Abstract
Many tumors contain heterogeneous populations of cells, only some of which exhibit increased tumorigenicity and resistance to anticancer therapies. Evidence suggests that these aggressive cancer cells, often termed “cancer stem cells” or “cancer stem-like cells” (CSCs), rely upon developmental signaling pathways that are important for survival and expansion of normal stem cells. Here we report that, in analogy to embryonic mammary epithelial biology, estrogen signaling expands the pool of functional breast CSCs through a paracrine FGF/FGFR/Tbx3 signaling pathway. Estrogen or FGF9 pretreatment induced CSC properties of breast cancer cell lines and freshly isolated breast cancer cells, whereas cotreatment of cells with tamoxifen or a small molecule inhibitor of FGFR signaling was sufficient to prevent the estrogen-induced expansion of CSCs. Furthermore, reduction of FGFR or Tbx3 gene expression was able to abrogate tumorsphere formation, whereas ectopic Tbx3 expression increased tumor seeding potential by 100-fold. These findings demonstrate that breast CSCs are stimulated by estrogen through a signaling pathway that similarly controls normal mammary epithelial stem cell biology.
More than 70% of breast cancers express high levels of the estrogen receptor (ERα), and many of these tumors require estrogen for sustained growth and progression. In recent years, multiple reports have shown that subpopulations of so-called cancer stem cells (CSCs; also called stem-like cells or tumor-initiating cells) are also required for sustained tumor growth and progression, and may be responsible for cancer recurrence and metastasis (1). Whether such CSCs in ERα+ breast cancers are sensitive to estrogen is currently unknown.
Breast CSCs, which are operationally defined based on the number of self-renewing cells required to initiate a tumor and drive long-term tumor growth when transplanted into mice, can be isolated from primary tumor tissue or cultured cells lines (2–7). In human breast cancers, CSCs appear to be enriched within cell subpopulations with a CD44+/CD24−/low/ESA+ surface marker profile, are better able to form colonies, or “tumorspheres,” under low-adherence conditions, and display increased resistance to chemotherapeutic compounds (2–7).
The molecular mechanisms that regulate breast CSC frequency, localization, and maintenance remain poorly understood. However, a fair amount is known about the spatio-temporal signaling dynamics that govern the specification and maintenance of normal mammary gland stem cells. Embryonic development of the mouse mammary gland begins when Wnt and FGF signaling proteins, which are secreted by the underlying mesenchyme, induce placode formation and localize mammary epithelial fate specification (8). FGF ligands, acting through cognate receptors, activate the Tbx3 transcription factor in both the mesenchymal and mammary placodes. Tbx3, in a positive-feedback loop, activates additional FGF secretion and also Wnt signaling (9–12).
During puberty, estrogen is responsible for maturation of the mammary gland by mediating ductal elongation (9–13). Interestingly, there is significant evidence to suggest that estrogen signaling does not act directly on adult mammary epithelial stem cells but, rather, activates their proliferation through paracrine signaling (14, 15). These data imply a two-component mammary stem cell niche in which estrogen signaling in the ERα+ non-stem cell compartment stimulates the proliferation of cells within the ERα− stem cell compartment.
In breast cancer, it is unclear whether stem-like cells are also regulated by specific hormone-growth factor paracrine signaling pathways. In this study, we discovered that estrogen regulates breast CSC numbers through the FGF/Tbx3 signaling pathway, which happens also to regulate normal embryonic breast stem cells.
Results
Estrogen Stimulation Induces Expansion of Breast Cancer Stem-Like Cell Subpopulations.
To study the signaling pathways that regulate breast CSC expansion and maintenance, we needed an experimental system that allowed for consistent modulation of breast CSC numbers through defined signaling perturbations. Tumor initiation by the MCF7 cell line appears to rely on estrogen signaling; these cells are very poor at forming tumors in ovariectomzed mice (16). However, we and others have found that MCF7 cells can proliferate in vitro in the absence of estrogen (E2) if serum (even charcoal-stripped serum) is supplemented in high enough concentrations (Fig S1A). MCF7 cells grown under these conditions maintain a low percentage of CSCs as gauged by flow cytometry (Fig. S1B) and are likewise poor at forming tumors in ovariectomized mice. Yet, intact ovaries or estrogen supplementation allows even an estrogen-deprived MCF7 line to form tumors, suggesting that estrogen induces the survival or expansion of MCF7 CSCs.
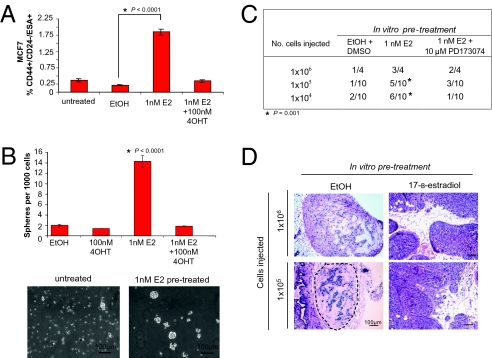
To determine whether estrogen could indeed induce CSC expansion, we treated MCF7 cells as well as other estrogen receptor-positive (ER+) cell lines (T47D, HCC1428) with 1 nM 17-β-estradiol or ethanol (vehicle control) for 6 d, and evaluated the proportion of stem-like cells by flow cytometry and sphere formation assays. We found that after estrogen stimulation, the proportion of CD44+/CD24−/ESA+ stem-like cells was nearly eightfold higher in ERα+ cultures, whereas no significant change in the proportion of CD44+/CD24−/ESA+ cells was observed when the same culture conditions were imposed on cells that lacked ER expression (Fig. 1A and Fig. S1C). When we challenged these MCF7 cultures to form tumorspheres, we found that the estrogen pretreated cultures formed sevenfold more tumorspheres than the ethanol-pretreated cultures (Fig. 1B). Moreover, we observed that the addition of the potent estrogen antagonist, 4-hydroxy tamoxifen (4OHT), could prevent the 17-β-estradiol induced expansion of CD44+/CD24−/ESA+ cells and sphere formation, indicating that these changes in marker expression and sphere formation were mediated through ER signaling.
