent activation of the conditional allele only after Agouti expression has already dropped below levels sufficient for pheomelanin production. However, DP cells harboring the activated allele persist through the hair cycle and follicles regenerated during the second cycle express the activated allele throughout the growth phase. The majority of hairs formed during this cycle lack a pheomelanin band and are completely black (94 ± 3.9% as opposed to 23 ± 3.8% in controls) (Fig. 5 B and C). In situ hybridization confirmed the suppression of Agouti expression in mice expressing constitutive activated β-catenin in the DP during the stages when Agouti transcripts would normally be at peak levels (Fig. 5 D–G and Fig. S5). Immunostaining for Corin reveals that although Corin levels are undetectable at telogen and high during midanagen in both wild type and mutant, Corin levels are substantially higher in the mutant during the pulse of Agouti expression in early anagen (Fig. 5 H and I). Thus, both gain and loss of function experiments illustrate the key role β-catenin plays in regulating a genetic network that controls pigment-type switching (Fig. 5J) and demonstrate that varying levels of β-catenin signaling in the DP can dictate a wide range of coat color variation.
Fig. 5.
Increased β−catenin activity in the DP results in black coat color. (A–C) A mouse expressing constitutively activated β−catenin in the DP (ΔEX3) and a littermate wild-type control (WT) are shown. In A, the hair coat after the first hair cycle (P20) is shown. In B, the dorsal fur was clipped at P20 to eliminate the hairs formed in the first cycle. In C, a newly formed hair coat after the second hair cycle (P50) is observed. (D and E) Composite figures composed of tiled micrographs from single sections show in situ hybridization for Agouti during the early anagen phase of the second hair cycle (P24) and reveal Agouti expression in wild-type mice (E) and its suppression in ΔEX3 mice (D). (F and G) Three times higher magnification of wild-type and ΔEX3 follicles are shown. Although the wild-type follicle is in a slightly later stage of the anagen phase than the ΔEX3 follicle, wild-type follicles at earlier stages express detectable levels of Agouti as well (see Fig. S5). (H and I) Immunostaining for Corin during early anagen (P24) reveals higher Corin levels in the DP of ΔEX3 mice. The same follicle is shown in Upper and Lower with YFP (green) marking the DP in Upper and Corin staining (red) in Lower. When the anti-Corin antibody was diluted 1:800 (H), Corin is reliably detected in ΔEX3 but not wild-type follicles. At lower dilutions (I, 1:200), weak staining is also observed in follicles from the same wild-type mouse. (J) Schematic representation of the genetic network that controls pigment-type switching. Grey wavy arrows represent alternative mechanisms by which the third signaling component may act to promote black pigment production (Discussion).
Discussion
This study reveals a genetic network that regulates pigment type switching. β-Catenin activity in the DP inversely controls the expression of both Corin and Agouti to coordinately regulate their levels. As Corin inhibits Agouti activity, this inverse regulation amplifies the effects of changes in β-catenin activity in the DP on coat color. This analysis also reveals the presence of an additional level of regulation to control eumelanogenic gene expression in the melanocyte that depends on β-catenin activity in the DP. However, the identity and mechanism of action of this component remain unknown.
The coordinate reduction in hair bulb size and melanocyte number in the mutant may be explained by an indirect mechanism in which an altered trophic environment of the hair bulb, whether directly influenced by the DP or indirectly influenced by keratinocytes, contributes to the regulation of melanocyte number. The more specific reduction in eumelanogenic gene expression within melanocytes may also be an indirect response or may represent a factor expressed by the DP that either acts as an agonist of Mc1r or enhances the activity of known agonists of Mc1r such as Pomc-derived α-MSH (Fig. 5J). Alternatively, this factor may act independently of the Mc1r signal transduction cascade to promote black pigment production or modify a downstream component of Mc1r signaling. Additional genetic studies will be required to distinguish between these possible mechanisms. Until this factor is identified, technical constraints prevent us from determining whether, like Corin and Agouti, the activity of this third mechanism is sensitive to levels of β-catenin activity in early anagen DP in the range that still promotes normal hair growth. If so, it would act with Corin to further amplify the response of the pigment system to small changes in β-catenin activity in the DP. If not, modification of its activity by a mechanism other than β-catenin activity in the DP would be expected to shift the set point of pigmentation and could thereby alter the range of coat color phenotypes that might be attained by changes in Agouti and Corin expression in response to changes in β-catenin signaling in the DP.
