Abstract
Cellular metabolism alters patterns of gene expression through a variety of mechanisms, including alterations in histone modifications and transcription factor activity. Nicotinamide adenine dinucleotide (NAD)–dependent proteins such as poly(ADP ribose) polymerases (PARPs) and sirtuin deacetylases play important roles in this regulation, thus NAD provides a crucial link between metabolism and these cellular signaling processes. Here, we found that lowering NAD levels in mouse primary cortical neurons led to decreased activity-dependent BDNF expression. The altered BDNF transcription occurred independently of Sirt or Parp activities; instead, low NAD levels promoted increased DNA methylation of the activity-dependent BDNF promoter. Increased methylation at this promoter triggered the dissociation of the insulator protein CTCF as well as the accompanying cohesin from the BDNF locus. The loss of these proteins resulted in histone acetylation and methylation changes at this locus consistent with chromatin compaction and gene silencing. Because BDNF is critical for neuronal function, these results suggest that age- or nutrition-associated declines in NAD levels as well as deficits in cohesin function associated with disease modulate BDNF expression and could contribute to cognitive impairment.
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophin family of growth factors. It plays important roles in regulating neurogenesis, synaptic plasticity, and neuronal survival—functions that are vital to learning, memory, and cognition (1, 2). In the CNS, BDNF transcription is regulated by neuronal activity with high expression in the hippocampus and cortex (3, 4). The BDNF gene contains multiple promoters that generate transcripts containing different noncoding exons spliced to a common single coding exon (4). Of the multiple BDNF mRNAs, transcription initiated from BDNF promoter IV is dramatically activated in neurons treated with KCl, which causes membrane depolarization and subsequent influx of calcium (5, 6). In addition to calcium signaling pathways, BDNF transcription is regulated epigenetically by DNA methylation and subsequent chromatin remodeling. Specifically, DNA methylation at promoter IV is involved in silencing BDNF gene expression via transcriptional repressor methyl-CpG-binding protein (MeCP2) (5–7). Abnormal BDNF transcription resulting from aberrant occupancy of methylated DNA binding sites in its promoter has been implicated in the etiology of Rett syndrome (8), and other abnormalities in BDNF are associated with neurodegenerative disorders, including Huntington, Alzheimer, and Parkinson diseases (2), as well as psychiatric disorders such as depression and schizophrenia (1).
Age-associated declines in BDNF levels are thought to contribute to impaired cognitive performance in older individuals (9), as are age-related changes in metabolism (10). Nicotinamide adenine dinucleotide (NAD) is a key molecule that links the metabolic state of the cell with gene expression and cell functions. These links are accomplished through its role as an electron carrier in redox reactions and as a substrate for enzymes such as the sirtuin deacetylases and poly(ADP ribose) polymerases (PARPs) (11). Because of this central role and the realization that NAD levels decline with age (12), it has been speculated that NAD contributes to aging-related deficits in multiple organ systems, including the brain (13). Moreover, individuals deprived of adequate amounts of the NAD precursor (vitamin B3) develop dementia as part of the symptomatology of pellagra (14). However, the molecular mechanisms linking NAD with cognitive function are unknown.
Here, we report that lowering intracellular NAD levels in cortical neurons inhibits activity-dependent BDNF transcription. Cortical neurons with low NAD levels had DNA hypermethylation at BDNF promoter IV, which, in turn, promoted the release of the DNA methylation–sensitive nuclear factor CCCTC-binding factor (CTCF). The binding of CTCF and accompanying cohesin components to the BDNF locus is important for sustaining a chromatin structure necessary for BDNF transcription, because loss of CTCF or cohesin is associated with an inactive chromatin structure and decreased BDNF transcription. These data shed light on the mechanistic relationship between NAD biosynthesis and BDNF gene regulation and suggest a link between BDNF and the development of mental retardation in patients with cohesinopathies.
