Abstract
Background
Nanotechnology and engineered nanomaterials (ENM) are here to stay. Recent evidence suggests that exposure to environmental particulate matter exacerbates symptoms of asthma. In the present study we investigated the modulatory effects of titanium dioxide particle exposure in an experimental allergic asthma.
Methods
Nonallergic (healthy) and ovalbumin-sensitized (asthmatic) mice were exposed via inhalation to two different sizes of titanium dioxide particles, nanosized (nTiO2) and fine (fTiO2), for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3. Different endpoints were analysed to evaluate the immunological status of the mice.
Results
Healthy mice elicited pulmonary neutrophilia accompanied by significantly increased chemokine CXCL5 expression when exposed to nTiO2. Surprisingly, allergic pulmonary inflammation was dramatically suppressed in asthmatic mice which were exposed to nTiO2 or fTiO2 particles - i.e. the levels of leucocytes, cytokines, chemokines and antibodies characteristic to allergic asthma were substantially decreased.
Conclusions
Our results suggest that repeated airway exposure to TiO2 particles modulates the airway inflammation depending on the immunological status of the exposed mice.
Background
The exploding market of nanobased products and nanotechnology as a whole have put the health professionals and regulatory authorities at an alert. There is already growing evidence on the potential adverse health effects on healthy individuals, but only part of the world's population can be categorized into this group. A large part of the population has impaired health conditions that will make them more susceptible to develop health problems from particulate exposure.
In industrialized countries asthma and allergies are increasingly prevalent. According to the European Academy of Allergy and Clinical Immunology (EAACI) one in three children today is allergic and 30-50% of them will develop asthma. It is estimated that by year 2015 half of all Europeans may be suffering from allergy [1]. Asthma is a product of both genetic predisposition and environmental conditions. Children in wealthy countries are more likely to develop allergy-related asthma than children in poorer nations [2]. Hygiene hypothesis suggests that lack of intense infections due to improved hygiene, vaccination and antibiotics has altered the immune system to improperly respond to neutral substances [3]. Approximately 80% of asthma cases today are caused by allergies. Evidence already exists that environmental particulate matter, such as air pollutants and diesel exhaust particles, enhances airway hyperresponsiveness and exacerbation of asthma as well as increases respiratory and cardiovascular mortality and morbidity [4-6]. The most susceptible population groups for these adverse health effects include elderly subjects with chronic cardiorespiratory disease, as well as children and asthmatic subjects of all ages.
Nanosized and larger particles of titanium dioxide (TiO2) are widely used in many fields of science and technology. According to the IARC [7] titanium dioxide accounts for 70% of the total production volume of pigments worldwide and is classified as possibly carcinogenic to human beings (ie, group 2B). TiO2 is used in various applications such as paints, coatings, UV protection, photocatalysis, sensing and electrochromics, photochromics as well as food colouring [8]. Brightness and high refractive index are properties that have made TiO2 the most widely used white pigment. Other properties of TiO2 include chemical stability, low toxicity and cheap price. Plain TiO2 nanoparticles are often altered to better and more specifically suit their uses. Alterations can be made by doping TiO2 with other elements or by modifying the surface with other semiconductor materials. TiO2 mostly occurs as rutile, anatase or brookite chrystalline polymorphs.
In the present study we explored the effects of repeated inhalation exposure in asthmatic and healthy mice to two different sizes of TiO2. We demonstrate that contrary to expectations exposure to fine or nanosized particles inhibits most soluble and cellular mediators of allergic asthma. The present study emphasizes that it is crucial to take into account the heterogeneity of the state of health of individuals in assessing health implications of nanoparticle exposure in humans.
Materials and methods
Test materials
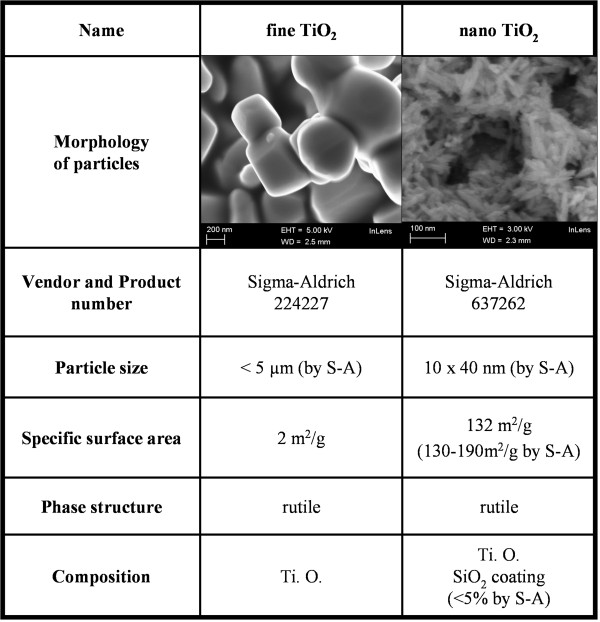
Two different types of titanium dioxide (TiO2) particles were used in our experiments. The other TiO2 was nanosized and the other coarser fine-sized. The fine rutile particle (fTiO2; product number 224227, Sigma-Aldrich, Steinheim, Germany) had an initial particle size of under 5 μm and nanosized rutile (nTiO2; product number 637262, Sigma-Aldrich) was silica coated, needle-like and ca. 10 × 40 nm in size. Both materials were thoroughly characterized before and during exposures (Figure 1, [9]). The size and morphology of the nanopowders were characterized by electron microscopy (Zeiss ULTRAplus FEG-SEM, Carl Zeiss NTS GmbH, Oberkochen, Germany and JEM 2010 TEM, Jeol Ltd., Tokyo, Japan) and their composition by energy dispersive spectroscopy (EDS; ThermoNoran Vantage, Thermo Scientific, Breda, The Netherlands). The crystallinity and phase composition of the nanopowders were characterized by powder x-ray diffraction analysis (Siemens D-500, Siemens AG, Kahrlruhe, Germany) and specific surface area of nanopowders was measured by nitrogen adsorption, using the Brunauer-Emmett-Teller method (Coulter Omnisorp 100CX, Florida, USA).
Figure 1.
Particle characteristics. Particles used in this study are listed in this table. All characteristics are measured by us as described in the materials and methods section unless stated otherwise in the table. Pictures of nanoparticles are taken using scanning electron microscopy[9].
Exposure system for aerosolized particles
The animals were exposed using a solid particle dispenser (Rotating Brush Generator, RBG 1000, Palas, Karlsruhe, Germany) as described earlier by Rossi et al. (2010).