Fig. 1.
Estrogen increases cancer stem cells in ERα+ cell lines. (A) Average percentage of CD44+/CD24−/ESA+ cells in the ERα+ cell line MCF7 following 6-d treatment with either 1 nM 17-β-estradiol (E2) or vehicle (EtOH); n = 5 biological replicates. Data are mean ± SEM. (B) MCF7 tumorsphere formation presented as the average number of spheres per 1,000 cells plated ± SEM; n = 3 biological replicates. Representative phase contrast micrographs of MCF7 spheres are shown. (C) Tumor formation of MCF7 cells pretreated with EtOH and DMSO (vehicles), E2, or E2 and PD173074 injected in limiting dilution into ovariectomized mice. *Nonparametric χ2 statistic was used to test the expected vs. observed frequencies of tumor formation at limiting dilution with a level of 0.001 (critical value, 10.83). (D) Representative H&E-stained sections of mammary glands injected with EtOH-pretreated or 1 nM estrogen (E2)-pretreated MCF7 cells are shown.
We next evaluated the ability of estrogen-pretreated MCF7 cultures to form tumors by injecting cells pretreated with estrogen in vitro for 6 d into the mammary glands of ovariectomized NOD/SCID mice in dilution series. Estrogen-pretreated cells were able to form tumors in mice 100-fold more efficiently when compared with the vehicle (EtOH+DMSO) treated cells (P = 0.001, Fig. 1C). Histological examination of tissue sections revealed that MCF7 cells pretreated in vitro with estrogen formed invasive ductal carcinomas (Fig. 1D). We also examined the injection sites of MCF7 cells pretreated with ethanol that had not formed tumors and observed viable cells within the mammary glands that formed only benign epithelial structures, suggesting that lack of tumor growth was not due to immune clearance of cells or increased cell death. These results indicate that estrogen-induced expansion of cancer stem-like cells in vitro leads to a functional increase in breast CSCs and tumorigenic phenotypes in vivo.
Estrogen Expands Breast CSCs via Paracrine-Acting Protein Factors.
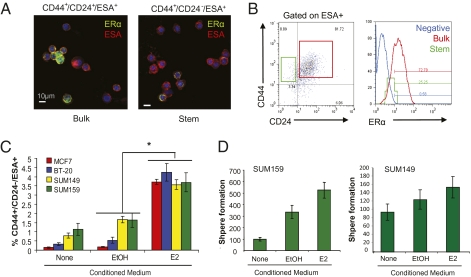
Because ER activity appeared necessary for the expansion of breast CSCs in response to estrogen, we next examined the levels of ERα expression in the CD44+/CD24−/ESA+ stem-like MCF7 subpopulation. Using both immunofluorescence on freshly sorted cytospun cells and four-color flow cytometry, we found that ≥70% of the cells in the bulk fraction (CD44+/CD24+/ESA+) were strongly ERα+, whereas only 20–25% of the CD44+/CD24−/ESA+ stem-like cells had detectable nuclear ERα staining (Fig. 2 A and B and Fig. S2A). We also observed that ERα+ cells in the CD44+/CD24−/ESA+ population had much lower levels of staining than ERα+ cells from the bulk fraction (Fig. 2B, histogram).
Fig. 2.
Paracrine factors produced in response to estrogen expand ERα− breast CSCs. (A) Immunofluorescence of sorted cytospun MCF7 cells for ERα (green) and ESA/EpCAM (red) expression, counterstained for nuclei with DAPI (blue). (B) Cytometric plots of ERα expression in CD44+/CD24+/ESA+ cells (red, bulk), and in CD44+/CD24−/ESA+ stem-like cells (green), which comprise 2% of the culture. (C) Average percentage of CD44+/CD24−/ESA+ cells in ERα− SUM149, SUM159, and BT20 cultures following treatment with conditioned media from either ethanol (EtOH) or E2-pretreated MCF7. MCF7 cells are shown for reference, *P < 0.0001, n = 4 biological replicates. Data are mean ± SEM. (D) Tumorsphere-forming potential of SUM149 or SUM159 cultures described in C; n = 4 biological replicates. Data are mean ± SEM.
