Abstract
Importance of the field
Retinal degenerations cause blindness. One potential therapy is cell replacement. Because the human retina lacks regeneration capacity, much attention has been directed towards searching for cells that can differentiate into retinal neurons.
Areas covered in this review
We discuss the possibility of using transcription factor genes to channel retinal pigment epithelial (RPE) cells’ capabilities of proliferation and plasticity towards the production of retinal neurons.
What the reader will gain
Experiments with chick embryos show that RPE cells – in the eye, in explant, or in a dissociated cell culture – can give rise to cells resembling retinal neurons when reprogrammed with regulatory genes involved in retinal neurogenesis. Depending on the regulatory gene used, reprogramming generates cells exhibiting traits of photoreceptor cells, amacrine cells and/or young ganglion neurons.
Take home message
Gene-directed reprogramming of chick RPE can efficiently generate cells that exhibit traits of retinal neurons. Remaining to be addressed is the question of whether the results from chicks apply to mammals. Since the RPE is located adjacent to the neural retina, RPE reprogramming, if successful in mammals, may offer an approach to repopulate the neural retina without involving cell transplantation.
Keywords: cell-replacement, photoreceptors, regeneration, retinal ganglion cells, transcription factors
1. Introduction
Vision begins in the neural retina, a thin tissue about 300 μm thick in the back of the human eye. In the neural retina, millions of neurons act in concert to produce visual input to the brain. Retinal neurons are categorized into groups according to their morphologies and functions. The primary, light-sensitive neurons are photoreceptors, which capture photons and generate electrophysiological signals. Those electrophysiological signals are then transmitted to and modulated by secondary, inner neurons, and finally relayed to the brain by retinal ganglion cells via their axons. These axons compose the optic nerve. As terminally differentiated cells, retinal neurons that die due to any cause cannot be replenished, leading to irreversible vision loss. The low quality of life caused by impaired vision has spurred intense interest in developing therapeutic approaches, including cell-replacement with endogenous cells generated by regeneration mechanisms (for a recent review, see [1]) or with exogenous cells delivered by cell transplantation (for a recent review, see [2]).
Intrinsic retinal regeneration would offer a straightforward approach to repair retinal damage caused by cell loss. In fish and chick, injuries to the retina induce the formation of new retinal neurons from progenitor/stem cells residing at the ciliary marginal zone [3–6]. However, this regeneration mechanism is lacking in the retina of mammalian species, including humans [7,8]. Although not canonical progenitor/stem cells, Müller glia in various species (including mammals) retain certain properties of retinal progenitor cells. For instance, in response to damage to retinal neurons, Müller cells will reenter the cell cycle, a proliferative response, and may give rise to retinal neurons, a regenerative capability [9–18]. Nonetheless, the regenerative capability of Müller cells in mammals is much lower than that in fish and birds [1], and challenges exist in employing Müller glia to efficiently repopulate a mammalian retina after retinal degeneration. The lack of effective retinal regeneration in the mammalian retina has fueled research exploring cells/tissues outside the neural retina, including the retinal pigment epithelium (RPE), as potential sources of new retinal neurons.
2. The RPE as a source of new retinal neurons
The RPE consists of darkly pigmented cells organized as a single-layered, transporting epithelium with important roles in retinal physiology. Anatomically, the RPE lies immediately adjacent to neural retina and forms the outer blood–retinal barrier. This anatomical location puts the RPE at a unique position for providing new neurons to repopulate a degenerating retina without cell transplantation. But a key biological question is whether the RPE, a non-neural tissue, is amenable to reprogramming to give rise to retinal neurons.
Developmentally, the non-neural RPE and the neural retina originate from the same structure – the optic vesicle. During development, the optic vesicle invaginates to form the double-layered optic cup, thus creating the anatomical separation of the RPE (the outer layer of the cup) and the neural retina (the inner layer). This common origin may bring about shared molecular and cellular characteristics and aid fate switches. Indeed, classic experiments have demonstrated an intriguing phenomenon: the RPE can give rise to a neural retina. In chick embryos, separating the RPE from the retina with a thread [19], or by surgically removing most of the retina [20], causes the RPE to develop into a neural retina. Later investigation found that basic fibroblast growth factor (bFGF) stimulates this remarkable transdifferentiation [21]. Rodent RPE from young embryos [22,23] and amphibian RPE [24,25] are also capable of RPE-to-neural retina trans-differentiation. Unfortunately, harnessing this biological phenomenon for retinal neuron production faces major obstacles, including a strict age-limitation: it takes place only during very early stages of embryonic development, prior to day 4.5 (E4.5) in chick [26] and prior to E13 in rodents [22,23].
Unlike retinal neurons, mature RPE cells of many species, including humans, can reenter the cell cycle to proliferate. In normal eyes, most RPE cells remain quiescent, probably in response to signals from the neural retina, and a small population located in the peripheral RPE undergoes proliferation [27]. Retinal detachment results in RPE cell proliferation [28]. In addition, certain pathological conditions and physical stimulations can cause RPE cells to proliferate. For instance, rat RPE cells reenter the cell cycle and proliferate in response to laser photocoagulation [29]. In the clinical setting, RPE’s proliferative response is an undesirable side-effect of surgery, because progeny cells may differentiate into cells with tractional force, causing retinal detachment and leading to visual impairment [30]. The proliferative responses of RPE and the plasticity of its progeny cells, nonetheless, raise an intriguing possibility of exploring the RPE as a convenient source of retinal neurons for replacement in situ.