Aerosol and particle characterization
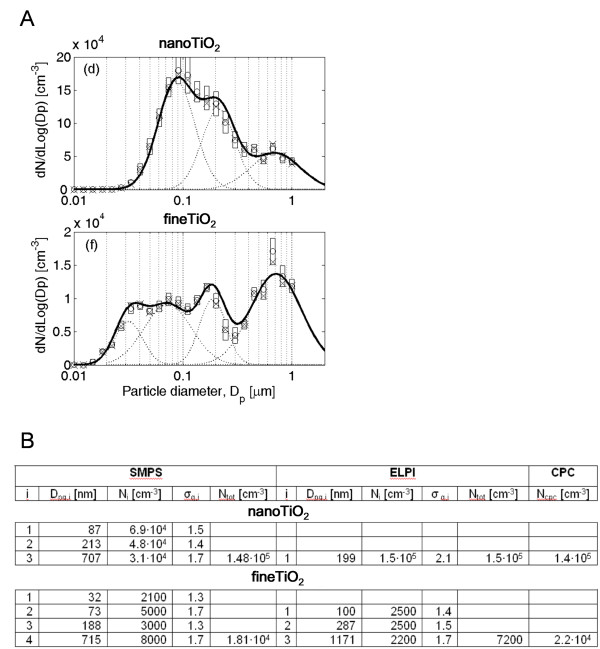
The aerosol size distributions were measured from 10 nm to 1000 nm with a scanning mobility particle sizer (SMPS) consisting of a 63Ni bipolar aerosol neutralizer, a Vienna type differential mobility analyzer (DMA) with length of 28 cm and TSI model 3010 condensation particle counter (CPC) [10]. Aerosol aerodynamic size distribution was measured from 15.9 nm to 10 μm with an electronic low pressure impactor (ELPI, Dekati) (Figure 2) [11]. The aerosol mass concentration was determined gravimetrically after collection on nitrocellulose filters (Millipore). Particle concentration inside the particle dispenser chamber was also monitored using a personal DataRAM (online mass concentration meter, pDR1000AN, Waltham, MA, USA).
Figure 2.
Particle and aerosol characteristics. A) Particle mobility and aerodynamic number size distributions measured with SMPS. Size bin concentrations are represented as mean (o), median (x) and standard deviation (box). Solid line represents mean of multi log-normal function and dotted lines are means of single modes. Measurements and fitting were done during steady-state condition. B) Multi log-normal fit parameters for Fig 2A. particle size distributions and mean particle concentration measured by CPC [32]. Dpg, i, Ni and σg, i are geometric mean diameter, particle concentration and geometric standard deviation of mode i, respectively, and Ntot is total particle concentration of size distribution.
Animals - sensitization and exposure protocol
Following a 1-week acclimation period, 7-week-old female BALB/c/Sca mice (Scanbur AB, Sollentuna, Sweden) were randomized into two exposure and control groups (8 mice/group). Mice were sensitized intraperitonally with 20 μg of ovalbumin (OVA) in alum (Sigma-Aldrich, St Louis, MO) in 100 μl of phosphate-buffered saline (PBS) on days 1 and 14 of the experiment. Control group was given alum in 100 μl of PBS. Exposure groups were exposed three times a week for 2 hours for the duration of the four week experiment. The exposure concentration was 10 ± 2 mg/m3 in all tests. This concentration was chosen to mimic occupational conditions where workers are exposed to concentrations of around 5 mg/m3 [12]. On days 25-27 all mice were challenged with 1% OVA solution via the airways for 20 min administered using the ultrasonic nebulizer (DeVilbiss, Glendale Heights, IL).
Measurement of airway responsiveness
Airway responsiveness was measured on day 28 using a single chamber, whole-body plethysmograph system (Buxco, Troy, NY) as described earlier [13]. Briefly, mice were exposed to increasing concentrations (1, 3, 10, 30 and 100 mg/ml) of metacholine (MCh; Sigma Aldrich) in PBS delivered via an AeroSonic 5000 D ultrasonic nebulizer (DeVilbiss, ITW, Glendale Heights, IL). Before MCh exposure the baseline is measured for three minutes. After baseline measurements the MCh is nebulized for 1.5 minutes and airway reactivity is measured for 5 minutes per concentration. Lung reactivity parameters were expressed as Penh (enhanced pause) values. After measurement of lung responsiveness, the mice were sacrificed using an overdose of isoflurane and samples were collected for analysis.
Collection of biological samples and preparation
Sample collection and preparation were done as described previously by Rossi et al. (2010). The following samples were collected: blood serum for antibody analysis, bronchoalveolar lavage (BAL) cells and supernatant for May Grünwald-Giemsa (MGG) staining and protein analysis and lung samples for RNA isolation and hematoxylin and eosin (H&E) and Periodic Acid-Schiff (PAS) staining. The spleens were also dissected from the mice onto 6-well plates with PBS for spleen cell stimulations.
Spleen cell stimulations
Spleens were mechanically broken down into paste and filtered to remove larger pieces. Cells were resuspended in RPMI media, counted under light microscopy and plated at a concentration of one million cells/ml. Cells to be stimulated were plated in RPMI containing 100 μg/ml ovalbumin. All cells were incubated for 48 hours after which the supernatant was collected and stored at -70°C prior to analysis.
mRNA and protein analyses
RNA isolation from the lung tissues
The lung samples were homogenized in a FastPrep FP120 (BIO 101, Thermo Savant, Waltham, Mass. USA) -machine and RNA was extracted using the FastRNA Pro Green Kit (Qbiogene/MP Biomedicals, Illkirch, France) and its instructions.
cDNA synthesis
cDNA was synthesized from 1 μg of total RNA in a 25 ul reaction using MultiScribe Reverse Transcriptase and random primers (The High-Capacity cDNA Archive Kit, Applied Biosystems, Foster City, CA) using the manufacturer's protocol. The synthesis was performed in a 2720 Thermal Cycler (Applied Biosystems, Carlsbad, California, USA) starting with 25°C for 10 minutes and continuing with 37°C for 120 minutes.
Polymerase chain reaction (PCR) amplification
PCR primers and probes were ordered as pre-developed assay reagents (18 S rRNA, TNFα, IL-1β, IL-4, IL-13, IL-10, foxp3, CCL3, CXCL5 and CXCL2) from Applied Biosystems (Carlsbad, California). The real-time quantitative PCR was performed as described previously by Rossi et al. (2010).
Enzyme-linked immunosorbent assay (ELISA)
TNF-α and IL-13 protein levels from spleen cell supernatants were determined using ELISA kits (eBioscience, San Diego, CA, US) used according to manufacturer's instructions. An ELISA plate microtiter reader (Multiskan MS, Labsystems, Helsinki, Finland) was used to read the results.