Results and Discussion
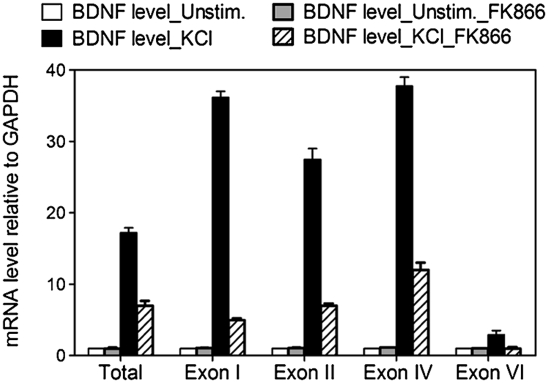
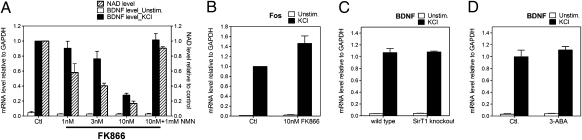
We hypothesized that alterations in NAD levels may modulate BDNF expression and thereby play a role in cognition. To investigate a functional link between intracellular NAD levels and BDNF transcription, we analyzed BDNF mRNA levels in mouse primary cortical neurons after treatment with the well-characterized nicotinamide phosphoribosyltransferase (Nampt) inhibitor FK866. FK866 decreases intracellular NAD levels via inhibition of Nampt, the rate-limiting step in NAD biosynthesis (15). Total BDNF transcripts and representative promoter-specific transcripts (i.e., promoters I, II, IV, and VI) (4) were assessed in neurons treated with KCl to induce membrane depolarization, calcium influx, and subsequent activation of BDNF expression (16). We observed reduced levels of all BDNF transcripts examined in neurons treated with FK866 (Fig. 1). To investigate the molecular mechanism of how reduced intracellular NAD levels inhibit BDNF transcription, we focused our analysis on transcripts initiated from the most well-characterized BDNF promoter, promoter IV. As expected, cortical neurons treated with FK866 showed a dose-dependent decrease in NAD levels (Fig. 2A). Although KCl treatment robustly increased BDNF transcripts initiated from promoter IV in neurons with normal NAD levels, a FK866-mediated decrease in NAD levels was mirrored by a reduced BDNF transcriptional response to membrane depolarization (Fig. 2A). The addition of nicotinamide mononucleotide, the product of the Nampt-catalyzed reaction, restored both intracellular NAD concentrations and BDNF transcription in FK866-treated cells (Fig. 2A), indicating the specificity of the FK866-induced changes. Moreover, the overall integrity or transcriptional capabilities of FK866-treated neurons were not compromised over the time frame of our experiments (Fig. S1 A and B). Furthermore, the FK866-mediated declines in NAD levels in these neurons did not invoke a general compromise in calcium-dependent signaling pathways or transcription because the membrane depolarization–dependent transcription of the immediate early gene, c-fos, was not inhibited (Fig. 2B).
Fig. 1.
NAD levels regulate activity-dependent BDNF transcription in cortical neurons. BDNF transcripts initiated from promoter I, II, IV, and VI, as well as the level of total BDNF transcripts, were measured by using qRT-PCR and normalized to GAPDH mRNA levels. Mouse cortical neurons were stimulated with KCl for 4 h to induce membrane depolarization and BDNF transcription. Neurons treated with Nampt inhibitor FK866 (10 nM) had decreased BDNF induction after KCl treatment.
Fig. 2.
Activity-dependent BDNF transcription initiated from promoter IV in cortical neurons is inhibited by FK866. (A) NAD levels and transcription from BDNF promoter IV in mouse cortical neurons treated with indicated concentration of the Nampt inhibitor FK866. Nicotinamide mononucleotide (NMN), the product of the Nampt-catalyzed reaction, was added where indicated. Total mRNA was isolated from naïve neurons or those stimulated with KCl for 4 h. BDNF transcripts initiated from promoter IV were measured with qRT-PCR and normalized to GAPDH mRNA levels. Neurons with low NAD levels had decreased BDNF induction after KCl treatment. (B) c-fos mRNA levels in neurons incubated with or without FK866 were determined as above and were not affected by FK866 treatment. (C) E15 primary cortical neurons from wild-type and Sirt1-deficient mice were cultured for 5DIV and stimulated with 55 mM KCl for 4 h. Sirt1-deficient neurons showed equivalent BDNF induction compared with wild-type controls. Quantification was performed from duplicate experiments from at least three mice for each genotype. (D) Mouse cortical neurons were incubated for 24 h with PARP inhibitor 3-aminobenzamide (3-ABA; 7 mM) before depolarization by KCl addition. Inhibition of PARPs had no impact on the magnitude of BDNF induction. Quantification was performed from three independent experiments.
The activity of two well-characterized NAD-dependent chromatin-modifying proteins, Sirt1 and Parp1, is decreased in cells with low NAD levels (11, 17). Because these proteins promote gene silencing, a decrease in their activity would be expected to lead to gene activation. Nevertheless, their central role in NAD-regulated gene expression led us to directly examine whether Sirt1 or Parps plays a role in this phenomenon. We cultured cortical neurons from Sirt1-deficient mice and found that BDNF induction was unaltered in response to membrane depolarization compared with wild-type controls (Fig. 2C). We next explored the involvement of PARP-1, a PARP that participates in transcriptional control by modulating chromatin structure (17), by treating cortical neurons for 24 h with the PARP inhibitor 3-aminobenzamide (7 mM). No changes in BDNF induction were observed in neurons treated with PARP inhibitor (Fig. 2D). These data indicate that PARP-1 and Sirt1 are unlikely to be involved in BDNF gene transcription and that lowered NAD concentrations silence BDNF gene transcription in a Sirt1- and PARP-1-independent manner.
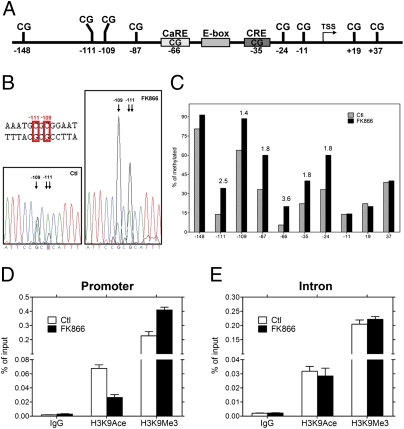
DNA methylation influences the transcriptional activity of BDNF promoter IV (5, 6). We therefore tested whether NAD levels influenced DNA methylation in this region. Bisulfite sequencing of DNA from untreated and FK866-treated neurons was used to assess the methylation status of BDNF promoter IV (Fig. 3A). We sequenced PCR fragments of the bisulfite-treated DNA directly and found a significant difference in the peak height, indicating increased methyl-cytosine in the FK866-treated samples (Fig. 3B). This result was confirmed and quantified by cloning these PCR products and sequencing individual clones. We found that 7 of 10 CpG sites (between base pairs −148 and +37) in BDNF promoter IV exhibited higher levels of methylation after FK866 treatment (Fig. 3 A–C). Consistent with previously published reports (5, 6), we found the CpG at position −148 was robustly methylated in both conditions, whereas methylation at six other CpG sites within the promoter region increased (1.4- to 3.6-fold) in response to NAD depletion (Fig. 3 B and C). These data indicate that alterations in NAD levels affect BDNF expression via changes in DNA methylation of the promoter.