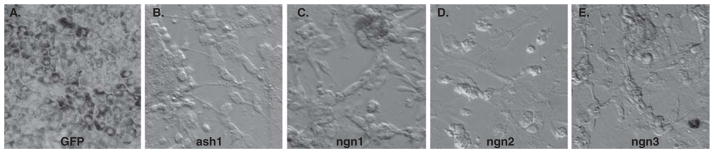
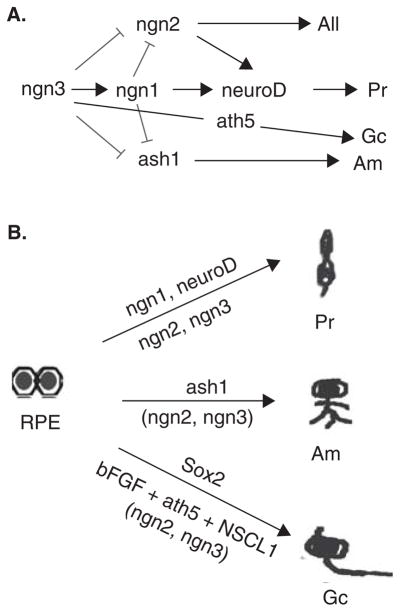
With mounting knowledge about the genetic control of retinal neurogenesis, an unconventional approach has been formulated for producing new retinal neurons – gene-directed reprogramming to channel RPE’s capabilities of proliferation and plasticity towards retinal neurogenesis [31–35]. In these studies, RPE is taken from chick embryos at E6 – E15, long after the developmental loss of the competence of bFGF-induced RPE to neural retina transdifferentiation, and the progeny cells of the RPE cultured as dissociated cells are transduced with genes encoding transcription factors that play instrumental roles in retinal neuron production. More than 20 transcription factors involved in regulating retinal development have been assayed for their ability to reprogram RPE to differentiate towards retinal neurons [35]. These include genes encoding transcription factors in the basic helix-loop-helix (bHLH) family and homeodomain family. The homeodomain genes include Pax6, Rax, six3 and chx10, which are known to play important roles in the development of the eye and/or the neural retina. Despite their demonstrated importance, these homeodomain genes displayed insignificant activities in reprogramming RPE to produce cells expressing markers for retinal neurons [35]. The negative outcome does not undermine the importance of these genes in retinal neurogenesis; rather, it shows their ineffectiveness in the context of RPE cells. Among the bHLH genes assayed, four (ash1, ngn1, ngn2 and ngn3) induced RPE cell cultures to give rise to neuron-like cells and neuron-like clusters apparent by light microscopy (Figure 1) [32,34,35].
Figure 1. Neuron-like cells and neuron-like clusters in RPE cell cultures after reprogramming by ash1 (B), ngn1 (C), ngn2 (D), and ngn3 (E).
Neuron-like cells and neuron-like clusters are absent in the GFP control (A).
3. Generating photoreceptor-like cells by RPE reprogramming
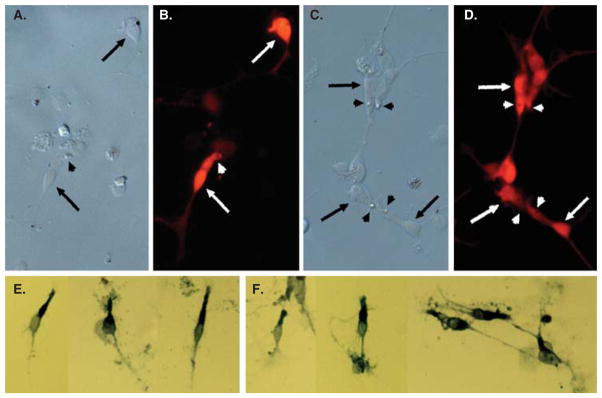
Cell typing analysis showed that photoreceptor-like cells were produced in large numbers in chick RPE cell cultures after reprogramming by ngn1, ngn2 or ngn3 (Figure 2) [32,35]. NeuroD, a bHLH gene involved in photoreceptor differentiation, also guided cultured RPE cells to differentiate into photoreceptor-like cells [31,36,37]. Among the genes with significant activity in inducing RPE to photoreceptor reprogramming, ngn1 and ngn3 emerged as the two front-runners, in terms of inducing the highest number of cells expressing visinin [35], an early marker of chick photoreceptors.
Figure 2. Photoreceptor-like cells produced from RPE reprogramming by ngn1 (A,B,E) or ngn3 (C,D,F).
A, C: Bright field view. B, D: Epifluorescence immunostaining for visinin. E, F: Immunostaining for red opsin. Arrows point to the cell body, and arrowheads point to a structural feature reminiscent of the lipid-droplet typically present in chick photoreceptors.
Analyses at the molecular, cellular and physiological levels showed that the reprogrammed cells display advanced photo-receptor traits [35,37]. The new cells expressed transcription factors crx, nr2e3, raxL, RXRγ and neuroD, which participate in initiating the photoreceptor differentiation program [35]. They also express components of phototransduction, including red opsin (Figure 2E and F) [35,37], the α-subunit of cyclic nucleotide gated channels and cone α-transducin. Red opsin+ cells displayed dot-like immunostaining at the apices of the cells, reminiscent of the immunolocalization of red opsin of in photoreceptors in the retina, indicating a proper localization of red opsin [35]. Morphologically, in contrast to the hexagonal RPE cells, visinin+ cells resembled photoreceptors, with an elongated cell body, an axon-like process, an inner segment-like compartment, and a lipid droplet-like structural feature (Figure 2A – D, arrowheads) [35]. EM analysis showed that reprogrammed cells developed cellular compartments rich in mitochondria [35,37]. On the apex of the inner segment, reprogrammed cells displayed ciliary expansions, reminiscent of the developing outer segments of retinal photoreceptors in the E17 eye or in culture [35]. Perhaps most excitingly, reprogrammed cells displayed physiological traits typical of retinal photoreceptors. Retinal photoreceptors exhibit two physiological hallmarks – light response and visual recovery. Both properties are reflected as changes in by cytosolic, free calcium (Ca2+) levels. Fluorescent calcium imaging showed that reprogrammed cells responded to light by decreasing their Ca2+ levels and responded to 9-cis-retinal by increasing their Ca2+ levels [35,37].