Luminex
For analysis of TNF-α and IL-13 proteins in BAL fluid supernatants we used a Milliplex Mouse Cytokine/Chemokine Immunoassay (Millipore Corporation, Billerica, MA) according to the manufacturers' protocol. 3% bovine serum albumin (BSA; Sigma-Aldrich, St Louis, MO) in PBS was added at a concentration of 0.5% to samples, controls and standards to ensure sufficient protein amounts for the assay. Assay was performed using Luminex xMAP Technology (Bio-Plex 200 System, BioRad, Hercules, CA).
Measurement of serum antibodies
For the analysis of OVA-specific IgE, ELISA plates were coated with Purified Rat Anti-mouse IgE antibody (BD Biosciences, San Jose, CA) and incubated overnight. On the second day the plates were blocked with 3% BSA in PBS, serum samples (1:10 and 1:20 dilution) were added to the plate and incubated overnight. Biotinylated OVA was added and streptavidin horseradish peroxidase (Pharmingen 13047E, BD Biosciences) followed by ABTS solution (ABTS Microwell Peroxidase Substrate System, KPL, Gaithersburg, MD). Absorption was read at 405 nm with ELISA plate absorbance reader (Multiskan MS, Labsystems, Helsinki, Finland).
For OVA-specific IgG2a analysis, ELISA plates were coated with ovalbumin (BD Biosciences) and incubated overnight. On the second day the plates were blocked with 3% BSA in PBS, serum samples (1:60, 1:180, 1:540 and 1:1620 dilution) were added to the plate and incubated overnight. Biotinylated anti-mouse IgG2a (Pharmingen 02012 D, BD Biosciences) was added and streptavidin horseradish peroxidase (BD Biosciences). ABTS solution and peroxidase were mixed 50:50 and added before reading absorption at 405 nm with ELISA plate absorbance reader.
Analysis of mucus producing cells
The lung tissue was analysed from formalin-fixed, paraffin-embedded sections. These sections were stained for mucus secreting goblet cells using Periodic acid-Schiff (PAS) -stain. The data was analyzed with Leica Image Manager IM50 version 4.0 (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK). PAS+ cells were counted as an average of PAS+ cells found in 100 μm of bronchus counted from three bronchioles of similar size per mouse (n = 8 mice per group).
Hydroxyl radical (•OH) formation capacity
The •OH formation capacity was determined using the benzoic acid probe as described earlier by Rossi et al. (2010).
Statistical analysis
The toxicological data was analyzed with the GraphPadPrism software (GraphPadPrism Software, Inc., San Diego, CA). For all statistical analysis we first performed analysis of variance using one-way ANOVA (nonparametric Kruskal-Walles test) and when the ANOVA was positive we performed post-testing. The different mice groups were compared using nonparametric Mann-Whitney U - test. P-values less than 0.05 were considered statistically significant.
Results
Inhalation exposure to TiO2 particles reduces leukocyte numbers and mucus secretion in the airways of asthmatic mice
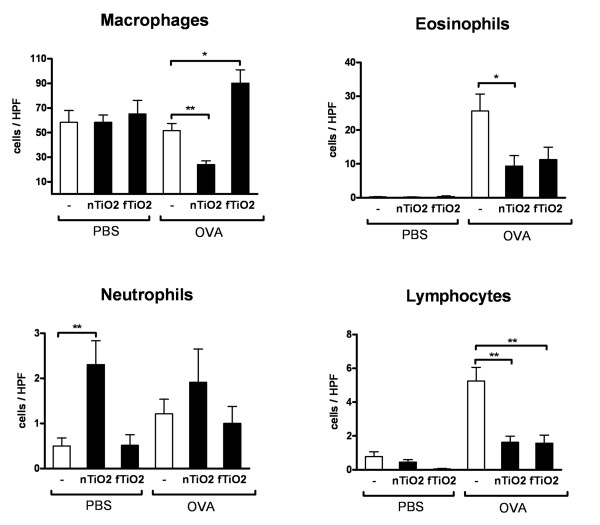
Asthmatic mice demonstrate increased numbers of eosinophils and lymphocytes in the airways compared to healthy controls. In healthy mice (PBS) there was a significant 4.6-fold increase in the influx of neutrophils following exposure to nTiO2 (Figure 3) whereas the numbers of macrophages, eosinophils and lymphocytes remained unaffected. Exposure to fTiO2 had no effects on any cells in the BAL of healthy mice. Surprisingly, the numbers of eosinophils and lymphocytes, characteristic features of allergic inflammation, were dramatically reduced in asthmatic mice (OVA) after exposure to both nTiO2 and fTiO2. Macrophages were reduced in half following nTiO2 and increased 1.7-fold following fTiO2 exposure in asthmatic mice. Changes in numbers of pulmonary neutrophils remained nonsignificant.
Figure 3.
Cell count in bronchoalveolar lavage fluid. The effect of titanium dioxide (TiO2) inhalation exposure performed for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 on macrophage, eosinophil, neutrophil and lymphocyte infiltration to bronchoalveolar lavage fluid (BALF) calculated from May-Grünwald-Giemsa (MGG)-stained cytospin slides with light microscopy (x80). Results are shown as cells per high power field (HPF) for healthy (PBS) and allergic (OVA) mice exposed to nanosized (nTiO2) or fine (fTiO2) TiO2 or left unexposed (-). From each slide the cells were counted from three fields from which an average was counted. N = 8 mice per group. The bars represent mean ± SEM; * P < 0.05 and ** P < 0.01 significantly different from unexposed control; Mann-Whitney U test.
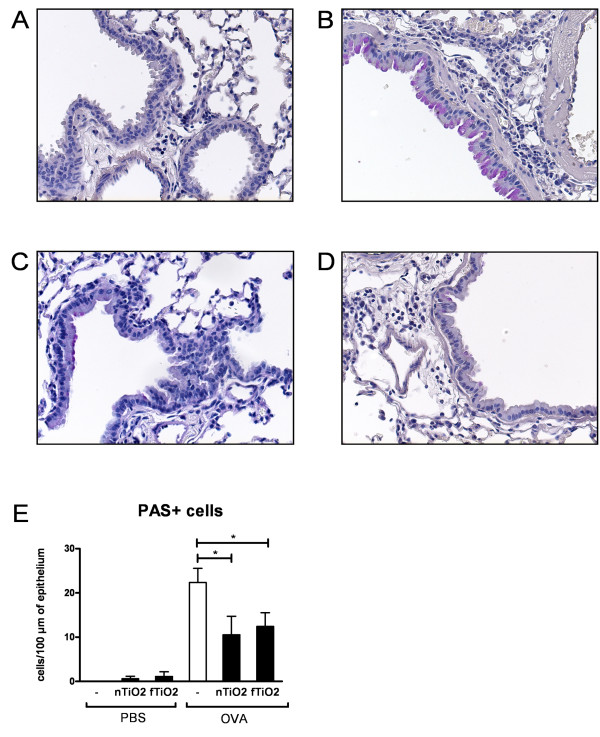
Goblet cell hyperplasia in the lungs is a common feature of allergic asthma and mucus secreting PAS+ goblet cells are usually not present in bronchioles and smaller conducting airways of mice [14]. Healthy mice showed clear lungs with no mucus secreting cells (Figure 4A) whereas PAS+ cells were abundant in the bronchial epithelium of allergic mice (Figure 4B). Both TiO2 particles caused a drastic reduction in the numbers of PAS+ cells in the allergic mice (Figure 4C nTiO2 and D fTiO2). Both exposed groups showed a statistically significant decrease in goblet cell numbers in the epithelium (Figure 4E) after the particle exposure.