Fig. 3.
FK866-treated cortical neurons show increased levels of DNA methylation at BDNF promoter IV. (A) A schematic depicting the regulatory elements within the mouse BDNF exon IV promoter (promoter IV) (6). (B) Direct sequencing of PCR products amplified with bisulfate-treated DNA as template. DNA samples were derived from 5DIV cortical neurons treated with or without FK866. DNA sequencing traces are shown for the −109 and −111 sites in BDNF promoter IV. Note the traces depict the antisense (reverse) strand; therefore, G is indicative of a methylated cytosine, and A is indicative of a nonmethylated cytosine. An increase in DNA methylation at −109 and −111 sites was observed in samples treated with FK866. (C) Mapping of the methylation status of the 10 CpG sites near the transcription start site (TSS) by bisulfate sequencing of genomic DNA. The number above the black bar represents the fold changes in methylated cytosine relative to control at the indicated position. (D and E) Neurons treated with or without FK866 (10 nM) for 24 h were subjected to ChIP with antibodies specific to trimethyl-H3 K9 (H3K9Me3), acetyl-H3 K9 (H3K9Ace), or control IgG followed by amplification with PCR primers specific for BDNF promoter IV and a downstream intronic region. Data show the representative result from four independent experiments.
The transcription of BDNF in response to membrane depolarization is accompanied by DNA methylation-related chromatin remodeling events at promoter IV (5, 6). Because DNA methylation in BDNF promoter IV was increased in neurons with low NAD levels, we tested whether this finding was accompanied by changes in the chromatin structure of the region. Chromatin immunoprecipitation (ChIP) analysis of BDNF promoter IV with histone 3 lysine 9 (H3K9) antibodies showed decreased acetylation of H3K9 (H3K9Ace; active chromatin marker) and concurrent increased methylation at the same residue (H3K9Me3; repressed chromatin marker) after FK866 treatment (Fig. 3D). These results indicate that the chromatin around BDNF promoter IV was switched to an inactive state in neurons with low NAD levels. These changes in chromatin structure were specific to promoter IV because similar changes in chromatin “marks” were not observed at a downstream intronic CpG island (Fig. 3E). Together, these data illustrate that lowering NAD levels in cortical neurons increases DNA methylation and chromatin compaction at BDNF promoter IV, consistent with its inhibitory effect on activity-dependent BDNF transcription.
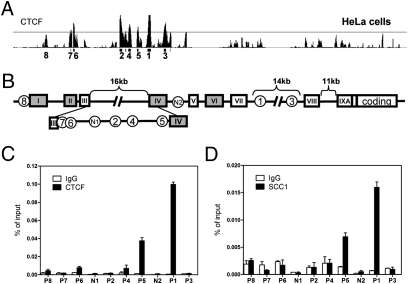
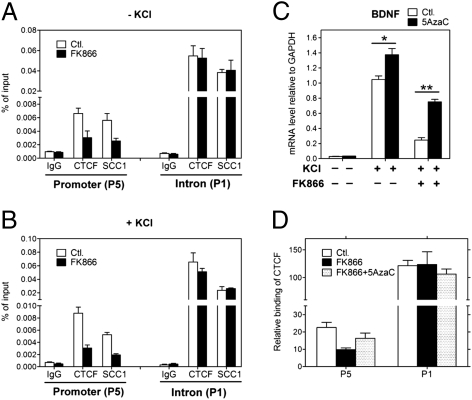
The extent of DNA methylation has been consistently observed to be inversely correlated with the level of gene expression (18). To remain active, the BDNF promoter IV must remain relatively hypomethylated in neurons with normal NAD levels. How this is accomplished at the BDNF locus is unknown, but in other systems, CTCF fulfills this function via its ability to act as a genome-wide “epigenetic shield” (19). CTCF is a highly conserved multifunctional protein that plays critical roles in transcriptional regulation, including promoter repression, activation, enhancer-blocking activity, chromatin insulation, and long-range chromatin interactions (19). Importantly, the interaction of CTCF with DNA is itself sensitive to CpG methylation (19). To investigate whether CTCF influences the BDNF locus, we scrutinized the previously published genome-wide survey of CTCF binding performed in human cervical cancer cells (HeLa) and found strong signals at the BDNF locus (20) (Fig. 4A). Cohesin, a highly conserved multicomponent complex, is functionally linked to CTCF as a regulator of gene expression and is also involved in chromosome segregation and DNA damage response (21). Interestingly, SCC1, a core component of cohesin, was also bound to the BDNF locus in this survey (20) (Fig. S2A). Cell type–specific CTCF binding has been observed (22, 23), thus prompting us to explore whether CTCF also occupies the BDNF locus in cortical neurons by using ChIP assays. Although we found slight differences between cortical neurons and HeLa cells, the strong CTCF binding sites at the BDNF locus within the activity-inducible promoter IV (site P5) and at site P1 located in an intronic region downstream of the transcriptional start site were conserved in cortical neurons (Fig. 4 B and C). These enrichments represented specific associations of CTCF at the BDNF locus because assays using preimmune IgG or primers spanning random segments (e.g., regions N1 and N2; Fig. 4 B and C) failed to yield significant signals in the ChIP analysis. Furthermore, consistent with findings that cohesin colocalizes with CTCF at multiple sites in the human genome (20), we found a similar binding pattern at the BDNF locus in cortical neurons for SCC1 (Fig. 4D). These results indicate that CTCF and cohesin are likely to participate in BDNF transcriptional regulation in cortical neurons.