Given these results, we hypothesized that in analogy to the normal mammary gland, paracrine factors released by the ERα+ cells in response to estrogen stimulation might induce the expansion of CD44+/CD24−/ESA+ stem-like cells. To evaluate this hypothesis, we harvested conditioned media from MCF7 cells that were pretreated with either vehicle (EtOH) or 1 nM 17-β-estradiol. We observed that MCF7 cultures fed estrogen-conditioned media for 6 d contained 20-fold more CD44+/CD24−/ESA+ cells than matched cultures fed vehicle conditioned media (Fig. S2B, P < 0.002). In addition, expansion of this subpopulation was significantly attenuated if the conditioned medium was boiled before treatment of recipient lines (P < 0.05), indicating that the factors promoting stem-like cell expansion were heat labile and thus likely to be secreted proteins.
We tested whether conditioned media from estrogen-treated MCF7 cells could increase CSC numbers in three ERα− breast cancer lines, SUM149, SUM159, and BT-20. We observed that exposure to conditioned media from estrogen-treated MCF7 cells induced a statistically significant expansion of the CD44+/CD24−/ESA+ stem-like cells in all three cell lines, yielding cultures that were more efficient at forming tumorspheres (Fig. 2 C and D). In sum, these data suggest that estrogen acts to induce secretion of paracrine acting proteins, which in turn increase percentages of CD44+/CD24−/ESA+ populations and corresponding cancer stem-like cell properties in many breast cancer cell lines.
Estrogen Induces FGF9/FGFR3 Signaling to Increase Cancer Stem-Like Numbers.
To identify the secreted proteins mediating breast cancer stem-like cell expansion following estrogen treatment, we examined the conditioned media from either 17-β-estradiol–treated or vehicle-treated MCF7 cells and quantitatively assayed for 164 secreted growth factors and cytokines using an antibody-based protein array. In addition to known estrogen-induced factors, we observed that the secretion of every assayed FGF family member (FGF2/bFGF, FGF4, FGF6, FGF7, and FGF9) was increased at least twofold upon estrogen treatment compared with ethanol-treated controls (Fig. S2C). Notably, FGF9, which is induced by estrogen in endometriosis and during embryonic mammary placode formation (17), was increased 14-fold following estrogen treatment of MCF7 cells.
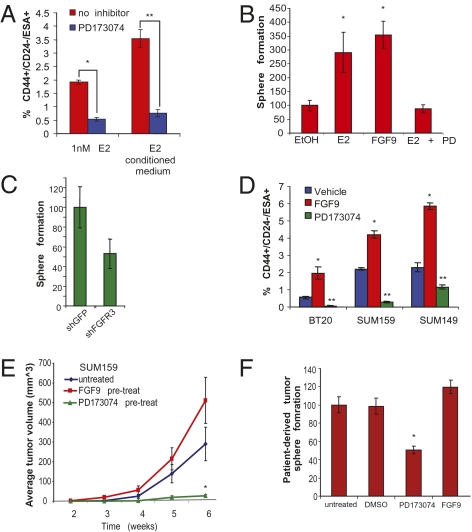
We next tested whether FGF signaling was necessary for the estrogen-induced expansion of the breast CSC-enriched subpopulation. Accordingly, we treated MCF7 cells with a chemical inhibitor of FGFR signaling, PD173074, together with either estrogen or conditioned medium from estrogen-pretreated cells. By flow cytometry, we observed that inhibition of FGF signaling prevented either estrogen or conditioned medium from estrogen-pretreated cells to elicit an increase in CD44+/CD24−/ESA+ cells (Fig. 3A). In contrast, the addition of recombinant FGF9 to serum-free cultures was sufficient to increase the CD44+/CD24−/ESA+ subpopulation (Fig. S2D) and to promote tumorsphere formation to levels comparable to those in estrogen-treated sphere cultures (Fig. 3B). FGF9 and estrogen appeared to have a synergistic effect on increasing MCF7 CSCs (Fig. 3A). When we tested two other ligands from the FGF family, FGF2 and FGF10, we saw that although these factors did not increase the basal levels of CD44+/CD24−/ESA+ cells in the cell line, they were able to slightly increase the effect of estrogen (Fig. S2E). In contrast, feeding candidate growth factors, including EGF, HRG, IGFII, BMP6, and SDF1β, failed to increase the proportion of CSCs in the presence or absence of estrogen supplementation (Fig. S2E).
Fig. 3.
FGFR signaling is necessary for estrogen CSC expansion. (A) Average percentage of CD44+/CD24−/ESA+ cells in MCF7 cultures treated with 1 nM 17-β-estradiol (E2) or E2-conditioned medium in the presence of the FGFR inhibitor PD173074. n = 6 Biological replicates for fresh media, *P < 0.0001; n = 4 biological replicates for conditioned media, **P < 0.005, Data are mean ± SEM. (B) MCF7 cells pretreated E2, FGF9 (100 ng/mL), or E2 and PD173074 were seeded for tumorspheres and resulting spheres, Data are mean ± SEM, n = 4 biological replicates. *P = 0.01 either E2 or FGF9 vs. EtOH. (C) Sphere formation of estrogen-pretreated MCF7 cultures transduced with indicated small hairpins. (D) Flow-cytometric analysis of CD44+/CD24−/ESA+ cells in ERα− SUM149, SUM159, BT20 cultures following treatment with either recombinant human FGF9 or the FGFR inhibitor, PD173074. MCF7 cells treated with E2 are shown as reference. Data are mean ± SEM; n = 4 biological replicates. *P < 0.004 FGF9 vs. vehicle; **P < 0.0005 PD vs. vehicle. (E) Tumor formation of 104 SUM159 cells pretreated with DMSO, FGF9, or PD173074 injected orthotopically into mice; n = 12 for each treatment. *P < 0.02 DMSO vs. PD. Data are mean ± SEM. (F) Tumorsphere formation of breast cancer cells isolated from a primary human breast cancer (TUM177) treated with FGF9 or the FGFR inhibitor PD173074, *P = 0.01 DMSO vs. PD. Data are mean ± SEM.