Figure 4.
Occurence of mucus producing cells in bronchioles. Periodic acid-Schiff (PAS) -stained mouse lung tissue, where mucus producing goblet cells can be seen in colour red around the bronchioles. Tissue samples from unexposed healthy (A) and asthmatic (B) mice and asthmatic mice exposed to nanosized (C; nTiO2) or fine (D; fTiO2) TiO2. (E) Effects of titanium dioxide exposure on the amount of mucus-producing cells around the bronchioles. Results are indicated as the average of PAS+ cells/100 μm of bronchus counted from three bronchioles per mouse (n = 8 mice per group) for healthy (PBS) and allergic (OVA) mice exposed to nanosized (nTiO2) or fine (fTiO2) TiO2 or left unexposed (-). N = 8 mice per group. The bars represent mean ± SEM; * P < 0.05 significantly different from unexposed control; Mann-Whitney U test.
Airway reactivity to inhaled metacholine is significantly affected by particle exposure
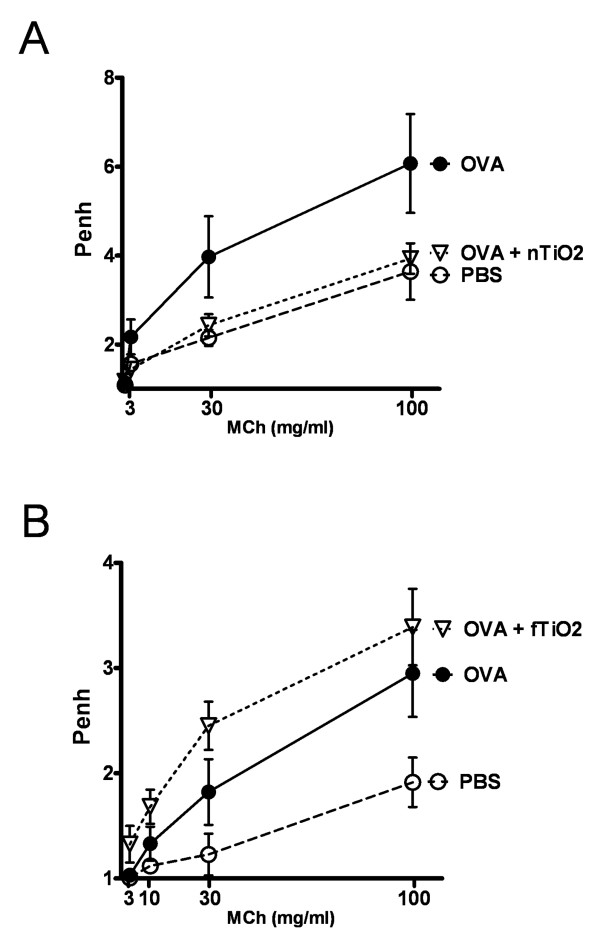
Airway hyperresponsiveness, a hallmark of asthma, was measured by airway reactivity to increased doses of inhaled metacholine (Mch). Exposure of OVA sensitized and challenged mice to nTiO2 (Figure 5A) reduced airway hyperreactivity (AHR) to the level of healthy mice. On the contrary, exposure to fTiO2 (Figure 5B) slightly increased the reactivity of the lungs, showing modest exacerbation of asthmatic symptoms. We also exposed PBS groups to TiO2 particles (data not shown) but no difference on airway reactivity to inhaled metacholine was found between them and unexposed PBS groups.
Figure 5.
Airway responsiveness to metacholine. Effect of airway responsiveness to metacholine (MCh) in allergic mice after exposure to nanosized (A; nTiO2) or fine (B; fTiO2) TiO2 for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 (n = 8 mice per group). Results are shown as enhanced pause (Penh) values, dimensionless values that we used to empirically monitor airway function, in relation to increasing doses (1-100 mg/ml) of aerosolized MCh for healthy (PBS) and allergic (OVA) mice.
Particle exposure results in a dramatic inhibition of proinflammatory, regulatory and Th2 cytokines in the lungs of asthmatic mice
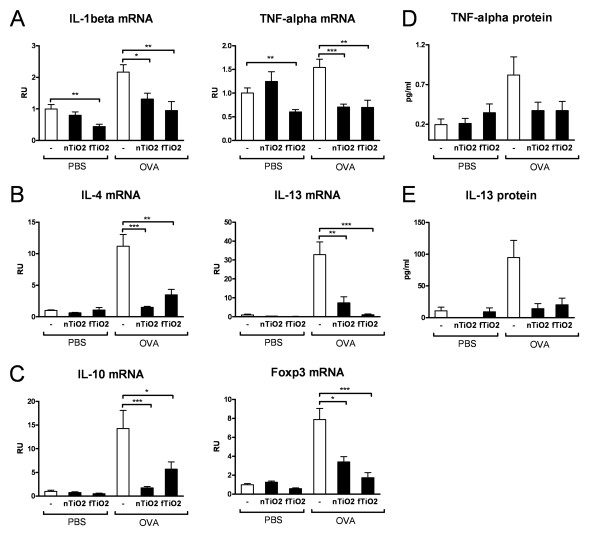
The mRNA expression of proinflammatory cytokines, IL-1β and TNF-α (Figure 6A), was downregulated after particle exposure to about half and third respectively in asthmatic mice when compared to the nonexposed control. IL-1β and TNF-α levels were also reduced in half in healthy (PBS) mice following exposure to fTiO2. Similarly, Th2 type cytokines IL-4 and IL-13 (Figure 6B), which are present in asthmatic but not in healthy mice, were significantly diminished after exposure to both nTiO2 and fTiO2. To examine effects of particle exposure on the regulatory machinery we measured mRNA expression of major suppressive cytokine IL-10 and Foxp3, a marker of regulatory T-cells. fTiO2 exposure reduced IL-10 levels by 2.5-fold and Foxp3 levels by 4.5-fold. nTiO2 exposure again reduced IL-10 levels by over 8-fold and Foxp3 levels by 2.3-fold (Figure 6C). We also looked at if suppression in cytokine expression could be seen at the protein level. Reduction in the protein levels of both TNF-α (Figure 6D) and IL-13 (Figure 6D) in the BAL from asthmatic mice exposed to TiO2 particles was observed with statistical significance (one-way ANOVA) in IL-13 levels.