Fig. 4.
CTCF and cohesin are associated with the BDNF locus in mouse cortical neurons. (A) Previously published (20) whole-genome ChIP data was visualized with the Integrated Genome Browser (Affymetrix) to identify CTCF binding sites at the BDNF locus. CTCF binds to multiple sites on BDNF locus in HeLa cells, and the numbers under the peaks indicate order of peak magnitude in BDNF locus; for example, 1 is the site with the highest binding and 8 is the lowest. (B) A schematic diagram illustrating the genomic structure of the mouse BDNF gene (4). Solid boxes represent exons, and encircled numbers indicate positions of CTCF binding sites labeled above relative to these exons. (C and D) ChIP assays using antibodies specific to CTCF or the core cohesin component SCC1 were performed to assess occupancy of indicated sites within BDNF locus in cortical neurons. CTCF and cohesin were enriched at BDNF promoter IV (P5) and at P1 in the intron. Fragments corresponding to N1 and N2 were used as negative control binding sites for CTCF and SCC1. Data show the representative result from six independent experiments.
To investigate the potential function of CTCF binding at the BDNF locus, we examined the correlation between CTCF occupancy and BDNF expression. Similar to previous findings that CTCF binding sites containing CpG dinucleotides retain the potential for methylation-based regulation in response to biological or environmental signals (19), we found that the methylation status of CpG dinucleotides within CTCF-binding sites of promoter IV remained responsive to intracellular NAD levels in cortical neurons (Fig. 3 B and C and Fig. S2 B and C). As CTCF DNA binding is influenced by DNA methylation, ChIP assays were used to compare DNA occupancy by CTCF at the BDNF locus in untreated and FK866-treated neurons. Consistent with previous results demonstrating that increased DNA methylation abrogates CTCF binding (19), we observed a dramatic decrease in CTCF occupancy at BDNF promoter IV in neurons with low NAD levels (Fig. 5A, site P5). To test whether similar effects on NAD-mediated DNA occupancy occurred in depolarized neurons, we repeated these ChIP experiments by using neurons treated with KCl. In depolarized neurons, we also found reduced CTCF occupancy at the BDNF promoter IV (Fig. 5B, site P5).
Fig. 5.
CTCF and cohesin binding to BDNF promoter IV is regulated by NAD via altered DNA methylation. (A and B) Cortical neurons treated with or without FK866 for 24 h (and with or without KCl for 90 min) were subjected to ChIP analysis with antibodies specific to CTCF or SCC1. Low NAD levels promoted the release of CTCF and SCC1 from the BDNF promoter IV (P5) but not from the intronic region (P1). (C) Restoration of BDNF gene expression after 5AzaC pretreatment of cortical neurons. KCl-induced BDNF transcription initiated at promoter IV was measured by qRT-PCR in neurons treated with FK866 in the absence (−) or presence (+) of 5AzaC. Results are mean ± SD. *P < 0.01, **P < 0.0001. Data are representative of at least four independent experiments. (D) ChIP analysis showed increased levels of CTCF occupancy at BDNF promoter IV (P5) but not at the intron (P1) in the presence of FK866 in neurons pretreated with 5AzaC.
The NAD-dependent loss of CTCF binding was specific to site P5 within BDNF promoter IV and was not observed at the P1 site in the downstream intron (Fig. 5 A and B, site P1), where no changes in methylation occurred after NAD depletion (Fig. S3 A and B). Indeed, the CpG dinucleotides in the intronic region containing site P1 are mostly unmethylated, providing insight into why CTCF exhibits stronger binding at P1 than P5 (Fig. S3 A and B). Western blot analysis confirmed that NAD depletion did not trigger a signaling pathway that affected CTCF levels or its ADP ribosylation, which influences its activities (24, 25) (Fig. S3C). We also examined SCC1 binding in NAD-depleted cortical neurons and found that cohesin binding to the BDNF promoter (P5), but not the distal intronic region (P1), was also reduced (Fig. 5 A and B). These results are consistent with previous reports demonstrating that CTCF is required for positioning cohesin on DNA (20, 26).
