The amyloid precursor protein (APP) is the precursor to the amyloid β-protein (Aβ), which is the major constituent of amyloid plaques found in brains of individuals with Alzheimer's disease (AD). Because amyloid (senile) plaques are one of the pathological hallmarks of AD, processing of APP and generation of Aβ from APP have been areas of intense study. Cloning of the APP cDNA in 1987 led to an immediate conundrum: release of Aβ from APP would involve at least two cleavages, because Aβ is not located at either N or C terminus (1). More troubling was the implication that one of the two predicted cleavages at the C terminus of Aβ would take place in the predicted transmembrane domain, a proteolytic event that did not have any biological precedence at that time. This intramembranous cleavage event was subsequently coined γ-secretase activity without knowledge of its identity.
In 1995, the presenilin-1 (PSEN1) gene was identified along with the description of a number of mutations in this gene associated with familial AD (FAD) (2). To date, more than 170 FAD-linked mutations in the PSEN1 gene have been reported worldwide and account for the majority of cases of hereditary AD (http://www.molgen.ua.ac.be/ADMutations). The first evidence that presenilins (PSs) are critically linked to γ-secretase activity was the finding that deficiency in PS1 resulted in markedly diminished production of Aβ peptides (3). Subsequently, it was shown that two aspartate residues within two predicted transmembrane regions are critical for activity, suggesting that PS was a novel aspartyl protease and may be the actual catalytic unit of γ-secretase (4). Further evidence was obtained when photoactivatable transition-state analog inhibitor probes were found to covalently bind to PS1 (5). Confusing matters, however, were the subsequent findings of obligatory partners to PS and γ-secretase activity. γ-Secretase activity requires association of PS with three other subunits—namely, nicastrin (NCT), anterior pharynx defective 1 (APH-1), and presenilin enhancer (PEN2), to form the catalytically active γ-secretase complex (6). In yeast, ectopic expression indicated that these four subunits are required and sufficient for function, and they appear to exist in a 1:1:1:1 stoichiometry (7, 8). Finally, it was recognized that endoproteolytic processing of PS, generating amino-terminal fragments (NTFs) and carboxyl-terminal fragments (CTFs), is required for activity (9). Given this complexity, establishing the precise role of each subunit of the γ-secretase complex has not been possible in the absence of an in vitro system that allows reconstitution of γ-secretase activity from purified components. This long-sought-after goal has been achieved, and the recent findings in PNAS by Ahn et al. (10) provide conclusive evidence that activated PS is catalytically competent by itself and therefore constitutes the catalytic core of the γ-secretase complex.
Technically, in vitro reconstitution of γ-secretase activity is a challenge not only because of the topology of PS, with eight to nine transmembrane domains (an issue that remains somewhat controversial), but also because the other three components have to be taken into account. If PS holoprotein alone possessed the enzymatic activity, this objective would perhaps not be as difficult. Given these considerations, Ahn et al. take a creative route by using a highly purified bacterially expressed recombinant PS1, harboring the naturally occurring FAD mutation lacking exon 9 (ΔE9), and incorporating the recombinant protein into artificial liposomes (10). The selection of this particular PS1 variant is one key to their success, because PS1ΔE9 lacks the endoproteolytic cleavage site yet remains constitutively active. The purified proteoliposomes were then tested for in vitro γ-secretase assay after detergent solubilization with an artificial truncated APP substrate. Validity of the reconstituted system was confirmed by a number of control experiments, and the results are strikingly clear: PS1ΔE9 incorporated into liposomes together with substrate produced bona fide intrinsic γ-secretase activity without the other three components of the γ-secretase complex.