The genetic alterations that underlay coat color variation illuminate mechanisms that drive evolutionary change. Genetic analysis of coat color variation in natural environments has repeatedly identified variants of the Mc1r and Agouti genes as sources of phenotypic diversity (2–4, 6, 7). Although the central roles that both Mc1r and Agouti play in pigment switching contributes to this phenomena, the fact that both genes have no known function outside of pigmentation and are therefore largely free of other constraints on variation is also relevant. The requirement for β-catenin in a broad array of tissues and developmental events places it at the opposite extreme of this continuum. However, although this general requirement may constrain the accumulation of mutations in β-catenin itself, the vast complexity of inputs modifying the canonical Wnt signal transduction pathway in which this protein functions as a node provides a wide array of opportunities for genetic divergence less subject to constraint. The requirement of β-catenin activity in the DP for hair morphogenesis (22) sets lower limits to the continuum along an effective signaling gradient to generate a lighter but otherwise normal hair coat. In contrast, the fact that maximal levels of activated β-catenin in the DP do not grossly affect hair structure provides no upper limit on the strength of pathway activation for effective darkening of the hair coat. The complementary regulation of Corin and Agouti amplify the impact of more modest changes in Wnt signaling activity on hair pigment. These mechanisms allow changes within one range of β-catenin activity to selectively modify hair pigmentation, while changes in an overlapping range modify both hair structure and color. In this way, dermal papilla niche cells exploit the same signal transduction pathway to direct production of signals that regulate two apparently independent biological processes.
Materials and Methods
Mice, in Situ Hybridization, Immunostaining, and Melanocyte Counts.
Mice used in this study and detailed procedures are described in SI Materials and Methods. For in situ hybridization, frozen sections were hybridized with dig-labeled RNA probe corresponding to nt 126–613 of Agouti (NM_015770). For immunostaining, fixed-skin sections were incubated with rabbit polyclonal anti-corin (21) diluted 1:800 or 1:200, mouse monoclonal anti-Mitf (30) diluted 1:10, and rabbit polyclonal anti-Tyr (31) diluted 1:500. For melanocyte count, skin sections were double immunostained for Mitf and Tyr and used to score Mitf-positive cells in follicle bulbs with a clear zone of Tyr staining.
Hair Shaft Analysis.
Hairs were plucked at the end of the first cycle at P20 and mounted on slides. To collect hairs formed in the second cycle, the hair-coat was shaved at P20 and newly formed hairs were plucked at P50 after the end of the second cycle. Hair shafts were photographed as described (21). Chemical analysis of hair shaft for total melanin was performed as described (27).
Real-Time PCR.
Middorsal skins of wild-type and mutant mice from P1–P10 were collected and used to prepare RNA. Normalized RNA quantities were reverse transcribed by using random hexamer primers and SuperScript First-Strand synthesis system III (Invitrogen). For real-time PCR, primer pairs from SuperArray were used and differences between samples were quantified based on the ΔΔCt method.
Supplementary Material
Acknowledgments
We thank David E. Fisher and Vincent Hearing for providing the C5 anti-Mitf and αPEP7 anti-Tyr antibodies, respectively, and R. Czyzewski for technical assistance. This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant 1R01AR055256 (to B.A.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007326107/-/DCSupplemental.