Figure 6.
mRNA and protein expression of cytokines. Relative mRNA-expression in the lung tissue of (A) proinflammatory cytokines, IL-1β and TNF-α, (B) Th2 type cytokines IL-4 and IL-13, (C) regulatory cytokine IL-10 and marker of regulatory T-cells Foxp3. Protein levels (pg/ml) of proinflammatory cytokine TNF-α (D) and Th2-type cytokine IL-13 (E) in BAL supernatant of mice. Results are shown in relative units (RU) or (pg/ml) for healthy (PBS) and allergic (OVA) mice exposed for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 to nanosized (nTiO2) or fine (fTiO2) TiO2 or left unexposed (-). N = 8 mice per group. The bars represent mean ± SEM; * P < 0.05, ** P < 0.01, and *** P < 0.001 significantly different from unexposed control; Mann-Whitney U test.
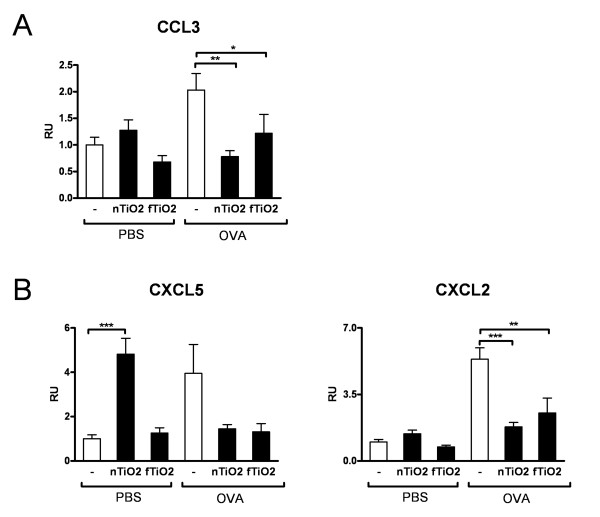
The same suppression phenomenon that was seen in cytokines could be seen with all chemokines examined. mRNA expression of proinflammatory CCL3 (Figure 7A) and neutrophil attracting CXCL5 and CXCL2 (Figure 7B) was decreased in asthmatic mice after exposure to both used particles. In healthy mice exposure to nTiO2 caused an almost 5-fold elevation of CXCL5 levels, corresponding to high levels of neutrophils seen in the BALF of the same mice.
Figure 7.
mRNA expression of chemokines. mRNA expression of proinflammatory chemokine CCL3 (A) and neutrophil attracting chemokines CXCL5 and CXCL2 (B) in the lung tissue of mice. Results are shown in relative units (RU) for healthy (PBS) and allergic (OVA) mice exposed for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 to nanosized (nTiO2) or fine (fTiO2) TiO2 or left unexposed (-). N = 8 mice per group. The bars represent mean ± SEM; * P < 0.05, ** P < 0.01, and *** P < 0.001 significantly different from unexposed control; Mann-Whitney U test.
Inhibition of cytokine production in OVA-stimulated spleen cells and reduction in OVA specific IgE levels suggests systemic immune suppression following particle exposure in asthmatic mice
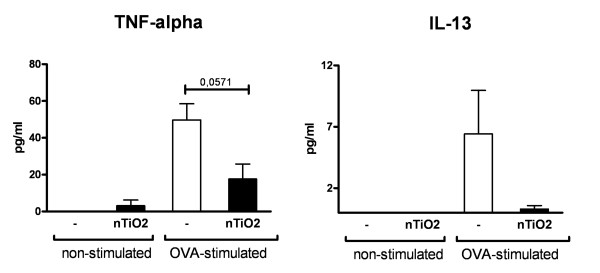
To evaluate possible systemic effects of particle exposure we also examined spleen cells from asthmatic nTiO2 exposed mice. Spleen cells were either stimulated with OVA or left untreated. OVA stimulated cells express TNF-α and IL-13 as expected, but when the mice had been exposed to nTiO2 the protein levels were reduced amply (Figure 8). These results suggest that there is indeed a systemic response that can be shown from the spleen cells.
Figure 8.
Protein levels of plated spleen cells. Protein levels (pg/ml) of TNF-α and IL-13 in the supernatant of plated spleen cells extracted from allergic mice exposed to nTiO2 for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 or left unexposed (-). Cells were either stimulated with OVA or left untreated. N = 8 mice per group. The bars represent mean ± SEM; * P < 0.05 significantly different from unexposed control; Mann-Whitney U test.
Increased levels of allergen specific IgE antibodies are characteristic feature of allergic asthma. To investigate effects of particle exposure on circulating antibodies we examined the allergen specific IgE and IgG2a levels. OVA sensitized mice show high levels of OVA specific IgE, but exposure to fTiO2 reduces the levels of OVA specific IgE significantly (Figure 9A). On the contrary, no significant changes in the levels of OVA specific IgE could be seen after exposure to nTiO2 particles (Figure 9A). Levels of OVA specific IgG2a were low and unaffected by particle exposure (Figure 9B).
Figure 9.
Expression of antibodies. Levels of ovalbumin (OVA) specific antibodies IgE (A) and IgG2a (B). Results are shown in relative units (RU) for healthy (PBS) and allergic (OVA) mice exposed for 2 hours a day, three days a week, for four weeks at a concentration of 10 mg/m3 to nanosized (nTiO2) or fine (fTiO2) TiO2 or left unexposed (-). N = 8 mice per group. The bars represent mean ± SEM; ** P < 0.01 significantly different from unexposed control; Mann-Whitney U test.
Characteristics of nanoparticles
Initial particle sizes of particles used in this study are for fTiO2 < 5 μm and for nTiO2 10 · 40 nm as given by the vendor. Aerosolized particles in our experiment occured as agglomerates. Specific surface area of nTiO2 is 132 m2/g while it's only 2 m2/g for fTiO2 (Figure 1). The aerodynamic number size distributions indicate that nTiO2 dispersed in air using the solid particle dispenser occurred mostly as agglomerates of 100 nm at a number concentration of 69 000 cm-3 whereas fTiO2 occurred as agglomerates of 1 μm at a number concentration of 8000 cm-3 (Figure 2A and 2B). fTiO2 distribution consist mainly of agglomerates of 0.1 - 2 μm as measured using ELPI. It can be concluded that in particle number concentration most fTiO2 are below 1 μm in size but particle mass concentration relies on over 1 μm particles. Analysis of the hydroxyl radical formation capacity using the benzoic acid probe showed that neither of the used particles produced •OH-radicals in a systematic dose-response pattern [9].