There are four FGF receptors, and MCF7 cells express high levels of FGFR3 (Fig. S3A), which binds with high affinity to FGF9 (17). To rule out a nonspecific effect of the PD173074 compound, we examined whether the knockdown of FGFR3 in MCF7 cells might also abolish estrogen-induced expansion of the breast cancer stem-like cell populations. Accordingly, we inhibited FGFR3 expression using lentiviral infection with targeted shRNAs. We observed a 76% reduction in FGFR3 protein expression in MCF7 cultures transduced with shFGFR3 (Fig. S3B). Similar to treatment with PD173074, inhibition of FGFR3 expression in MCF7 cells led to a fourfold reduction in the proportion of CD44+/CD24−/ESA+ cells and a twofold reduction in sphere formation in response to estrogen treatment (Fig. S3C and Fig. 3C) without reducing estrogen-induced proliferation in adherent cultures (Fig. S3D).
To functionally assess whether inhibition of FGF signaling in the presence of estrogen affected tumor formation, MCF7 cells were pretreated for 6 d in vitro with estrogen in the presence of PD173074 and injected into mice. Tumor-initiating potential conferred by 17-β-estradiol pretreatment alone was abolished in the presence of FGFR-inhibition (Fig. 1C, P = 0.001). These data indicate that estrogen expands breast cancer stem cell numbers at least in part through the FGF/FGFR signaling pathway.
To determine whether the FGF signaling pathway also regulates stem-like cell populations in ERα− breast cancer cell lines, we added either recombinant FGF9 or PD173074 to SUM149, SUM159, and BT-20 cultures. Treatment with FGF9 induced an average 2.5-fold expansion of the stem-like cells and enhanced tumorsphere formation, whereas inhibition of FGFR signaling with PD173074 decreased the proportion of CD44+/CD24−/ESA+ stem-like cells by eightfold and reduced sphere formation (Fig. 3D and Fig. S3E). In addition, SUM159 cells pretreated in vitro with PD173074 or FGF9 were injected orthotopically into immunocompromised mice to evaluate tumor initiation. Indeed, PD173074 pretreatment significantly inhibited SUM159-derived tumor growth in vivo (P < 0.02, Fig. 3E).
We also isolated patient-derived breast carcinoma cells, and treated these cells with either FGF9 or PD173074 in sphere culture. We observed a modest 1.2-fold increase in tumorsphere formation in response to treatment with FGF9 but a statistically significant twofold reduction in sphere formation in the presence of PD173074 (P < 0.01, Fig. 3F). Similarly, when we dissociated freshly isolated tumors from human-in-mouse tumor generated tissues (SI Materials and Methods), we found that these cells grew significantly fewer sphere colonies in the presence of PD173074 than in the presence of FGF9 (Fig. S3F). Collectively, these data demonstrate that FGF/FGFR signaling is an important regulator of breast cancer stem-like cells.
Estrogen and FGF Signaling Induce Tbx3 Expression.
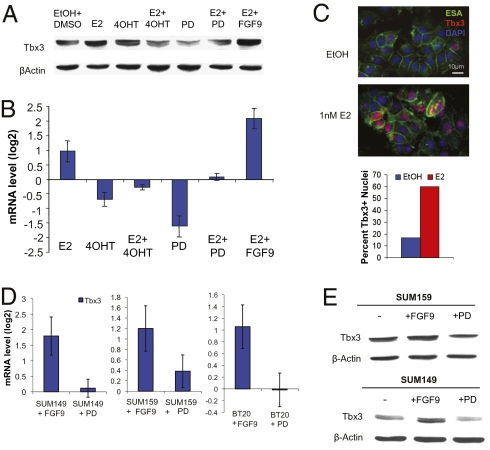
The Tbx3 transcription factor has been reported to activate FGF signaling but also act downstream of FGF signaling, where it is required for propagation of FGF and Wnt signals in the rudimentary mammary epithelium (8–10). Therefore, we wanted to determine whether levels of Tbx3 correlated with estrogen or FGF signaling. Accordingly, we examined Tbx3 expression in MCF7 cultures treated with combinations of estrogen, tamoxifen, FGF9, or PD173074. Indeed, Tbx3 mRNA and protein expression were increased in MCF7 cells treated with estrogen and further increased by FGF9 stimulation (Fig. 4 A and B). This induction was effectively inhibited by 4OHT or PD173074. Tbx3 protein was also visualized by immuno-fluorescence, revealing nuclear localization for the Tbx3 transcription factor in 60% of the MCF7 culture following estrogen treatment (Fig. 4C).
Fig. 4.