Ever since the FAD-linked PS1 mutations have been identified, most if not all of the mutations that have been analyzed show two consistently perturbed phenotypes: slight to significant reduction in γ-secretase activity and an increase in the ratio of Aβ42/Aβ40 peptides (6). It has been known for some time that Aβ peptides are heterogenous. Though the majority of Aβ peptides produced is the 40-aa isoform (Aβ40), it has been hypothesized that it is the longer more fibril-prone and neurotoxic 42-aa isoform (Aβ42) that is pathogenic. In this context, virtually all PS mutations increased the ratio of the long vs. short Aβ species (Aβ42/Aβ40). A second major finding of the Ahn et al. study (10) is that the PS1ΔE9 mutation increased the Aβ42/Aβ40 ratio while slightly reducing Aβ40 levels when compared with a PS1ΔE9 variant with the C290S mutation—the latter predicted to mimic wild-type PS1 activity. Two other FAD PS1 mutations, when incorporated into the PS1ΔE9 backbone, were able to augment Aβ42/Aβ40 ratios. Last, certain APP mutations are known to alter Aβ profiles, and in this in vitro system, PS1ΔE9 recombinant protein was also able to elevate Aβ42/Aβ40 ratios when coincorporated into proteoliposomes with APP substrate carrying these mutations. Together, these findings show that the PS1 mutations themselves, without other cellular processes, were responsible for the perturbations in activity reflected in the Aβ42/Aβ40 ratios.
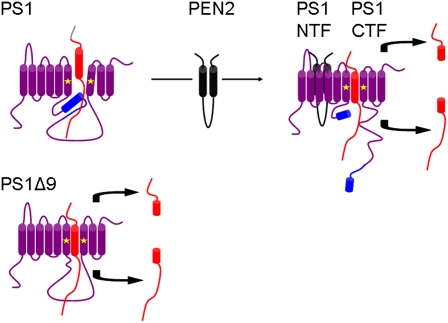
The third important finding from Ahn et al. (10) concerns wild-type PS1. The authors state that they failed to detect γ-secretase activity when wild-type full-length recombinant PS1 was tested in their in vitro system. At face value, this finding seems to confirm the notion that PS1 holoprotein is inactive. Because PEN2 has been suggested to play an essential role in PS endoproteolysis (11), Ahn et al. (10) added purified recombinant PEN2 into proteoliposomes containing PS1 holoprotein and APP substrate. In this setting, not only were PS1 NTF and CTF detected, indicating endoproteolysis, but bona fide γ-secretase activity was also observed. Therefore, these findings suggest that PS1 is normally an inactive zymogen that requires processing and activation (Fig. 1), a step that requires PEN2. Unresolved in this experiment is what it means for PS1 to be activated (is it simply endoproteolysis, or something else?) or whether activation by PEN2 renders PS1 autocatalytic, allowing it to cleave itself as well as other γ-secretase substrates. Alternatively, it remains possible that PEN2 possesses an as-yet unrecognized enzymatic activity, representing the so-called presenilinase.
Fig. 1.
Model of PS1 activation. Uncleaved PS1 holoprotein may obstruct positioning of the substrate with respect to the catalytic aspartate residues, but removal of exon 9 in the PS1ΔE9 mutation allows access to the catalytic site. Interaction of PEN2 with PS1 holoprotein results in endoproteolytic cleavage of PS1 to generate PS1 NTF and PS1 CTF. Once PS1 is cleaved, the γ-secretase substrate can reach the catalytic aspartate residues where an intracellular domain and an extracellular/lumen peptide (Aβ) are released after proteolysis. PS1 (purple), exon 9 of PS1 (blue), catalytic aspartates (asterisks), PEN-2 (black), substrate (red).