Previous studies showed that MeCP2, a methylated-DNA binding transcriptional repressor, silences BDNF gene expression in cortical neurons via binding to methylated motifs within BDNF promoter IV (5, 6). Because DNA methylation increased within BDNF promoter IV in neurons with low NAD levels, we tested whether this finding was accompanied by changes in MeCP2 binding at this region. Indeed, in contrast to the NAD effects on CTCF binding, we observed an increase in MeCP2 occupancy at the BDNF promoter IV in neurons treated with FK866, which suggests that alterations in MeCP2 DNA binding also contribute to the inhibitory effects on BDNF expression induced by lower NAD levels (Fig. S4).
To further examine the hypermethylation-associated decrease in CTCF binding and its relationship with BDNF transcription, we used the DNA-methyltransferase inhibitor 5′-aza-cytidine (5AzaC) to inhibit DNA methylation. As expected, neurons treated with 5AzaC alone showed a slight increase in BDNF transcription (27) (Fig. 5C). However, neurons pretreated with 5AzaC before addition of FK866 showed a significant restoration of BDNF induction after membrane depolarization (Fig. 5C). We found that the FK866-induced decrease in CTCF binding was also largely reversed by treatment with 5AzaC (Fig. 5D). These data indicate that FK866-induced decreases in BDNF expression are accompanied by increased BDNF promoter methylation and subsequent loss of CTCF and cohesin occupancy.
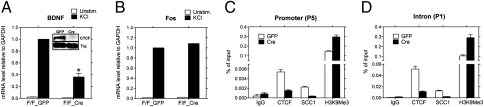
To directly assess the role of CTCF in regulating BDNF transcription, we studied cortical neurons from mice with a conditional CTCF allele (CTCFf/f) (28). CTCFf/f neurons were infected with lentivirus expressing EGFP (control) or Cre recombinase to excise the mutant CTCF allele. No evidence of neuronal death or axonal degeneration was observed in CTCF-deficient neurons (Fig. S5 A and B). However, we observed a severe reduction in membrane depolarization–induced BDNF expression in CTCFf/f neurons expressing Cre versus EGFP (Fig. 6A). As previously observed with FK866 treatment, the induction of c-fos was not impaired in CTCF-deficient neurons (Fig. 6B). Moreover, loss of CTCF binding led to increased levels of the inactive chromatin marker H3K9Me3 at the BDNF locus (Fig. 6 C and D). Hence, our studies indicate that CTCF binding at the BDNF locus is critical for normal BDNF transcription—results that are in concert with others indicating that CTCF loss leads to formation of an inactive chromatin structure, loss of long-range chromatin interactions, and transcriptional silencing (25, 29).
Fig. 6.
CTCF and cohesin proteins are essential for BDNF transcription in mouse cortical neurons. (A) Western blot analysis of lysates prepared from CTCFf/f cortical neurons infected with lentivirus expressing EGFP or Cre recombinase. Note the complete loss of CTCF in neurons expressing Cre, indicating efficient excision of the mutant allele (Insert). BDNF transcripts initiated at promoter IV in control or CTCF-depleted neurons were measured by qRT-PCR. Neurons lacking CTCF had significantly decreased levels of BDNF mRNA after KCl treatment. Results are mean ± SD. *P < 0.0001 (vs. F/F_GFP treated with KCl). The results shown are representative of those obtained in five independent experiments. (B) RT-PCR showing normal levels of c-fos mRNA in KCl-stimulated cortical neurons lacking CTCF. (C and D) ChIP analysis of CTCF, SCC1, and H3K9Me3 (inactive chromatin marker) binding at BDNF promoter IV (P5) and intronic region (P1) in CTCFf/f cortical neurons infected with lentivirus expressing EGFP or Cre recombinase. Note that loss of CTCF resulted in loss of SCC1 at these sites and generated an inactive chromatin structure in this region.
Previous studies demonstrated that cohesin colocalizes with CTCF sites in mammalian genomes (20). Cohesin is required for proper CTCF function (21) and is important for chromatin remodeling (30). The core of the cohesin complex includes a heterodimer composed of SMC1 and SMC3 and two non-structural maintenance of chromosomes (SMC) subunits, SCC1 and SCC3. In addition to having roles in cohesion during cell cycle, cohesin is also involved in genome stability, DNA repair and recombination, and gene expression (21). Patients with cohesin mutations, such as in Cornelia de Lange syndrome or Roberts syndrome, have severe mental retardation and other behavioral abnormalities (21), suggesting that cohesin is important for proper neural development and function.