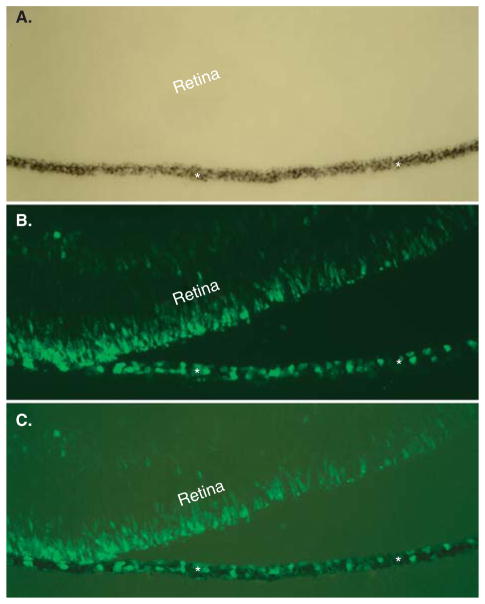
To be a convenient source of photoreceptor cells, the RPE in the eye needs to be reprogrammable. To test this, experimental chick embryos were examined for visinin expression in the RPE layer. In control E7.5 chick eyes, visinin+ cells were confined within the neural retina at the prospective outer nuclear layer (ONL). In eyes infected with replication competent avian sarcoma-leukosis virus long terminal repeat with splice acceptor (RCAS)-ngn3, visinin+ cells were present in both the RPE and the neural retina (Figure 3) [38]. The RPE layer in RCAS-ngn1-infected embryos contained far fewer visinin+ cells. This was unexpected, because with dissociated RPE cells ngn1 was no less effective than ngn3. To test whether the eye with an intact retina exhibited some inhibition on ngn1-RPE to produce otherwise surplus photoreceptor cells, the RPE from the embryos was separated from the neural retina and cultured as an explant, to unleash the RPE’s potential to produce visinin+ cells. Indeed, RPE explants from embryos infected with RCAS-ngn1 produced layers of visinin+ cells, while the GFP control did not [38]. As with dissociated cell cultures, reprogrammed cells from RPE explants expressed genes associated with advanced photoreceptor differentiation [38].
Figure 3. Visinin+ cells in the RPE layer after reprogramming by ngn3.
Shown are bright-field (A), visinin immunofluorescence (B), and simultaneous view of both (C) of an E7.5 eye infected with RCAS-ngn3. Asterisks mark the RPE.
Another important issue is whether mature RPE is reprogrammable. Li et al. [38] reported that layers of visinin+ cells emerged from the RPE explants from chick at E12, when the RPE is close to maturity, and abundant visinin+ cells emerged in RPE explants from chick at E18, when the visual system is functional. These results suggest that mature RPE is reprogrammable.
4. Reprogramming RPE towards other retinal neurons
Variations were observed in the cell products from the reprogramming by bHLH genes. Reprogramming of RPE cells by ngn1 and neuroD generates mostly cells displaying photo-receptor traits [31,35,36,38]. On the other hand, reprogramming by ngn2 results in the genesis of cells of molecularly and morphologically diverse types, which resemble photoreceptor cells, ganglion cells, and, in a smaller number, amacrine cells [32]. Similarly, reprogramming by ngn3 produces diverse types of cells, with photoreceptor cells and, to a lesser extent, ganglion cells as the main products [35], and some cells displaying similarities to amacrine cells (Figure 4A and B).
Figure 4.
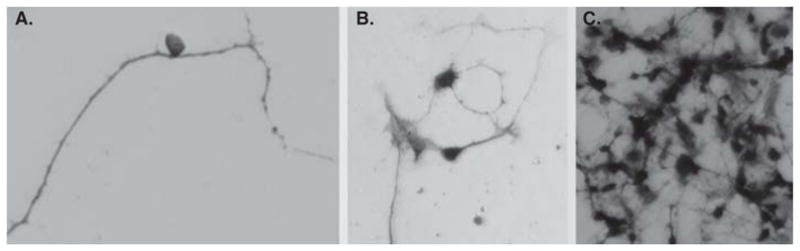
An RA4+ cell (A) and calretinin+ positive cells (B, C) produced by reprogramming cultured RPE cells by ngn3 (A, B) or by ash1 (C).
Different populations of cells were produced from reprogramming by ash1 (Figure 4C) [34]. In ash1-reprogrammed RPE culture, the new cells developed elaborate processes characteristic of neurons and expressed genes/markers that identify different types of retinal neurons [34]. The most prevalently expressed neural marker was calretinin, which in the chick retina identifies amacrine, ganglion, and horizontal cells. In an assay for the presence of functional, ionotropic glutamate receptors that lead to a rise in the cytosolic free calcium (Ca2+) concentration, the reprogrammed cells responded to glutamate and N-methyl-D-aspartate by increasing their Ca2+ concentrations, and after reaching a peak level, returned to the basal level. The study [34] suggests that chick RPE progeny cells can be reprogrammed by ash1 to develop molecular, morphological, and physiological properties that are characteristic of retinal neurons, probably amacrine cells.
5. Reprogramming RPE towards retinal ganglion-like neurons
Sox2 and bFGF have also been assayed for activities to reprogram RPE cells to differentiate towards retinal neurons. Sox2 belongs to the SoxB1 subfamily of transcription factors, which are characterized by a high mobility group (HMG) DNA-binding domain. It is one of four genes used to convert adult fibroblasts into induced pluripotent stem cells in mice and in humans [39–42]. Sox2 is expressed in the neural plate and in neural stem cells and progenitors during both embryonic and adult neurogenesis, in which sox2 plays an important role in maintaining neural progenitor/stem cell properties [43–46]. In the developing retina, sox2 is mostly expressed in proliferating progenitor cells [47,48], and the RPE appears to lack sox2 expression [48]. In the mouse retina, decreasing the expression of sox2 results in progenitor cells that cannot proliferate or differentiate [49]. In humans, sox2 mutations cause anophthalmia [50]. These findings show that sox2 plays an important role in the wellbeing of retinal progenitor cells. This particular role of sox2 prompted the idea that sox2 might reprogram RPE to differentiate towards retinal neurons. This idea has been experimentally tested in chick RPE cells [48].