References
- 1.Hoekstra HE. Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity. 2006;97:222–234. doi: 10.1038/sj.hdy.6800861. [DOI] [PubMed] [Google Scholar]
- 2.Nachman MW, Hoekstra HE, D'Agostino SL. The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci USA. 2003;100:5268–5273. doi: 10.1073/pnas.0431157100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP. A single amino acid mutation contributes to adaptive beach mouse color pattern. Science. 2006;313:101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- 4.Steiner CC, Weber JN, Hoekstra HE. Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biol. 2007;5:e219. doi: 10.1371/journal.pbio.0050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson TM, et al. Molecular and evolutionary history of melanism in North American gray wolves. Science. 2009;323:1339–1343. doi: 10.1126/science.1165448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kingsley EP, Manceau M, Wiley CD, Hoekstra HE. Melanism in peromyscus is caused by independent mutations in agouti. PLoS ONE. 2009;4:e6435. doi: 10.1371/journal.pone.0006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linnen CR, Kingsley EP, Jensen JD, Hoekstra HE. On the origin and spread of an adaptive allele in deer mice. Science. 2009;325:1095–1098. doi: 10.1126/science.1175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barsh G, Gunn T, He L, Schlossman S, Duke-Cohan J. Biochemical and genetic studies of pigment-type switching. Pigment Cell Res. 2000;13(Suppl 8):48–53. doi: 10.1034/j.1600-0749.13.s8.10.x. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, et al. Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology. 2005;146:1245–1253. doi: 10.1210/en.2004-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smart JL, Low MJ. Lack of proopiomelanocortin peptides results in obesity and defective adrenal function but normal melanocyte pigmentation in the murine C57BL/6 genetic background. Ann N Y Acad Sci. 2003;994:202–210. doi: 10.1111/j.1749-6632.2003.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 11.Geschwind II. Change in hair color in mice induced by injection of alpha-MSH. Endocrinology. 1966;79:1165–1167. doi: 10.1210/endo-79-6-1165. [DOI] [PubMed] [Google Scholar]
- 12.Healy E, et al. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet. 2001;10:2397–2402. doi: 10.1093/hmg/10.21.2397. [DOI] [PubMed] [Google Scholar]
- 13.Jackson IJ, Budd PS, Keighren M, McKie L. Humanized MC1R transgenic mice reveal human specific receptor function. Hum Mol Genet. 2007;16:2341–2348. doi: 10.1093/hmg/ddm191. [DOI] [PubMed] [Google Scholar]
- 14.Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998;12:316–330. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunn TM, et al. The mouse mahogany locus encodes a transmembrane form of human attractin. Nature. 1999;398:152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- 16.Phan LK, Lin F, LeDuc CA, Chung WK, Leibel RL. The mouse mahoganoid coat color mutation disrupts a novel C3HC4 RING domain protein. J Clin Invest. 2002;110:1449–1459. doi: 10.1172/JCI16131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He L, et al. Spongiform degeneration in mahoganoid mutant mice. Science. 2003;299:710–712. doi: 10.1126/science.1079694. [DOI] [PubMed] [Google Scholar]
- 18.He L, Eldridge AG, Jackson PK, Gunn TM, Barsh GS. Accessory proteins for melanocortin signaling: attractin and mahogunin. Ann N Y Acad Sci. 2003;994:288–298. doi: 10.1111/j.1749-6632.2003.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 19.He L, et al. A biochemical function for attractin in agouti-induced pigmentation and obesity. Nat Genet. 2001;27:40–47. doi: 10.1038/83741. [DOI] [PubMed] [Google Scholar]
- 20.Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121:3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- 21.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–225. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enshell-Seijffers D, Lindon C, Kashiwagi M, Morgan BA. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev Cell. 2010;18:633–642. doi: 10.1016/j.devcel.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vrieling H, Duhl DM, Millar SE, Miller KA, Barsh GS. Differences in dorsal and ventral pigmentation result from regional expression of the mouse agouti gene. Proc Natl Acad Sci USA. 1994;91:5667–5671. doi: 10.1073/pnas.91.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerns JA, et al. Linkage and segregation analysis of black and brindle coat color in domestic dogs. Genetics. 2007;176:1679–1689. doi: 10.1534/genetics.107.074237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Candille SI, et al. A -defensin mutation causes black coat color in domestic dogs. Science. 2007;318:1418–1423. doi: 10.1126/science.1147880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharov AA, et al. Bone morphogenetic protein (BMP) signaling controls hair pigmentation by means of cross-talk with the melanocortin receptor-1 pathway. Proc Natl Acad Sci USA. 2005;102:93–98. doi: 10.1073/pnas.0408455102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozeki H, Ito S, Wakamatsu K, Hirobe T. Chemical characterization of hair melanins in various coat-color mutants of mice. J Invest Dermatol. 1995;105:361–366. doi: 10.1111/1523-1747.ep12320792. [DOI] [PubMed] [Google Scholar]
- 28.Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King R, et al. Microphthalmia transcription factor. A sensitive and specific melanocyte marker for MelanomaDiagnosis. Am J Pathol. 1999;155:731–738. doi: 10.1016/S0002-9440(10)65172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiménez M, Tsukamoto K, Hearing VJ. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991;266:1147–1156. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.