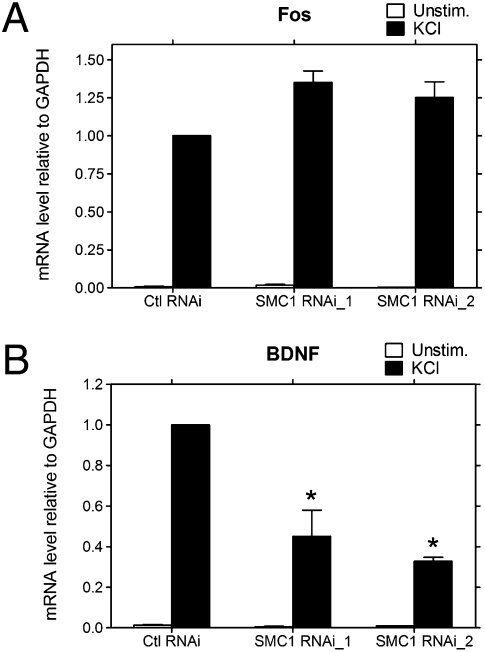
Although cohesin plays numerous roles in the nervous system (21), a role in BDNF transcription is particularly intriguing because BNDF is critical for learning and memory and is associated with several mental disorders, such as Rett syndrome (8). Using cortical neurons, we found that cohesin colocalizes with CTCF within the BDNF locus at both promoter IV and an intronic region (Fig. 4D). In addition, as reported by others in nonneuronal cells (20, 26), loss of CTCF occupancy was also accompanied by loss of SCC1 binding at the BDNF locus in these neurons (Figs. 5 A and B and 6 C and D). The correlation between CTCF occupancy and BDNF expression in cortical neurons led us to test whether cohesin was also critical for BDNF transcription. We focused on the core cohesin component SMC1 because the mutations found in Cornelia de Lange syndrome patients are consistent with a loss of function (31) and were therefore likely to be mimicked by SMC1 knockdown. SMC1 was depleted in cortical neurons by infecting with SMC1 small interfering RNA (siRNA) lentivirus. Two siRNAs were identified that reduced SMC1 mRNA levels by 60% and 85%, respectively (Fig. S6A). Neurons with reduced levels of SMC1 had no apparent morphological deficits or abnormalities in calcium-dependent signaling or transcription as judged by KCl-mediated c-fos induction (Fig. 7A and Fig. S6B). However, SMC1 depletion caused significant reductions in membrane depolarization–induced BDNF transcription (Fig. 7B), similar to that observed in CTCF-depleted neurons (Fig. 6A). These data support the idea that alterations in BDNF expression could contribute to the defective cognition observed in patients with cohesinopathies.
Fig. 7.
Neurons lacking SMC1 have defects in activity-induced BDNF transcription. Cortical neurons were infected with lentivirus expressing siRNAs targeting luciferase (control) or SMC1 and stimulated with KCl for 4 h. c-fos (A) and BDNF (B) mRNA levels were measured by using qRT-PCR. Neurons depleted of SMC1 had normal KCl-mediated induction of c-fos but BDNF induction was greatly decreased, similar to that observed in neurons lacking CTCF. The error bars indicate the ± SD. *P < 0.0001 (vs. Ctl RNAi treated with KCl). Data represent at least three independent experiments.
The methylation of CpG dinucleotides recruits methylated DNA–specific binding proteins that, in turn, attract histone-modifying enzymes and generate a silenced chromatin state. Aberrant epigenetic changes, such as promoter hypermethylation, are associated with inappropriate gene silencing and the development of diseases, including cancer and mental retardation (18). Our findings reveal an alternative pathway allowing for NAD metabolism–mediated epigenetic gene regulation. In cortical neurons, the binding of the DNA methylation–sensitive factor CTCF and associated cohesin components to the BDNF locus is critical for establishing the chromatin structure required for BDNF transcription, because neurons lacking either CTCF or cohesin proteins possess an inactive chromatin structure at the BDNF locus and manifest a decrease in BDNF transcription. Similar to previous reports (5, 6), we found that the CpG at −148 within BDNF promoter IV is highly methylated in cortical neurons, whereas other CpGs closer to the transcriptional start site (i.e., −109, −111, or −87) are less frequently methylated. However, lowering NAD levels, as might occur during cellular stress, induce increased methylation at these latter CpG sites, thereby promoting the release of CTCF and cohesin in this region of the BDNF promoter IV. Interestingly, in addition to the loss of CTCF/cohesin binding, we found that the increased methylation of this locus also recruited MeCP2. This increase presumably triggers the formation of the transcriptional repressor complex containing MeCP2/HDAC1/Sin3A at the BDNF promoter and contributes to the inhibitory effects on BDNF transcription in neurons with lower NAD levels (5, 6). Because these effects are abolished by treatment with a DNA-methyltransferase inhibitor, regulation of DNA-methyltransferase activity may be involved in NAD modulation of BDNF transcription. The less-frequently methylated CpGs we identified may serve as sensors to link activity-dependent BDNF expression with the metabolic state of the neuron.
Our functional data showing changes in BDNF transcription from multiple promoters in response to NAD levels suggest that CTCF/cohesin may have a more global influence on the BDNF locus. Because one important function of CTCF is to mediate or maintain long-range chromatin architecture (19, 32), changes in the CTCF/cohesin DNA binding at a subset of its binding sites could have a profound impact on the overall BDNF chromatin architecture. Moreover, we recently found that the neuron-restrictive silencer element (NRSE) located between exons I and II is occupied by CTCF/cohesin in cortical neurons (Fig. S7 A–C). The NRSE is an important regulatory element involved in metabolic regulation of BDNF transcription through the glycolysis pathways (33). Interestingly, we found that CTCF/cohesin binding to the NRSE is also sensitive to NAD levels, which, of course, are modulated by glycolytic activity (Fig. S7 D and E). The interactions by these multiple proteins located at different BDNF locus will be an important influence on BDNF levels. In aged or nutritionally deficient individuals, the altered BDNF levels could then, in turn, play a role in the gradual decline in cognition.