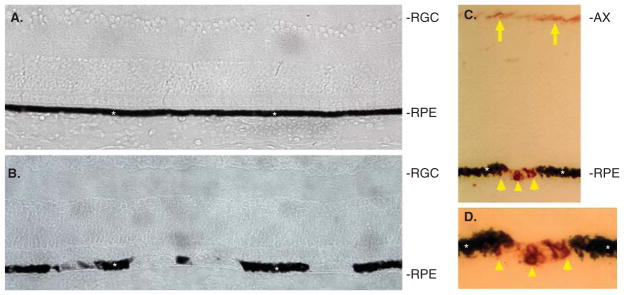
Ectopic expression of sox2 in the chick RPE makes the experimental eyes ‘spotty’ in general appearance [48]. Closer examination has shown that the RPE cells in the ‘spotty’ regions partially or completely lack their usual dark pigmentation (Figure 5A and B) [48]. In the affected regions, BrdU incorporation was detected, indicating that those cells had reentered the cell cycle [48]. In addition, over 50% of the cells in the depigmented regions of the RPE are positive for RA4 immunoreactivity (Figure 5C and D) [48], a marker of newborn ganglion cells [51]. Some of the RA4+ cells exhibited neural morphologies. Cells in the depigmented regions were also immunopositive for 3A10 and 4H6, two monoclonal antibodies against a neurofilament-associated protein and a neurofilament, respectively [48]. Thus, sox2 was able to reprogram cells in the RPE layer of the eye to lose their normal appearance, to take on neural-like morphology, and to express markers atypical of RPE but typical of retinal ganglion neurons.
Figure 5. Sox2 induced the loss of pigmentation and the presence of RA4+ cells in the RPE layer of chick eyes.
A: Cross-section of a control E15 retina infected with RCAS-GFP. B: Cross-section of an experimental E15 retina infected with RCAS-Sox2. C: RA4 immunostaining of an experimental retina. D: Higher magnification of C. Asterisks mark the RPE. Arrows point to RA4+ axons of retinal ganglion cells. Arrowheads point to RA4+ cells in the RPE layer.
AX: Axons of retinal ganglion cells; RGC: Retinal ganglion cells; RPE: Retinal pigment epithelium.
As in the eye, sox2 guided RPE cells in culture away from their usual path and towards becoming retinal neurons. Dissociated RPE cell cultures subjected to sox2 reprogramming failed to become repigmented, which occurs in the control (Figure 6A and B) [48]. In the sox2-reprogrammed RPE cell cultures, RA4+ (Figure 6C and D) and 3A10+ cells were present in large numbers, accounting for more than 50% and more than 30%, respectively, of the total cells present. Morphologically, some of the RA4+ (Figure 6E and F) or 3A10+ cells exhibited long processes, markedly deviating from the hexagonal or fibroblast-like morphologies of RPE cells in culture. Reprogramming by sox2 decreased the expression of Mitf, Otx2 and Mmp115, genes with important roles in maintaining RPE properties, and increased the expression of neural genes Pax6 and Npy-1 [48].
Figure 6. Sox2 induced hypopigmentation and generation of RA4+ cells in dissociated RPE cell cultures.
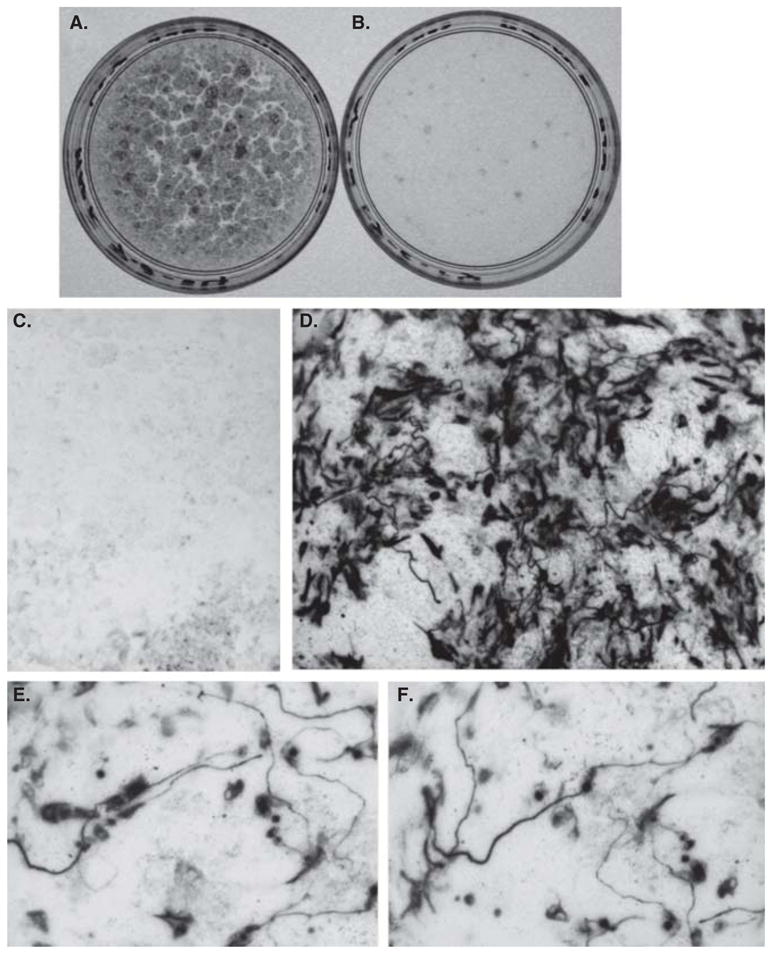
A, B: Two 35-mm dishes with RPE cell cultures infected with control RCAS-GFP (A) or RCAS-Sox2 (B). C, D: RA4 immunostaining of a control culture (C) and an experimental culture (D). E, F: Individual RA4+ cells viewed at a high magnification.