The identification of the γ-secretase complex has been immensely important for AD research, but has also led to the recognition that the intramembrane proteolysis is crucial to other cellular processes. Intramembrane cleaving proteases (i-CLIPs) are now recognized as proteases that cleave a myriad of membrane protein substrates (12). Signal peptide peptidase (SPP) is an i-CLIP and bacterial SPP known to function without obligatory partners (12). Thus, the findings by Ahn et al. (10) bring the functional homology of SPP and PS a step closer together. At another cellular level, this cleavage within the transmembrane domain, an event also termed regulated intramembrane proteolysis (RIP),releases the cytosolic domain of these type I membrane proteins
PS is catalytically competent by itself and therefore constitutes the catalytic core of the γ-secretase complex.
from their membrane tether. In doing so, it allows the now-released cytosolic domains to translocate into the nucleus to transmit nuclear signals, such as in the case for the Notch receptor, among many proposed functions. Interestingly, more than 50 membrane proteins have been noted to be substrates for γ-secretase cleavage, and they all share an antecedent proteolytic processing in the ectodomain, leaving short domains but, surprisingly, little sequence specificity (12). As such, RIP allows the released peptide domains to perform specific cellular functions, but it has also been proposed that this regulated proteolytic process is simply part of the degradation pathway of membrane proteins (13).
The reconstitution system described by Ahn et al. (10) displays many of the essential “signatures” of γ-secretase activity. Although their findings convincingly show the isolated catalytic activity of PS, a number of critical questions remain unanswered. Nevertheless, having an in vitro system at hand should aid in resolving these pressing issues: (i) How does PS function at the atomic level and how is the catalytic core protected from the hydrophobic environment of the membrane? (ii) What are the precise roles of the other three associated subunits (NCT, APH-1, and PEN2) and how do they facilitate the assembly of PS into a stable and fully active enzyme complex? (iii) What are the conformational changes necessary to activate PS from its zymogen form? (iv) How do the disease-associated mutations cause the changes in γ-secretase cleavages to alter Aβ generation? (v) Is the processive model of Aβ generation from APP correct? This latter model, derived largely from a series of elegant studies by Ihara and colleagues, proposes that an initial ε-cleavage of APP takes place near the cytosolic membrane interface, followed by sequential cuts occurring every three amino acids along the α-helical face of APP within the plasma membrane, resulting in progressively shorter Aβ peptides, finally ending in their release from the cell (14). In summary, much still remains to be done in unraveling the mysteries of this enigmatic series of proteolytic events. Nevertheless, the PNAS study by Ahn et al. (10) opens up the possibility of exploiting molecular dynamics simulations of this simpler enzymatic process using 1H NMR in combination with high-resolution X-ray crystallographic analyses. It is not the end, but rather the end of the beginning, in the long journey to a comprehensive understanding of γ-secretase activity and the spectrum of physiological and pathophysiological roles it serves.
Footnotes
The authors declare no conflict of interest.
See companion article on page 21435.
References
- 1.Kang J, et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 2.Sherrington R, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 3.De Strooper B, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe MS, et al. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 5.Li YM, et al. Photoactivated gamma-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 6.De Strooper B. Proteases and proteolysis in Alzheimer disease: A multifactorial view on the disease process. Physiol Rev. 2010;90:465–494. doi: 10.1152/physrev.00023.2009. [DOI] [PubMed] [Google Scholar]
- 7.Sato T, et al. Active gamma-secretase complexes contain only one of each component. J Biol Chem. 2007;282:33985–33993. doi: 10.1074/jbc.M705248200. [DOI] [PubMed] [Google Scholar]
- 8.Edbauer D, et al. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 9.Thinakaran G, et al. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 10.Ahn K, et al. Activation and intrinsic γ-secretase activity of presenilin 1. Proc Natl Acad Sci USA. 2010;107:21435–21440. doi: 10.1073/pnas.1013246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo WJ, et al. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J Biol Chem. 2003;278:7850–7854. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- 12.Wakabayashi T, De Strooper B. Presenilins: Members of the gamma-secretase quartets, but part-time soloists too. Physiology (Bethesda) 2008;23:194–204. doi: 10.1152/physiol.00009.2008. [DOI] [PubMed] [Google Scholar]
- 13.Kopan R, Ilagan MX. Gamma-secretase: Proteasome of the membrane? Nat Rev Mol Cell Biol. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- 14.Takami M, et al. gamma-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci. 2009;29:13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]