FGF/Tbx3 signaling is intact human breast cancer cells. (A) Western blot of Tbx3 in MCF7 cultures treated with vehicle (EtOH+DMSO), 1 nM E2, 100 ng/mL FGF9, 100 nM 4OHT, or FGFR inhibitor 10 μM PD173074. (B) Quantitative RT- PCR of Tbx3 expression in the same MCF7 cells assayed in A. Data are represented as average delta (deltaCt) ± SEM; n = 4 experiments. (C) Immunofluorescence of MCF7 cells treated with 1 nM E2 or EtOH vehicle; ESA/EpCAM (green), Tbx3 (red), and DAPI (blue) show nuclear localization of Tbx3. Quantification is shown below. (D) Quantitative RT-PCR analysis of DUSP6 and Tbx3 expression in SUM149, SUM159, and BT-20 cultures treated with FGF9 or PD173074 relative to expression in cultures treated with DMSO. Data are represented as average delta (deltaCt) ± SEM; n = 4 biological replicates. (E) Western blot analysis of Tbx3 expression in SUM149 and SUM159 cells described in D.
We also examined the FGF-Tbx3 signaling axis in ERα− SUM149, SUM159, and BT-20 breast cancer cells treated with recombinant FGF9 or PD173074. Consistent with the findings in MCF7 cells, Tbx3 mRNA and protein expression was induced in response to FGF9 treatment (Fig. 4 D and E). Although treatment of the cultures with PD173074 did not affect the basal Tbx3 mRNA levels, protein levels appeared to be modestly decreased. Taken together, these data indicate that: (i) estrogen stimulates expansion of tumorigenic breast cancer cells in part through FGF signaling, (ii) inhibition of FGF/FGFR signaling decreases tumorigenic breast stem-like cells, and (iii) estrogen causes induction of Tbx3 expression breast cancer cells and is a likely mechanism through which FGF signaling is perpetuated.
Tbx3 Expression Is Sufficient for Breast CSC Expansion.
Because Tbx3 is known to be necessary for the specification and expansion of normal mammary stem cells, we next examined whether Tbx3 might also be necessary for the expansion of breast cancer stem-like cells. Using an RNAi knock-down approach in three different breast cancer cell lines (MCF7, SUM149, and SUM159), we were able to reduce endogenous Tbx3 mRNA and protein levels 60–85% (Fig. S4 A and B). It is known that the DUSP6 phosphatase is activated following FGF signaling, and that the spatiotemporal expression pattern of DUSP6 in the developing mammary gland is similar to that of Tbx3 (10). Therefore, we also assayed expression of DUSP6 and found that DUSP6 mRNA expression was reduced an average of 2.4-fold in the shTbx3 transduced cultures. These results are analogous to observations in embryonic mammary epithelial cells showing that FGF signaling is required for Tbx3 and DUSP6 expression and that Tbx3 expression is important for further FGF production and signal propagation (8–10).
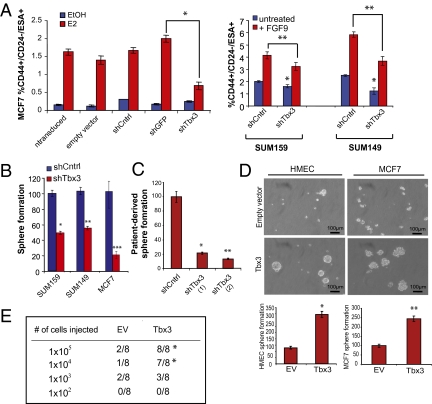
To assess the role of Tbx3 in breast CSC maintenance, we performed flow cytometry and tumorsphere assays on cells exhibiting the greatest inhibition of Tbx3 expression. We found no significant difference in the proliferation rates of SUM149, SUM159, or MCF7 breast cancer cells upon Tbx3 knockdown (Fig. S4 C and D). However, the ability of MCF7 cells transduced with shTbx3 to increase the proportion of CD44+/CD24−/ESA+ cells or increase sphere formation following estrogen was significantly attenuated (Fig. 5 A and B). Likewise, a 20–50% reduction in cancer stem-like cells and tumorsphere formation was observed in shTbx3 transduced SUM149 and SUM159 lines (Fig. 5 A and B). Furthermore, Tbx3 was inhibited using two different hairpins in patient-derived cancer cells and also resulted in a significant reduction in sphere formation (Fig. 5C).
Fig. 5.
Tbx3 is necessary and sufficient for breast CSC expansion. (A) (Left) Average percentage of CD44+/CD24−/ESA+ cells in MCF7 cultures transduced with lentiviruses encoding short hairpins targeting a scrambled sequence (Cntrl), GFP, or Tbx3 and treated with 1 nM 17-β-estradiol (E2) or vehicle (EtOH). *P < 0.0015. (Right) Average percentage of CD44+/CD24−/ESA+ cells in SUM149 and SUM159 cultures transduced with lentiviruses encoding short hairpins targeting Tbx3 and treated with recombinant FGF9. Data are mean ± SEM; n = 4 biological replicates. *P < 0.003; **P < 0.007. (B) Normalized tumorsphere-forming potential of SUM159, SUM149, or MCF7 cultures transduced with hairpins targeting a scramble sequence (Cntrl) or Tbx3. Data as mean ± SEM; n = 4 experiments. *P < 0.001; **P < 0.005; ***P < 0.02. (C) Normalized tumorsphere formation of breast cancer cells isolated from a primary human breast cancer (TUM177) transduced with lentiviruses containing two different short hairpin sequences targeting Tbx3. *P < 0.002; **P < 0.0008. (D) Normalized sphere-forming ability of immortalized human mammary epithelia cells (HMEC) or MCF7 cells ectopically overexpressing human Tbx3; n = 4 experiments, 2 biological replicates. *P = 0.002; **P = 0.003. (E) Tumor formation of MCF7 cells overexpressing Tbx3 or empty vector (EV) injected in limiting dilution into NOD/SCID mice. *Nonparametric χ2 statistic was used as described in Fig. 1.