Notably, Sox2-initiated RPE reprogramming produced cells with a somewhat limited extent of neural differentiation, both in the eye and in RPE cell cultures. This is not surprising, considering that Sox2 is well known to promote progenitor/stem cell status and inhibit neural differentiation. This limited extent of differentiation suggests that Sox2 redirects the differentiation of RPE cells towards retinal neurons, but continued neural differentiation requires additional factors. Future studies aiming for advanced neural differentiation may include regulating Sox2 expression and incorporating factors/genes that promote neural differentiation. Previous studies have shown that ath5 and NSCL1, which are bHLH genes transiently expressed in differentiated retinal ganglion cells, promote bFGF-induced RPE reprogramming towards retinal ganglion cells [33,52]. Because of the expression of differentiation markers, the reprogrammed cells were unlikely to be progenitor cells.
The lack of re-pigmentation and the presence of RA4+ cells in dissociated RPE cell cultures have previously been observed with the administration of bFGF to the culture [53]. This prompted an investigation of a possible link between sox2 and bFGF. The spatial patterns of expression of sox2 and bFGF overlapped in the developing retina, and their co-expression was detected in some retinal cells, particularly in Müller glia during late stages of development. Retinal expression of both sox2 and bFGF, assayed by in situ mRNA hybridization, was reduced by Müller glia atrophies and was enhanced by NMDA damage. Furthermore, RT-PCR analyses showed that sox2 was induced in RPE cell cultures treated with bFGF, while bFGF expression was enhanced by sox2 in the retina and in Müller glia culture. These results suggest a positive relationship between sox2 and bFGF and imply their involvement in Müller glial responses to retinal damage.
Significant variations exist in terms of the efficiency at which cells expressing ganglion markers emerged from reprogrammed RPE cell cultures. Sox2 is the highest, rivaled only by bFGF. However, in bFGF-initiated RPE reprogramming, the neural differentiation, if any, is very primitive [53]. All the RA4+ cells in bFGF-treated RPE cell cultures lack any commensurate neural morphologies, but rather retain the flat, fibroblast-like morphologies characteristic of cultured RPE cells before reaching confluency. The bFGF-reprogrammed differentiation towards retinal ganglion cells can be enhanced by bHLH genes ath5 and NSCL1, each alone [52] or more pronouncedly in combination [33]. In ngn2- or ngn3-induced RPE reprogramming, the molecular and cellular differentiation towards retinal ganglion cells is advanced, but less than 1% of the cells in ngn2-reprogrammed cultures [32] and 5 – 10% of the cells in ngn3-reprogrammed cultures [35] exhibit such differentiation. In addition, these cells are far outnumbered by those with photoreceptor traits, creating a problem of a mixed population of cell types. In comparison, sox2-reprogrammed cultures contain no photoreceptor-like cells [48], and more than 50% of the cells are positive for retinal ganglion cell marker RA4. Thus, sox2-induced RPE reprogramming is comparatively better suited for generating non-photoreceptor neurons, particularly ganglion cells.
6. Specificity of gene-directed RPE reprogramming
The variations in the products of gene-directed RPE reprogramming indicate not only that the RPE cells are responsive to the gene-directed reprogramming, but also that the products of reprogramming display gene-specificities. A comparative analysis indicates that the product population from the RPE reprogramming more or less agrees with the known or implicated roles of each corresponding gene in retinal development of the chick (Figure 7). Studies of the expression of bHLH genes in the developing chick retina showed spatial overlaps of ngn3, ngn1 and neuroD expression [54,55]. Functional studies suggest that ngn1 may play a major role in steering a progenitor cell towards the photoreceptor path during retinal neurogenesis in the chick [54]. On the other hand, ngn2 showed a temporal and spatial pattern of expression consistent with its role in progenitor cells that may later differentiate into all major types of retinal cells, while ash1 is expressed in progenitor cells likely to adopt an amacrine cell fate [56,57]. Investigation into their genetic relationships showed induction of ngn1 by ngn3, but not by ngn2 or neuroD, while ngn3 or ngn1 inhibited the expression of ngn2 and ash1 [54,55]. Overall, there appears an inductive pathway of ngn3 to ngn1 to neuroD and an inhibition of ngn2 (and ash1) by ngn1 and by ngn3 (Figure 7A). Thus, retinal neurogenesis seems to employ a complex network of bHLH regulatory factors that are expressed during different time frames and participate in the genesis of diverse cell types, for balanced production of all retinal cell populations. In the chick retina, sox2 is expressed in progenitor cells and later in Muller glia and a small number of cells in the amacrine cell layer and ganglion cell layer [47,48], consistent with its know importance for maintaining retinal progenitor cell properties [49]. In addition, the limited neural differentiation of in sox2-induced RPE reprogramming is consistent with the well known role of sox2 in inhibiting neural differentiation.
Figure 7.
A: Sketch showing the proposed genetic relationship among bHLH genes in chick retinal neurogenesis. B: Diagram of gene-directed reprogramming of RPE showing genes that have been assayed and the cell types that the major products mostly closely resemble. Parentheses indicate that the cell type constitutes a minor component of the resultant reprogramming products. All, all other types of retinal cells.
Am: Amacrine cells; Gc: Retinal ganglion cells; Pr: Photoreceptors.
7. Other potential sources of new retinal neurons
The recently-demonstrated multipotency of adult stem cells has spurred interest in testing ocular cells outside the neural retina for their capacity to generate retinal neurons. Ocular tissues/cells being explored include the iris pigment epithelium [58–62], the ciliary body [61,63–65], the limbal epithelium [66], and the RPE [31,32,35,37,38,52,67]. With the exception of the RPE, all these ocular cells/tissues give rise to disappointingly low numbers of photoreceptor cells. Notably, early reports of the presence of retinal stem cells in the ciliary epithelium of the mammalian eye [63,64] have recently been contested [68].