Discussion
We reported recently that repeated inhalation exposure to silica coated rutile titanium dioxide nanoparticles (nTiO2) induces pulmonary neutrophilia accompanied by expression of relevant cytokines and chemokines in healthy mice [9]. Out of five different TiO2 particles this was the only one that was slightly toxic. The purpose of the present study was to examine immunomodulatory effects of inhalation exposure to different sized particles, nTiO2 and fine TiO2 (fTiO2) in OVA-sensitized, allergic mice. Interestingly, inhalation exposure to both nanosized and fine TiO2 caused local and systemic inhibition of several features of experimental asthma. Our results indicate that particle exposure can modulate airway inflammation and airway hyperreactivity in quite distinct ways depending on the immune status of the animals.
Present results obtained from healthy mice were in line with results from our previous study. Exposure to nTiO2 particles caused an influx of pulmonary neutrophils and an expression of neutrophil attracting chemokine CXCL5. When healthy mice inhaled fTiO2 the mRNA expression of two proinflammatory cytokines, TNF-α and IL-1β respectively, was suppressed. There have been a limited number of relevant studies done with inhalation and the results have been contradictory. Some studies have reported pulmonary neutrophilia after exposure to TiO2 nanoparticles whereas others have not [15-20]. Comparison of these ENM is difficult due to different or insufficient methods of particle characterisation. There are also few cases of inhalation exposure reported in humans reporting that inhalation of titanium dioxide may cause metal fume fever [21] or respiratory symptoms accompanied by reduction in pulmonary function [22].
Epidemiological studies have shown that underlying respiratory disease may critically affect the severity of the symptoms when exposed to particulate air pollution [4-6]. The most susceptible population groups for these adverse effects include especially asthmatic subjects of all ages. In the present study we examined whether the asthmatic mice were more susceptible to develop airway inflammation in response to exposure to TiO2 nanoparticles when compared to healthy mice. To our surprise exposure to fTiO2 and nTiO2 significantly downregulated Th2 type inflammation in allergic mice by preventing the infiltration of eosinophils and lymphocytes to the lungs, inhibiting the expression of Th2 cytokines in the BAL as well as decreasing the numbers of mucus producing goblet cells in the airway epithelium. To find out whether the observed suppression was also a systemic phenomenon we stimulated spleen cells from the asthmatic mice with OVA. Stimulated cells derived from asthmatic mice readily expressed TNF-α and IL-13 protein, but cells from asthmatic mice that had been exposed to nTiO2 lacked expression of these proteins suggesting that suppression was indeed a systemic effect.
Increased airway hyperreactivity (AHR) to inhaled methacholine (MCh) is one of the hallmarks of allergic asthma. It was of interest that different size TiO2 particles acted differently on AHR in the present study. Exposure to nTiO2 reduced OVA induced AHR to the baseline level of non-sensitized mice whereas exposure to fTiO2 did not have any suppressive effect - actually fTiO2 exposed asthmatic mice exhibited slightly higher levels of AHR compared to asthmatic mice without fTiO2 exposure. Since IL-13 is known to be closely involved in the development of AHR [23] it can be speculated that reduction of AHR seen in nTiO2 exposed mice may at least partly be explained by diminished levels of IL-13 in the airways. On the other hand, fTiO2 exposed mice also demonstrated reduced levels of IL-13 mRNA and protein in the airways but their AHR was not suppressed. Thus, yet unidentified factors regulating AHR are likely to play an important role in the modulation of AHR responses during TiO2 particle exposure. Th2 cytokines IL-13 and IL-4 also control mucus production and their suppression may explain the clear-cut reduction of goblet cells in the airway epithelia. It is worth mentioning that some differences in the absolute values of Penh were observed in PBS and OVA groups between the two experiments (i.e. Figure 5A and 5B). We want to emphasize that although the scale of Penh values differed between the two experiments, the relative difference between PBS and OVA groups was almost identical. We can therefore quite reliably conclude that the particles studied exhibited different effects on airway reactivity in relation to PBS and OVA groups in these study settings.
Suppression of inflammation similar to the one seen in the present study has been previously reported in the context of exposure to soot and iron oxide [24], oak dust [25], cigarette smoke [26] and fullerenes [27]. Exposure of asthmatic mice to oak dust resulted in suppression of airway reactivity, IL-13, IFN-γ, CXCL5, CXCL2/3 and neutrophils [25]. Thacher et al. (2008) reported reduction in eosinophil numbers, PAS+ cells, IL-5, IL-4 and total IgE. Fullerene exposure caused inhibition of anaphylaxis [27]. In contrast to present findings Larsen et al. [28] reported that TiO2 nanoparticles exhibited a strong adjuvant effect on the development of Th2 dominant immune response in the ovalbumin allergic asthma model. The particles were delivered intraperitonally (IP), which is an unnatural exposure route to nanoparticles in real life. In our study we examined the effects of airway exposure to TiO2 particles, which better mimics the real-life exposure scenario. It is important to note that unlike the particles that people more often get exposed to, such as air pollutants and mold, the particles we studied were free of bioactive organic matter. Organic matter contains pathogen-associated molecular patterns that activate innate immunity and cause an enhanced response in allergic mice. In line with this we reported previously [29] that mold exposure together with allergen sensitization caused dramatically enhanced and qualitatively different pulmonary inflammation. The present results demonstrate that exposure to microbe free TiO2 particles may not increase Th2 associated airway inflammation and allergic airway hyperreactivity. It should be noted, however, that asthmatic symptoms are not always Th2 associated and therefore responses of exposure to TiO2 particles in patients with non-allergic asthma may differ from the present study.
It can be hypothesized that anti-inflammatory Th2 response caused by allergen sensitization may be suppressed by the competing proinflammatory response elicited by TiO2 exposure. On the other hand, it has been suggested that T-cell dysfunction results in systemic immune suppression in mice exposed to multiwalled carbon nanotubes [30]. Also in the case of cigarette smoke exposure, reduced T helper function was considered as one possible reason for the suppression of allergic symptoms. Furthermore, consensus exists that Foxp3+ regulatory T-cells are able to control the inflammation thus preventing overactive inflammatory process that harms hosts' own tissue. We therefore also investigated whether the exposure to TiO2 particles induces elevated levels of Foxp3, a marker of regulatory T-cells, as well as regulatory cytokine IL-10. Expression levels of Foxp3 and IL-10 were, however, significantly inhibited in asthmatic mice by the particle exposure excluding the possibility that suppression was mediated mainly via regulatory T-cells and regulatory cytokines.