Notably, we were unable to maintain efficient knockdown of Tbx3 in any cell line for more than two passages following selection; therefore, we could not assess in vivo tumor seeding ability of shTbx3 cells. Consequently, we took an alternative approach and ectopically overexpressed Tbx3 in normal human mammary epithelial cells (HMECs) and MCF7 cancer cells to determine whether Tbx3 expression would suffice to promote stem-like cell behavior. Indeed, expression of Tbx3 resulted in a ∼twofold increase in the number of spheres formed by HMEC cells and increased the proportion CD44+/CD24−/ESA+ cancer stem-like cells in MCF7 cells by ninefold (Fig. 5D and Fig. S4E). Furthermore, overexpression of Tbx3 in MCF7 cells led to a robust twofold increase in tumorsphere formation (Fig. 5D). Consistent with the expansion of cancer stem-like cells, overexpression of Tbx3 in MCF7 cells resulted in a 100-fold increase in tumor-initiation compared with control cells (Fig. 5E, P = 0.001). Collectively, these findings indicate that Tbx3 is sufficient to promote normal and cancer stem-like cell phenotypes.
Expression of FGFR3 and Tbx3 in Human Breast Cancers.
Our results suggest that paracrine FGF signaling mediated through Tbx3 is important in regulating the proportion of CSCs within cultured breast cancer populations. To determine whether this mechanism might also operate within the context of primary human breast cancers, we queried a gene expression database that encompasses more than 18,000 human cancer gene expression microarrays (18, 19) for FGFR3 and TBX3 expression. We found that TBX3 was highly expressed in many subtypes of breast cancer when compared with normal tissue, and that Tbx3 expression correlated with ER-positive tumors. Furthermore, Tbx3 expression was highly correlated with metastatic recurrence at both 3 and 5 y, whereas Stage III tumors had a high correlation with genomic amplification of the Tbx3 locus (Figs. S5 and S6 A and B). These data are consistent with and support other recent findings that TBX3 is up-regulated in human breast cancers (16). In addition, we found that breast tumors that responded to chemotherapy expressed significantly lower levels of Tbx3 than nonresponders, and that cell lines that are sensitive to chemotherapies likewise have much lower Tbx3 expression relative to chemotherapy-resistant cell lines (Fig. S6 C and D). Furthermore, ERα expression levels were strongly correlated with FGFR3 expression in a majority of primary tumor samples (P = 0.001, Fig. S5). These data are compatible with the notion that the E2/FGF/Tbx3 signaling axis is activated in many primary breast cancers.
Discussion
Here, we identify the estrogen/FGF/Tbx3 signaling axis as an important modulator of CSC properties both in vitro and in vivo. While much of our data were collected using the experimental system of the ERα+ MCF7 cell line, we were able to observe up-regulation of Tbx3 in many different primary human tumor datasets, suggesting the relevance of this pathway in primary tumor samples. In addition, we observed conservation of the FGF/FGFR3/Tbx3 signaling pathway in basal-type ERα− cell lines, as well in freshly dissociated patient tissue, indicating that this pathway may be important for growth of many subtypes of breast cancers other than the common ERα+ subtype.
The experiments described here also demonstrate that the regulation of breast CSCs are influenced by the same regulatory pathways that control stem cells in the developing mammary gland. Although the underlying basis for this connection is unclear, several other groups have observed a conservation or re-expression of developmental signaling programs in cancers and cancer stem cells (20–22). Based on the results described here, we propose a model in which Tbx3 expressing cancer cells promote the expansion of CSCs through paracrine FGF signaling (Fig. S7). In ERα+ breast cancer cell lines, estrogen (E2) binds to the estrogen receptor to induce FGF9 secretion and Tbx3 expression in the non-CSC compartment. Expression of Tbx3 leads to further expression of Wnts and FGFs to perpetuate signaling, which ultimately leads to expansion of the CSC pool. In breast cancers that do not express ERα, Tbx3 expression stabilizes paracrine FGF and Wnt signaling to regulate cancer stem cell (CSC) subpopulations. This model is consistent with prior work showing that Wnt signaling is necessary for maintenance of subpopulations with stem-like properties in normal mammary tissues and breast cancer (23). Clearly, further studies will be required to determine whether Tbx3 expression is induced by Wnt signaling in cancer and whether inhibition of this pathway will have a clinical impact.
The experiments described here demonstrate that estrogen can also influence the representation of breast CSCs within cancer cell populations, in part through its effects on the extracellular signaling milieu. Similar observations have been made in the normal mouse mammary gland, in which epithelial stem cell function is controlled in part through RANKL and Wnt4, which are secreted in response to both estrogen and progesterone (24, 25).