Mammalian stem cells of various origins have been explored for their ability to produce retinal neurons. Initial studies tested stem cells isolated from adult brain and bone marrow and met with very limited success [69–73]. Recently, significant progress has been reported in guiding human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells to differentiate into retinal neurons [74–80]. ES cells can give rise to retinal progenitor cells that exhibit similar gene expression profiles such as those derived from human fetal retina [74]. These ES cell-derived retinal progenitors can further differentiate into inner retinal neurons with functional glutamate receptors [74]. Other study shows that after transplantation into genetically modified mice, ES-derived photo-receptors can restore some visual function [74,76]. When subjected to a targeted and stepwise differentiation protocol, ES and iPS cells progressively narrow their potential fates to the eye field, then to the optic vesicle, the optic cup, retinal progenitors and finally to differentiated cells of the retina, particularly RPE cells and cones [75,77–79]. These new developments are very exciting and support the prospect of generating specific types of retinal cells from ES cells and especially from iPS cells for autologous cell transplantation as a therapy for retinal degeneration [81]. Notably, cell replacement is just one aspect of cell therapies, which may include the use of cells derived from embryonic stem cells and iPS cells to prevent or to slow down the degeneration of a particular type of retinal neuron without producing such a specific type of retinal neuron from the stem cells [82–84].
8. Conclusion
Studies with the chick system show that genes encoding transcription factors participating in retinal neurogenesis can guide RPE cells to differentiate towards retinal neurons. To produce ganglion-like cells, sox2 can be used, but additional genes/factors that promote neural differentiation are needed. To produce photoreceptor-like cells, the bHLH genes ngn1 or ngn3 are thus far the best choice to reprogram RPE cells. The new cells produced from ngn1-induced RPE reprogramming express a spectrum of photoreceptor genes, including transcriptional factors that set in motion the photoreceptor differentiation program and components of the phototransduction pathway. These cells exhibit structural features typical of developing photoreceptors. Perhaps most excitingly, they develop physiological traits that are hallmarks of photoreceptors, such as response to light and to 9-cis-retinal after light-bleaching. These results support the exciting possibility of deriving functional photoreceptors from the RPE.
9. Expert opinion
In this article we have discussed gene-directed ‘RPE to retinal neurons’ reprogramming as a possible approach to produce new retinal neurons. The theme builds upon RPE’s known abilities – cell proliferation and plasticity – and channels them towards the production of new retinal neurons, especially photoreceptor cells and ganglion cells. Photoreceptor-like cells are produced at high efficiency with RPE reprogramming by bHLH genes ngn1 and ngn3, while cells expressing early ganglion markers are efficiently produced from sox2-initiated RPE reprogramming. RPE reprogramming by bHLH genes showed advanced photoreceptor differentiation at the molecular, cellular, and physiological levels. On the other hand, reprogramming by sox2 produced cells with limited extents of neural differentiation. Importantly, ngn3 and sox2 can initiate RPE reprogramming in vivo in the eye, in addition to in vitro with cultured RPE cells, supporting the possibility of deploying the RPE as a convenient source of retinal neurons for cell replacement in situ in the eye without the involvement of cell transplantation.
While the results from studies with the chick system are interesting, it will be imperative to show that the results from chick studies are applicable to human cells. If the finding apply to humans, there is tremendous scientific interest and societal significance. The convenient location of the RPE makes the approach attractive for developing cell-replacement therapies to treat blindness due to retinal degeneration without cell transplantation. Additionally, some questions critically important to the prospect of the RPE reprogramming approach need to be addressed. i) Will there still be RPE remaining after the reprogramming? RPE plays important roles to the well-being of retinal neurons. Current information supports a belief that the RPE may regenerate itself during the process by deploying its proliferation and wound-healing traits, but studies directly addressing this concern are needed. ii) Will gene-directed reprogramming using insertional viral vectors cause mutagenesis and tumorigenesis? iii) Will advanced ganglion cell differentiation be achieved by regulating sox2 expression and incorporating factors/genes that promote neural differentiation? iv) Will the reprogrammed cells detach from the RPE, migrate to the appropriate place, and establish circuitry connections with partner cells to fulfill their functional roles? These are just some of critical questions that still need to be addressed in investigating the feasibility of RPE reprogramming as an approach to produce new retinal neurons for cell replacement studies.
Article highlights.
The biological properties and the anatomical location of the retinal pigment epithelium (RPE) make it attractive as a convenient source of new retinal neurons to repopulate the retina afflicted with retinal degeneration without cell transplantation.
Proneural basic helix-loop-helix (bHLH) genes that participate in the genetic hierarchy of photoreceptor genesis in the neural retina can effectively reprogram RPE to give rise to photoreceptor-like cells. This reprogramming can occur in the eye, in RPE explants, and in dissociated RPE cell cultures.
The reprogrammed cells can develop advanced photoreceptor traits.
Sox2-initiated RPE reprogramming gives rise to cells expressing markers of retinal ganglion cells.
Imperative future studies will include whether human or mammalian RPE is amenable to gene-directed reprogramming to give rise to photoreceptor-like or ganglion-like neurons.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
This paper has been sponsored by a National Institutes of Health/National Eye Institute (NIH/NEI) grant R01 EY011640, the EyeSight Foundation of Alabama, and Research to Prevent Blindness (to the Department of Opthalmology, University of Alabama at Birmingham).