Conclusions
Assessing risks associated with particle exposure is complicated. This study accentuates that attention has to be paid not only to various characteristics of particles but to various health conditions of people being exposed to them. An interesting study emphasizes these results where pregnancy of healthy mice enhanced lung inflammatory responses to otherwise inert TiO2 particles and caused increased allergic susceptibility in their offspring [31]. Very little is known about the physicochemical characteristics which are associated with harmful toxicological potential of nanoparticles. In the present study, the inflammatory potential was not associated with particle size. Even though results slightly varied between the two TiO2 particles examined the main mechanism induced by particle exposure in the context of allergic asthma was suppression of Th2 type inflammation. Further studies are needed to provide a better foundation for easier and more reliable assessment and management of risks of nanosized and larger particles.
Abbreviations
ABTS: 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid); AHR: airway hyperreactivity; BAL: bronchoalveolar lavage; BSA: bovine serum albumin; cDNA: complementary DNA; CCL3: C-C motif chemokine 3; CPC: condensation particle counter; CXCL2: C-X-C motif ligand 2; CXCL5: C-X-C motif chemokine 5; DMA: differential mobility analyzer; EAACI: European Academy of Allergy and Clinical Immunology; EDS: energy dispersive spectroscopy; ELPI: electronic low pressure impactor; ENM: engineered nanomaterials; foxp3: forkhead box P3; fTiO2: fine titanium dioxide; H&E: hematoxylin and eosin; IARC: The International Agency for Research on Cancer; IgE: immunoglobulin E; IgG2a: immunoglobulin G, subclass 2a; IL-1β: interleukin 1 beta; IL-4: interleukin 4; IL-10: interleukin 10; IL-13: interleukin 13; MCh: metacholine; MGG: May Grünwald-Giemsa; nm: nanometer; nTiO2: nanosized titanium dioxide; OH: hydroxyl radical; OVA: ovalbumin; PAS: Periodic Acid-Schiff; PBS: phosphate-buffered saline; PCR: Polymerase chain reaction; Penh: enhanced pause; RPMI: Roswell Park Memorial Institute medium; rRNA: ribosomal ribonucleic acid; SMPS: scanning mobility particle sizer; TNF-α: tumor necrosis factor-alpha.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
EMR was substantially involved in design of the study, acquisition and analysis of data for all in vivo and in vitro work, statistical analyses, interpretation of results, and drafted the manuscript.
LP was substantially involved in design of the study, interpretation of results, assisted in acquisition of data for in vivo work and revised the manuscript critically. AJK was responsible for the exposure setup and monitoring and characterisation of used particles and aerosols as well as drafting of the manuscript regarding exposure data. HN was substantially involved in acquisition of all in vivo related data. HW was an expert interpreting the histopathological samples in the study. KS was involved in the study as an expert providing expertise on the initial planning of the study and commenting of the manuscript. HA was substantially involved in design of the study, interpretation of results, drafting of the manuscript regarding discussion of data and revised the manuscript critically. All authors have read and approved the final manuscript.
Contributor Information
Elina M Rossi, Email: Elina.Rossi@ttl.fi.
Lea Pylkkänen, Email: Lea.Pylkkanen@ttl.fi.
Antti J Koivisto, Email: Joonas.Koivisto@ttl.fi.
Heli Nykäsenoja, Email: hnykasen@cc.hut.fi.
Henrik Wolff, Email: Henrik.Wolff@ttl.fi.
Kai Savolainen, Email: Kai.Savolainen@ttl.fi.
Harri Alenius, Email: Harri.Alenius@ttl.fi.
Acknowledgements
Skilled technical assistance from Piia Karisola, Minnamari Vippola, Keld Jensen and Esa Vanhala is greatly appreciated. This work was supported by the European Union, Inflammatory and genotoxic effects of engineered nanomaterials, NANOSH [NMP2-CT-2006-032777] and by the Academy of Finland FinNano-program, Engineered Nanoparticles: Synthesis, Characterization, Exposure and Health Hazards (NANOHEALTH)-project.
References
- Lötvall J, Frew A, for the European Academy of Allergology and Clinical Immunology. Allergy: an epidemic that must be stopped. Brussels: European Academy of Allergology and Clinical Immunology; 2006. http://www.eaaci.net/media/PDF/E/820.pdf [Google Scholar]
- Weinmayr G, Weiland SK, Bjorksten B, Brunekreef B, Buchele G, Cookson WOC, Garcia-Marcos L, Gotua M, Gratziou C, van Hage M, von Mutius E, Riikjarv M-A, Rzehak P, Stein RT, Strachan DP, Tsanakas J, Wickens K, Wong GW. Isaac Phase Two Study Group. Atopic Sensitization and the International Variation of Asthma Symptom Prevalence in Children. Am J Respir Crit Care Med. 2007;176:565–574. doi: 10.1164/rccm.200607-994OC. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, Parasites, and the Hygiene Hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- Gavett SH, Koren HS. The Role of Particulate Matter in Exacerbation of Atopic Asthma. International Archives of Allergy and Immunology. 2001;124:109–112. doi: 10.1159/000053685. [DOI] [PubMed] [Google Scholar]
- Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001;17:909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- Pandya RJ, Solomon G, Kinner A, Balmes JR. Diesel Exhaust and Asthma: Hypotheses and Molecular Mechanisms of Action. Environmental Health Perspectives. 2002;110:103–112. doi: 10.1289/ehp.02110s1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Cogliano V. Carcinogenicity of carbon black, titanium dioxide, and talc. The Lancet Oncology. 2006;7:295–296. doi: 10.1016/S1470-2045(06)70651-9. [DOI] [PubMed] [Google Scholar]
- Chen X, Mao SS. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem Rev. 2007;107:2891–2959. doi: 10.1021/cr0500535. [DOI] [PubMed] [Google Scholar]
- Rossi EM, Pylkkanen L, Koivisto AJ, Vippola M, Jensen KA, Miettinen M, Sirola K, Nykasenoja H, Karisola P, Stjernvall T, Vanhala E, Kiilunen M, Pasanen P, Mäkinen M, Hämeri K, Joutsensaari J, Tuomi T, Jokiniemi J, Wolff H, Savolainen K, Matikainen S, Alenius H. Airway Exposure to Silica-Coated TiO2 Nanoparticles Induces Pulmonary Neutrophilia in Mice. Toxicol Sci. 2010;113:422–433. doi: 10.1093/toxsci/kfp254. [DOI] [PubMed] [Google Scholar]