The experiments described here as well as in other studies have demonstrated that normal and cancer breast stem cell pools lack abundant ERα expression (24–26, 12). This suggests that the successes of tamoxifen and aromatase inhibitors, such as letrozole, for the treatment estrogen-sensitive breast tumors may be attributed to the inhibition of paracrine factors released by ERα+ breast cancer cells but not to the eradication of ER-negative CSCs. Indeed, residual breast cancer cells in tumor tissues treated with letrozole exhibited a pronounced enrichment of cells exhibiting CSC phenotypes (27). This observation, combined with our findings, suggests that resistance to antiestrogen therapies and recurrence of ERα+ breast cancers could arise from genetic or epigenetic alterations that allow for the acquisition of FGF/Tbx3 activity in the absence of continued estrogen stimulation. In support of this notion, tamoxifen resistance by breast cancer cells is accompanied by increases in DUSP6 expression (28), as well as mesenchymal transdifferentiation (29). Furthermore, studies have shown that overexpression of FGF ligands subverts the requirement for estrogen to drive tumor formation (30–32).
Although our experiments focused on breast cancer CSC expansion stimulated by FGF9, we found that other FGF ligands are also capable of influencing CSC numbers. We did not address here whether other FGFRs in addition to FGFR3 can contribute to breast CSC expansion. Nevertheless, an important prediction of our model is that the acquisition of resistance to anti-hormone therapies might be accompanied by an increase in FGF/FGFR/Tbx3 signaling and a concomitant increase in the proportion of CSCs. Therefore, targeting the FGF/FGFR/Tbx3 pathway may be a useful therapeutic strategy for hormone-therapy refractory luminal (ERα+) breast cancers.
Materials and Methods
Detailed methods are described in SI Materials and Methods.
Cells and Tissue Culture.
Cell line procurement and culture is described in SI Materials and Methods.
All human breast tissue was obtained in compliance with the laws and institutional guidelines, as approved by the institutional institutional review board committee from Tufts University School of Medicine. An ER+, Her2− tumor was obtained from discarded material, and noncancerous breast tissue was obtained from patients undergoing elective reduction mammoplasty at Tufts Medical Center. Cells were manipulated as described in SI Materials and Methods.
Flow Cytometry.
Antibodies used are EpCAM (ESA)-FITC (clone VU-ID9, AbD Serotec), CD24-PE (clone ML5, BD Pharmingen), and CD44-APC (clone G44-26, BD Pharmingen). When staining for ERα-FITC (clone SP1, Abcam) cells were stained sequentially with EpCAM (clone VU- ID9, Abcam), rat–anti-mouse PerCP (BD Pharmingen) and CD24-PE/CD44-APC (BD Pharmingen) before cells were fixed in 4% paraformaldehyde and 0.1% Saponin and incubated with ERα-FITC.
Tumorsphere Assays.
Cells were trypsinized and mechanically separated and, when necessary, passed through 40-μm filters to obtain single cell suspensions that were plated at less than 10,000 cells per mL in super–low-attachment plates in normal growth media (with supplements where indicated). Quantification of mammosphere and tumorsphere numbers was accomplished using a Multisizer 3 Coulter Counter (Beckman-Coulter) that provided number and size distributions of particles between 40 μm and 336 μm.
Conditioned Medium Experiments.
Subconfluent MCF7 cultures grown in standard phenol red containing DMEM with 10% FBS were washed and switched to phenol-red–free DMEM + 10% charcoal-dextran stripped FBS supplemented with 1 nM 17-β-estradiol or EtOH for 6 d. Cultures were then washed five times with PBS and incubated with fresh serum-free phenol-red–free DMEM. Conditioned medium (CM) was harvested 72 h later, passed through a 0.2-μm filter, and frozen at −80°C. For each experiment, at least three distinct batches of CM were combined and supplemented with 2 mM l-glutamine and 10% charcoal-dextran–stripped FBS and fed to naive cells for a total of 6 d, with media changed every 2 d, after boiling for 5 min where specified.
Western Blot and Immunofluorescence.
Antibodies used for IF were ERα-FITC (clone SP1, Abcam), EpCAM (clone B29.1, Abcam), and Tbx3 (rabbit, Aviva). Antibodies used for Western blotting were Tbx3 (mouse, Abcam), FGFR3 (rabbit, Sigma), and β-actin (clone mAbcam 8226, Abcam).
Isolation of RNA, Microarray, and Quantitative RT-PCR.
Cells were harvested by trypsinization of fluorescence-activated cell sorting and pelleted by centrifugation, and RNA isolation was performed using the RNAeasy kit (Qiagen) in accordance with the manufacturer's protocol. The RNA samples were then reverse transcribed using the iScript cDNA kit (Bio-Rad), and quantitative PCR was performed with Sybr green (Bio-Rad) on a Bio- Rad iCycler. Primers are listed in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Ina Klebba and Dr. Lisa Arendt for surgical assistance and maintenance of the animal colony. We thank Allen Parmelee and Steve Kwok for expert technical assistance with cell sorting. We thank Josh LaBaer at Harvard Medical School (Boston, MA) for generously providing us with human Tbx3 cDNA. This work was supported grants from the American Cancer Society–New England Division–Broadway on Beachside Postdoctoral Fellowship PF-08-101-01-CSM (to P.J.K.), Breast Cancer Research Foundation (to C.K. and C.M.F.), Raymond and Beverly Sackler Foundation (to C.K.), and National Institutes of Health/National Cancer Institute Grant R01CA125554 (to C.K.). C.K. is a Raymond and Beverly Sackler Foundation Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.M.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1007863107/-/DCSupplemental.