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Lamba DA, Karl MO, Reh TA. Strategies for retinal repair: cell replacement and regeneration. Prog Brain Res. 2009;175:23–31. doi: 10.1016/S0079-6123(09)17502-7. [DOI] [PubMed] [Google Scholar]
- 2.West EL, Pearson RA, MacLaren RE, et al. Cell transplantation strategies for retinal repair. Prog Brain Res. 2009;175:3–21. doi: 10.1016/S0079-6123(09)17501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44:289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 4.Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci. 2000;17:789–97. doi: 10.1017/s0952523800175121. [DOI] [PubMed] [Google Scholar]
- 5.Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220:197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- 6.Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232:62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 7••.Kubota R, Hokoc JN, Moshiri A, et al. A comparative study of neurogenesis in the retinal ciliary marginal zone of homeothermic vertebrates. Brain Res Dev Brain Res. 2002;134:31–41. doi: 10.1016/s0165-3806(01)00287-5. This study reports that the ciliary marginal zone (CMZ), which produces new neurons in a functioning retina, has been gradually diminished during vertebrate evolution, on the basis that the quail has a reduced CMZ in comparison with the chicken, the opposum has only a few CMZ-like cells, and the mouse retina has no detectable CMZ. [DOI] [PubMed] [Google Scholar]
- 8.Ohta K, Ito A, Tanaka H. Neural stem/progenitor cells in the vertebrate eye. Dev Growth Differ. 2008;50:253–9. doi: 10.1111/j.1440-169X.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 9•.Fischer AJ, Reh TA. Müller glia are a potential source of neuronal regeneration in the postnatal chicken retina. Nat Neurosci. 2001;4:247–52. doi: 10.1038/85090. This study found that in the chick retina, progeny cells from Müller glia proliferation after acute damage express transcription factors of embryonic retinal progenitors, transiently express neurofilament, and localize throughout the inner and outer nuclear layers of the retina. [DOI] [PubMed] [Google Scholar]
- 10•.Ooto S, Akagi T, Kageyama R, et al. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–9. doi: 10.1073/pnas.0402129101. The authors report that after chemically induced neural damage in adult rat retina, Müller glial cells proliferate and produce limited numbers of bipolar cells and rod photoreceptors. Treatment with retinoic acid and ectopic expression of transcription factors improves the yield of retinal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer AJ, Wang SZ, Reh TA. NeuroD induces the expression of visinin and calretinin by proliferating cells derived from toxin-damaged chicken retina. Dev Dyn. 2004;229:555–63. doi: 10.1002/dvdy.10438. [DOI] [PubMed] [Google Scholar]
- 12•.Yurco P, Cameron DA. Responses of Müller glia to retinal injury in adult zebrafish. Vision Res. 2005;45:991–1002. doi: 10.1016/j.visres.2004.10.022. This report describes evidence supporting the suggestion that proliferative Müller glia serve as stem/precursor cells for retinal regeneration in lesioned retina of adult zebrafish. [DOI] [PubMed] [Google Scholar]
- 13•.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. The authors report that following injury, Müller glia in adult zebrafish retina are associated with multipotent stem cells that regenerate retinal neurons and thus both CMZ and the microenvironment around some Müller glia are specialized niches that sustain retinal stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Das AV, Mallya KB, Zhao X, et al. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. This report describes how Müller glial cells enriched from mammalian retina can self-renew in culture and can give rise to cells expressing markers of retinal neurons in vitro and in vivo after transplantation. [DOI] [PubMed] [Google Scholar]
- 15•.Osakada F, Ooto S, Akagi T, et al. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27:4210–9. doi: 10.1523/JNEUROSCI.4193-06.2007. This study found that treatment of Müller glia-derived retinal progenitors with Wnt3a increases their proliferation more than 20-fold in photoreceptor-damaged retina and that the addition of retinoic acid or valproic acid induces differentiation of these cells primarily into photoreceptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Lawrence JM, Singhal S, Bhatia B, et al. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–43. doi: 10.1634/stemcells.2006-0724. The authors report that cells derived from human adult neural retina propagate under normal culture conditions and express neural stem cell markers and markers of postmitotic retinal neurons when cultured in the presence of extracellular matrix and fibroblast growth factor-2 or retinoic acid, or transplanted into the subretinal space of rat eye. [DOI] [PubMed] [Google Scholar]
- 17•.Takeda M, Takamiya A, Jiao JW, et al. alpha-Aminoadipate induces progenitor cell properties of Muller glia in adult mice. Invest Ophthalmol Vis Sci. 2008;49:1142–50. doi: 10.1167/iovs.07-0434. In this study, mature Müller glia in adult mice was found to respond to alpha-aminoadipate treatment and proliferate, dedifferentiate, migrate and give rise to new retinal neurons and photoreceptor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Karl MO, Hayes S, Nelson BR, et al. Stimulation of neural regeneration in the mouse retina. Proc Natl Acad Sci USA. 2008;105:19508–13. doi: 10.1073/pnas.0807453105. The author report that a subset of progeny cells from Müller glia’s proliferation, in response to NMDA-induced degeneration of ganglion and amacrine cells, differentiate into amacrine cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orts-Llorca F, Genis-Galvez JM. Experimental production of retinal septa in the chick embryo. Differentiation of pigment epithelium into neural retina. Acta Anat. 1960;42:31–70. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- 20.Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 21.Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Div Biol. 1989;134:201–5. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat pigment epithelium to neural retina. Brain Res. 1995;677:300–10. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]
- 23.Sakami S, Etter P, Reh TA. Activin signaling limits the competence for retinal regeneration from the pigmented epithelium. Mech Dev. 2008;125:106–16. doi: 10.1016/j.mod.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- 25.Vergara MN, Del Rio-Tsonis K. Retinal regeneration in the Xenopus laevis tadpole: a new model system. Mol Vis. 2009;15:1000–13. [PMC free article] [PubMed] [Google Scholar]
- 26.Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–88. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- 27••.Al-Hussaini H, Kam JH, Vugler A, et al. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Mol Vis. 2008;14:1784–91. This study found that in rat eyes, mature RPE cells in the periphery are positive for proliferating cell markers, Ki67, proliferating cell nuclear antigen, and bromodeoxyuridine incorporation. The number of RPE cells positive for proliferating cell markers is 10-fold higher in albino than in pigmented rats. Ki67-positive cells are detected in human RPE. [PMC free article] [PubMed] [Google Scholar]