Finally, our data demonstrating a relationship between NAD metabolism and the DNA methylation–sensitive CTCF/cohesin may have broad implications for gene regulation. For instance, transformed fibroblasts have lower NAD levels than their normal counterparts (34), and aberrant promoter hypermethylation is an important mechanism silencing tumor suppressor genes (18). With the renewed focus on the metabolic underpinnings of cancer, the discovery of a dynamic interplay among NAD, DNA methylation, chromatin compaction, and gene regulation may provide additional avenues for the development of chemotherapeutic agents.
Materials and Methods
Primary Cultures of Cortical Neuronal Cells and Drug Treatment.
Cortical neurons from embryonic day 15 (E15) CD-1 mice (Charles River Laboratories) were processed as described previously for mouse dorsal root ganglion primary cultures (35). Neurons were plated and cultured in Neurobasal medium (Invitrogen, 21103-049) with B27 serum-free supplement (Invitrogen, 17504-044), glutamine (5 mM), and penicillin/streptomycin. Cells were typically seeded at a density of one forebrain per 24-well plate. Neurons were stimulated with KCl (55 mM) at 5 d in vitro (DIV) for 4 h as previously described (5).
FK866 was obtained from the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (NIMH code F-901). Indicated concentrations of FK866 (diluted from 0.1 M DMSO stock) were added to the culture medium 24 h before stimulation with KCl. Nicotinamide mononucleotide (Sigma, N3501) was added at the same time as FK866. 5AzaC (10 μM; Sigma, A2385) freshly prepared in DMSO was added 48 h before KCl stimulation (which was 24 h before FK866 addition). Medium containing 5AzaC was replenished every 24 h to ensure maintenance of adequate levels.
Quantitative RT-PCR (qRT-PCR) and Western Blot Analyses.
RNA isolation, qRT-PCR, and normalization were performed as described previously (5). The primers used for amplification are listed in Table S1. All primers were intron-spanning to avoid coamplification of genomic DNA. Relative expression level of each gene was determined by using a standard curve and normalized to GAPDH mRNA levels. Normalization among experiments was performed according to the method described previously (36). Western blots were performed by using standard methods.
ChIP.
For ChIP analysis, cortical neurons grown on a 6-well plate were processed as described in the ChIP Assay kit protocol from Millipore (17-295). Typically, 10% of the forebrain from E15 + 5DIV mice was used for a single immunoprecipitation experiment. The chromatin was immunoprecipitated with the following antibodies: anti-CTCF (Millipore, 07729), anti-Scc1 (Abcam, ab992), anti–acetyl-histone H3K9 (Millipore, 17-658), and anti–trimethyl-histone H3K9 (Abcam, 8898). The precipitated DNA fragments were measured by qRT-PCR under the conditions described above. Primers specific for each segment of interest are listed in Table S2. To normalize PCR efficiency for different primer pairs (P5 vs. P1), the intensity of the PCR products from the chromatin immunoprecipitates were normalized against the intensity of the PCR products of the genomic DNA input amplified by the same primer pairs.
HPLC Measurement of NAD Levels.
Cortical neurons treated for 24 h with indicated concentrations of FK866 were washed with PBS, and NAD was measured as described previously (37).
Smc1 siRNA Knockdown.
Oligonucleotides targeting Smc1 were designed by the siRNA design program siDRM (http://sirecords.umn.edu/siDRM; ref. 38). The two independent target regions are RNAi_1: 5′-AGAAGAAAGAGCTGGGAAA-3′ and RNAi_2: 5′-AGAAGAAGCTAGAGGGAGA-3′. Oligonucleotides targeting the luciferase gene were used as control. Lentiviral vectors encoding siRNA were generated as described previously (37). Lentivirus production and neuronal infection were performed as previously described (37). More than 90% of the neurons were infected (i.e., EGFP-positive). To ensure effective CTCF or SMC1 depletion, experiments were performed 6 d after infection with virus.
Methylation Analysis.
Neuronal genomic DNA was isolated by using DNeasy Blood and Tissue Kit (Qiagen, 69504). Cells were directly lysed with PBS/proteinase K/lysis buffer AL on the plate without a PBS wash step. Purified genomic DNA (500 ng) was bisulfite-converted by using the EZ DNA Methylation Kit (Zymo, D5001). Bisulfate-treated samples were amplified by using primers that span the CpG islands in the promoter IV (P5) and intron (P1) regions and hybridize regardless of methylation status: promoter IV (P5), forward 5′-ACCGCGGTGGCGGCCGCAATATTTATAAAGTATGTAATGTTTTGGAA-3′ and reverse 5′- ATCCACTAGTTCTAGAACTCCATTTAATCTAAACAAAAACTAAAAA-3′; and intron (P1), forward 5′- ACCGCGGTGGCGGCCGCAGGGGAGGGTTAGGAGAGTTGAGTTA-3′ and reverse 5′- ATCCACTAGTTCTAGAAAAACCCTCAAATAAAATCAAAAAAATCCT-3′. The purified PCR products were cloned into pBluescript KS(+) vector by using In-Fusion PCR Cloning (Clontech). The percentage methylation of each CpG site was determined by examining the sequencing results from 38 to 40 individual clones for each treatment from three independent cultures.
Statistical Analysis.
Statistical analysis was carried out with a two-way ANOVA using Graphpad Prism.