References
- 1.Clarke MF, et al. Cancer stem cells—Perspectives on current status and future directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 2.Al-Hajj M, Wicha MS, Ito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginestier C, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponti D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 5.Yu F, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–1123. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 6.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008;10:1–13. doi: 10.1186/bcr1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson GW. Cooperation of signalling pathways in embryonic mammary gland development. Nat Rev Genet. 2007;8:963–972. doi: 10.1038/nrg2227. [DOI] [PubMed] [Google Scholar]
- 9.Rowley M, Grothey E, Couch FJ. The role of Tbx2 and Tbx3 in mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2004;9:109–118. doi: 10.1023/B:JOMG.0000037156.64331.3f. [DOI] [PubMed] [Google Scholar]
- 10.Eblaghie MC, et al. Interactions between FGF and Wnt signals and Tbx3 gene expression in mammary gland initiation in mouse embryos. J Anat. 2004;205:1–13. doi: 10.1111/j.0021-8782.2004.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dillon C, Spencer-Dene B, Dickson C. A crucial role for fibroblast growth factor signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:207–215. doi: 10.1023/B:JOMG.0000037163.56461.1e. [DOI] [PubMed] [Google Scholar]
- 12.Coleman-Krnacik S, Rosen JM. Differential temporal and spatial gene expression of fibroblast growth factor family members during mouse mammary gland development. Mol Endocrinol. 1994;8:218–229. doi: 10.1210/mend.8.2.8170478. [DOI] [PubMed] [Google Scholar]
- 13.Rosen JM, Humphreys R, Krnacik S, Juo P, Raught B. The regulation of mammary gland development by hormones, growth factors, and oncogenes. Prog Clin Biol Res. 1994;387:95–111. [PubMed] [Google Scholar]
- 14.Brisken C, Duss S. Stem cells and the stem cell niche in the breast: An integrated hormonal and developmental perspective. Stem Cell Rev. 2007;3:147–156. doi: 10.1007/s12015-007-0019-1. [DOI] [PubMed] [Google Scholar]
- 15.Mallepell S, Krust A, Chambon P, Brisken C. Paracrine signaling through the epithelial estrogen receptor alpha is required for proliferation and morphogenesis in the mammary gland. Proc Natl Acad Sci USA. 2006;103:2196–2201. doi: 10.1073/pnas.0510974103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noel A, Simon N, Raus J, Foidart JM. Basement membrane components (matrigel) promote the tumorigenicity of human breast adenocarcinoma MCF7 cells and provide an in vivo model to assess the responsiveness of cells to estrogen. Biochem Pharmacol. 1992;43:1263–1267. doi: 10.1016/0006-2952(92)90501-9. [DOI] [PubMed] [Google Scholar]
- 17.Wing LY, Chuang PC, Wu MH, Chen HM, Tsai SJ. Expression and mitogenic effect of fibroblast growth factor-9 in human endometriotic implant is regulated by aberrant production of estrogen. J Clin Endocrinol Metab. 2003;88:5547–5554. doi: 10.1210/jc.2003-030597. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes DR, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yarosh W, et al. TBX3 is overexpressed in breast cancer and represses p14 ARF by interacting with histone deacetylases. Cancer Res. 2008;68:693–699. doi: 10.1158/0008-5472.CAN-07-5012. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Porath I, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassan KA, Chen G, Kalemkerian GP, Wicha MS, Beer DG. An embryonic stem cell-like signature identifies poorly differentiated lung adenocarcinoma but not squamous cell carcinoma. Clin Cancer Res. 2009;15:6386–6390. doi: 10.1158/1078-0432.CCR-09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somervaille TC, et al. Hierarchical maintenance of MLL myeloid leukemia stem cells employs a transcriptional program shared with embryonic rather than adult stem cells. Cell Stem Cell. 2009;4:129–140. doi: 10.1016/j.stem.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiMeo TA, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asselin-Labat ML, et al. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 25.Joshi PA, et al. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz KB, Dye WW, Harrell JC, Kabos P, Sartorius CA. Rare steroid receptor-negative basal-like tumorigenic cells in luminal subtype human breast cancer xenografts. Proc Natl Acad Sci USA. 2008;105:5774–5779. doi: 10.1073/pnas.0706216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Creighton CJ, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Y, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: A mechanism of tamoxifen resistance. Cancer Res. 2006;66:5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiscox S, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 30.McLeskey SW, et al. Fibroblast growth factor 4 transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res. 1993;53:2168–2177. [PubMed] [Google Scholar]
- 31.Zhang L, et al. MCF-7 breast carcinoma cells overexpressing FGF-1 form vascularized, metastatic tumors in ovariectomized or tamoxifen-treated nude mice. Oncogene. 1997;15:2093–2108. doi: 10.1038/sj.onc.1201386. [DOI] [PubMed] [Google Scholar]
- 32.Hajitou A, et al. Progression in MCF-7 breast cancer cell tumorigenicity: Compared effect of FGF-3 and FGF-4. Breast Cancer Res Treat. 2000;60:15–28. doi: 10.1023/a:1006302602261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.