- Wang SC, Flagan RC. Scanning Electrical Mobility Spectrometer. Aerosol Science and Technology. 1990;13:230. doi: 10.1080/02786829008959441. [DOI] [Google Scholar]
- Marjamäki M, Keskinen J, Chen D-R, Pui DYH. Performance evaluation of the Electrical Low-Pressure Impactor (ELPI) Journal of Aerosol Science. 2000;31:249–261. doi: 10.1016/S0021-8502(99)00052-X. [DOI] [Google Scholar]
- Boffetta P, Soutar A, Cherrie JW, Granath F, Andersen A, Anttila A, Blettner M, Gaborieau V, Klug SJ, Langard S, Luce D, Merletti F, Miller B, Mirabelli D, Pukkala E, Adami H-O, Weiderpass E. Mortality Among Workers Employed in the Titanium Dioxide Production Industry in Europe. Cancer Causes and Control. 2004;15:697–706. doi: 10.1023/B:CACO.0000036188.23970.22. [DOI] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Tadeka K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive Measurement of Airway Responsiveness in Allergic Mice Using Barometric Plethysmography. Am J Respir Crit Care Med. 1997;156:766–767. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Harkema J, Mariassy A, St. George J, Hyde D, Plopper C. In: The Airway Epithelium: Physiology, Pathophysiology and Pharmacology. Farmer S, Hay D, editor. New York: Dekker; 1991. Epithelial cells of the conducting airways: a species comparison; pp. 3–39. [Google Scholar]
- Hext PM, Tomenson JA, Thompson P. Titanium Dioxide: Inhalation Toxicology and Epidemiology. Ann Occup Hyg. 2005;49:461–472. doi: 10.1093/annhyg/mei012. [DOI] [PubMed] [Google Scholar]
- Warheit DB, Yuen IS, Kelly DP, Snajdr S, Hartsky MA. Subchronic inhalation of high concentrations of low toxicity, low solubility participates produces sustained pulmonary inflammation and cellular proliferation. Toxicology Letters. 1996;88:249–253. doi: 10.1016/0378-4274(96)82678-6. [DOI] [PubMed] [Google Scholar]
- Grassian VH, O'Shaughnessy PT, Adamcakova-Dodd A, Pettibone JM, Thorne PS. Inhalation exposure study of Titanium dioxide nanoparticles with a primary particle size of 2 to 5 nm. Environmental Health Perspectives. 2007;115:397–402. doi: 10.1289/ehp.9469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int Arch Occup Environ Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- Geiser M, Casaulta M, Kupferschmid B, Schulz H, Semmler-Behnke M, Kreyling W. The Role of Macrophages in the Clearance of Inhaled Ultrafine Titanium Dioxide Particles. Am J Respir Cell Mol Biol. 2008;38:371–376. doi: 10.1165/rcmb.2007-0138OC. [DOI] [PubMed] [Google Scholar]
- Lee K, Yang Y-S, Kwon S-J, Lee J-S, Choi S-J, Seo H-S, Kang M-S, Lee B-C, Kim S-N, Yang H-S, Han Y-A, Ryu H-J, Heo J-D, Cho K-H, Song C-W, Cho K-H. Lung injury study by 15 days inhalation exposure of titanium dioxide nanoparticles in rats. Toxicology Letters. 2009;189:S186–S186. doi: 10.1016/j.toxlet.2009.06.647. [DOI] [Google Scholar]
- Otani N, Ishimatsu S, Mochizuki T. Acute group poisoning by titanium dioxide: inhalation exposure may cause metal fume fever. The American Journal of Emergency Medicine. 2008;26:608–611. doi: 10.1016/j.ajem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Garabrant D, Fine L, Oliver C, Bernstein L, Peters J. Abnormalities of pulmonary function and pleural disease among titanium metal production workers. Scand J Work Environ Health. 1987;13:47–51. doi: 10.5271/sjweh.2087. [DOI] [PubMed] [Google Scholar]
- Eum S-Y, Maghni K, Tolloczko B, Eidelman DH, Martin JG. IL-13 may mediate allergen-induced hyperresponsiveness independently of IL-5 or eotaxin by effects on airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2005;288:L576–584. doi: 10.1152/ajplung.00380.2003. [DOI] [PubMed] [Google Scholar]
- Last JA, Ward R, Temple L, Pinkerton KE, Kenyon NJ. Ovalbumin-Induced Airway Inflammation and Fibrosis in Mice Also Exposed to Ultrafine Particles. Inhalation Toxicology. 2004;16:93–102. doi: 10.1080/08958370490265077. [DOI] [PubMed] [Google Scholar]
- Määttä J, Haapakoski R, Lehto M, Leino M, Tillander S, Husgafvel-Pursiainen K, Wolff H, Savolainen K, Alenius H. Immunomodulatory Effects of Oak Dust Exposure in a Murine Model of Allergic Asthma. Toxicological Sciences. 2007;99:260–266. doi: 10.1093/toxsci/kfm145. [DOI] [PubMed] [Google Scholar]
- Thatcher TH, Benson RP, Phipps RP, Sime PJ. High-dose but not low-dose mainstream cigarette smoke suppresses allergic airway inflammation by inhibiting T cell function. Am J Physiol Lung Cell Mol Physiol. 2008;295:L412–421. doi: 10.1152/ajplung.00392.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JJ, Bateman HR, Stover A, Gomez G, Norton SK, Zhao W, Schwartz LB, Lenk R, Kepley CL. Fullerene Nanomaterials Inhibit the Allergic Response. J Immunol. 2007;179:665–672. doi: 10.4049/jimmunol.179.1.665. [DOI] [PubMed] [Google Scholar]
- Larsen ST, Roursgaard M, Jensen KA, Nielsen GD. Nano Titanium Dioxide Particles Promote Allergic Sensitization and Lung Inflammation in Mice. Basic Clin Pharmacol Toxicol. 2010;106:114–117. doi: 10.1111/j.1742-7843.2009.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leino MS, Alenius HT, Fyhrquist-Vanni N, Wolff HJ, Reijula KE, Hintikka E-L, Salkinoja-Salonen MS, Haahtela T, Makela MJ. Intranasal Exposure to Stachybotrys chartarum Enhances Airway Inflammation in Allergic Mice. Am J Respir Crit Care Med. 2006;173:512–518. doi: 10.1164/rccm.200503-466OC. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Wal RV, Gigliotti A, Burchiel SW, McDonald JD. Pulmonary and Systemic Immune Response to Inhaled Multiwalled Carbon Nanotubes. Toxicol Sci. 2007;100:203–214. doi: 10.1093/toxsci/kfm196. [DOI] [PubMed] [Google Scholar]
- Fedulov AV, Leme A, Yang Z, Dahl M, Lim R, Mariani TJ, Kobzik L. Pulmonary Exposure to Particles during Pregnancy Causes Increased Neonatal Asthma Susceptibility. Am J Respir Cell Mol Biol. 2008;38:57–67. doi: 10.1165/rcmb.2007-0124OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein T, Maso MD, Petäjä T, Koponen IK, Paatero P, Aalto PP, Hämeri K, Kulmala M. Evaluation of an automatic algorithm for fitting the particle number size distributions. Boreal Env Res. 2005;10:337–355. [Google Scholar]