Supplementary Material
Acknowledgments
We thank Andreu Valls, Rachel Darken, and Scott T. Isbell for reading this manuscript; Kelli Simburger, Amber Nielson, and Nina Panchenko for help with DNA cloning, sequencing, and animal husbandry; Katsuhiko Shirahige (Laboratory of Genome Structure and Function Center for Epigenetic Disease, Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo) for providing the CTCF/cohesin ChIP–ChIP data; and Li-wei Chang for help with ChIP–ChIP data processing. This work was supported by National Institutes of Health Neuroscience Blueprint Center Core Grant P30 NS057105 to Washington University, the Hope Center for Neurological Disorders, and by National Institutes of Health Grants CA111966 and AG013730 (to J.M.).
Footnotes
Conflict of interest statement: The authors and Washington University may derive benefit from a licensing agreement with Sirtris Pharmaceuticals, which did not provide any support for this work.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1002130107/-/DCSupplemental.
References
- 1.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuccato C, Cattaneo E. Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol. 2009;5:311–322. doi: 10.1038/nrneurol.2009.54. [DOI] [PubMed] [Google Scholar]
- 3.West AE. Biological functions of activity-dependent transcription revealed. Neuron. 2008;60:523–525. doi: 10.1016/j.neuron.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 4.Timmusk T, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 5.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 6.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 7.Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoghbi HY. Rett syndrome: What do we know for sure? Nat Neurosci. 2009;12:239–240. doi: 10.1038/nn0309-239. [DOI] [PubMed] [Google Scholar]
- 9.Tapia-Arancibia L, Aliaga E, Silhol M, Arancibia S. New insights into brain BDNF function in normal aging and Alzheimer disease. Brain Res Brain Res Rev. 2008;59:201–220. doi: 10.1016/j.brainresrev.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- 11.Koch-Nolte F, Haag F, Guse AH, Lund F, Ziegler M. Emerging roles of NAD+ and its metabolites in cell signaling. Sci Signal. 2009;2:mr1. doi: 10.1126/scisignal.257mr1. [DOI] [PubMed] [Google Scholar]
- 12.Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai SI. “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim Biophys Acta. 2010;1804:1584–1590. doi: 10.1016/j.bbapap.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams AC, Ramsden DB. Pellagra: A clue as to why energy failure causes diseases? Med Hypotheses. 2007;69:618–628. doi: 10.1016/j.mehy.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- 16.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 17.Kim MY, Mauro S, Gévry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. doi: 10.1038/nrg1655. [DOI] [PubMed] [Google Scholar]
- 19.Phillips JE, Corces VG. CTCF: Master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendt KS, et al. Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature. 2008;451:796–801. doi: 10.1038/nature06634. [DOI] [PubMed] [Google Scholar]
- 21.Dorsett D. Cohesin, gene expression and development: Lessons from Drosophila. Chromosome Res. 2009;17:185–200. doi: 10.1007/s10577-009-9022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TH, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuddapah S, et al. Global analysis of the insulator binding protein CTCF in chromatin barrier regions reveals demarcation of active and repressive domains. Genome Res. 2009;19:24–32. doi: 10.1101/gr.082800.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu W, et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet. 2004;36:1105–1110. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- 25.Witcher M, Emerson BM. Epigenetic silencing of the p16(INK4a) tumor suppressor is associated with loss of CTCF binding and a chromatin boundary. Mol Cell. 2009;34:271–284. doi: 10.1016/j.molcel.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio ED, et al. CTCF physically links cohesin to chromatin. Proc Natl Acad Sci USA. 2008;105:8309–8314. doi: 10.1073/pnas.0801273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson ED, Kavalali ET, Monteggia LM. Activity-dependent suppression of miniature neurotransmission through the regulation of DNA methylation. J Neurosci. 2008;28:395–406. doi: 10.1523/JNEUROSCI.3796-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heath H, et al. CTCF regulates cell cycle progression of αβ T cells in the thymus. EMBO J. 2008;27:2839–2850. doi: 10.1038/emboj.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Majumder P, Gomez JA, Chadwick BP, Boss JM. The insulator factor CTCF controls MHC class II gene expression and is required for the formation of long-distance chromatin interactions. J Exp Med. 2008;205:785–798. doi: 10.1084/jem.20071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hakimi MA, et al. A chromatin remodelling complex that loads cohesin onto human chromosomes. Nature. 2002;418:994–998. doi: 10.1038/nature01024. [DOI] [PubMed] [Google Scholar]
- 31.Revenkova E, et al. Cornelia de Lange syndrome mutations in SMC1A or SMC3 affect binding to DNA. Hum Mol Genet. 2009;18:418–427. doi: 10.1093/hmg/ddn369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wallace JA, Felsenfeld G. We gather together: Insulators and genome organization. Curr Opin Genet Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garriga-Canut M, et al. 2-Deoxy-d-glucose reduces epilepsy progression by NRSF-CtBP–dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JP, Passonneau JV, Johnson GS, Pastan I. The effect of growth conditions on NAD+ and NADH concentrations and the NAD+:NADH ratio in normal and transformed fibroblasts. J Biol Chem. 1974;249:4138–4143. [PubMed] [Google Scholar]
- 35.Chen Y, et al. NS21: Re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong W, et al. Integrated siRNA design based on surveying of features associated with high RNAi effectiveness. BMC Bioinformatics. 2006;7:516. doi: 10.1186/1471-2105-7-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.