- 28••.Anderson DH, Stern WH, Fisher SK, et al. The onset of pigment epithelial proliferation after retinal detachment. Invest Ophthalmol Vis Sci. 1981;21:10–6. The authors found that cell positive for 3H-thymidine incorporation are absent 12 hours after experimental detachment of the cat retina, but are detected at 24 and 48 hours after the detachment. During the period from 24-hour to 48-hour, the number of 3H-thymidine+ nuclei is doubled. The proliferative response of the RPE is limited to the detached region. [PubMed] [Google Scholar]
- 29.Zhang NL, Samadani EE, Frank RN. Mitogenesis and retinal pigment epithelial cell antigen expression in the rat after krypton laser photocoagulation. Invest Ophthalmol Vis Sci. 1993;34:2412–24. [PubMed] [Google Scholar]
- 30.Crisant S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Invest Ophthalmol Vis Sci. 1995;36:391–405. [PubMed] [Google Scholar]
- 31.Yan R-T, Wang S-Z. neuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. J Neurobiol. 1998;36:485–96. [PMC free article] [PubMed] [Google Scholar]
- 32.Yan R-T, Ma W, Wang S-Z. neurogenin 2 elicits the genesis of retinal neurons from cultures of non-neural cells. Proc Natl Acad Sci USA. 2001;98:15014–9. doi: 10.1073/pnas.261455698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie W, Yan R-T, Ma W, Wang S-Z. Enhanced retinal ganglion cell differentiation by ath5 and NSCL1 coexpression. Invest Ophthalmol Vis Sci. 2004;45:2922–8. doi: 10.1167/iovs.04-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao W, Yan R-T, Wang S-Z. Reprogramming chick RPE progeny cells to differentiate towards retinal neurons by ash1. Mol Vis. 2008;14:2309–20. [PMC free article] [PubMed] [Google Scholar]
- 35••.Yan R-T, Liang L, Ma W, et al. Neurogenin1 effectively reprograms cultured chick RPE cells to differentiate towards photoreceptors. J Comp Neurol. 2010;518:526–46. doi: 10.1002/cne.22236. The authors report that neurogenin 1 can reprogram more than 80% of the cells in a dissociated RPE cell culture derived from day 6 chick embryos to express a photoreceptor marker and that reprogrammed cells display advanced photoreceptor traits, including light response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan R-T, Wang S-Z. Expression of an array of photoreceptor genes in chick embryonic RPE cell cultures under the induction of neuroD. Neurosci Lett. 2000;280:83–6. doi: 10.1016/s0304-3940(99)01003-4. [DOI] [PubMed] [Google Scholar]
- 37.Liang L, Yan R-T, Li X, et al. Reprogramming progeny cells of embryonic RPE to produce photoreceptors: development of advanced photoreceptor traits under the induction of neuroD. Invest Ophthalmol Vis Sci. 2008;49:4145–53. doi: 10.1167/iovs.07-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38••.Li X, Ma W, Zhuo Y, et al. Using neurogenin to reprogram chick RPE to produce photoreceptor-like neurons. Invest Ophthalmol Vis Sci. 2010;51:516–25. doi: 10.1167/iovs.09-3822. This study report that mature RPE from chick embryos at advanced stages are amenable to ngn1- and ngn3-induced reprogramming and give rises to layers of cells expressing photoreceptor markers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:86–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 42.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–6. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 43.Zappone MV, Galli R, Catena R, et al. Sox2 regulatory sequences direct expression of a beta-geo transgene to telencephalic neural stem cells and precursors of the mouse embryo, revealing regionalization of gene expression in CNS stem cells. Development. 2000;127:2367–82. doi: 10.1242/dev.127.11.2367. [DOI] [PubMed] [Google Scholar]
- 44.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes & Dev. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferri AL, Cavallaro M, Braida D, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–19. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 46.Graham V, Khudyakov J, Ellis P, et al. SOX2functions to maintain neural progenitor identity. Neuron. 2003;39:749–65. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 47.Le RD, Rayner K, Rex M, et al. The transcription factor cSox2 and neuropeptide Y define a novel subgroup of amacrine cells in the retina. J Anat. 2002;200:51–6. doi: 10.1046/j.0021-8782.2001.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48••.Ma W, Yan R-T, Li X, Wang S-Z. Reprogramming RPE cell differentiation in vivo and in vitro with Sox2. Stem Cells. 2009;27:1376–87. doi: 10.1002/stem.48. This report describes studies showing that ectopic expression of Sox2 induce embryonic chick RPE cells in the eye and in dissociated cell culture to loss RPE properties and gain the expression of markers of retinal ganglion cells and that a genetic, inductive relationship exist between Sox2 and bFGF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taranova OV, Magness ST, Fagan MB, et al. SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes & Dev. 2006;20:1187–202. doi: 10.1101/gad.1407906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hagstrom SA, Pauer GJ, Reid J, et al. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet A. 2005:13895–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- 51.Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;4:117–24. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 52.Ma W, Yan R-T, Xie W, Wang S-Z. bHLH genes cath5 and cNSCL1 promote bFGF-stimulated RPE cells to transdifferentiate towards retinal ganglion cells. Dev Biol. 2004;265:320–8. doi: 10.1016/j.ydbio.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 53.Yan R-T, Wang S-Z. Differential induction of gene expression by basic fibroblast growth factor and neuroD in cultured retinal pigment epithelial cells. Vis Neurosci. 2000;17:157–64. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yan R-T, Li H, Wang S-Z. Pro-photoreceptor activity of chick neurogenin1. Invest Ophthalmol Vis Sci. 2009;50:5567–76. doi: 10.1167/iovs.09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma W, Yan R-T, Mao W, Wang S-Z. Neurogenin3 promotes early retinal neurogenesis. Mol Cell Neurosci. 2009;40:187–98. doi: 10.1016/j.mcn.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jasoni CL, Walker MB, Morris MD, Reh TA. A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development. 1994;120:769–83. doi: 10.1242/dev.120.4.769. [DOI] [PubMed] [Google Scholar]
- 57.Mao W, Yan R-T, Wang S-Z. Proneural gene ash1 promotes amacrine cell production in the chick retina. Dev Neurobiol. 2009;69:88–104. doi: 10.1002/dneu.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Haruta M, Kosaka M, Kanegae Y, et al. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat Neurosci. 2001;4:1163–4. doi: 10.1038/nn762. The authors report that the iris tissue in the adult rat eye can generate cells expressing marker for differentiated neurons. Cells expressing rod photoreceptor proteins are produced from iris-derived cells under the guidance of Crx. [DOI] [PubMed] [Google Scholar]
- 59•.Akagi T, Akita J, Haruta M, et al. Iris-derived cells from adult rodents and primates adopt photoreceptor-specific phenotypes. Invest Ophthalmol Vis Sci. 2005;46:3411–9. doi: 10.1167/iovs.04-1112. This study found that under the induction of Crx, adult rat iris-derived cells express photoreceptor-specific proteins. A combination of Crx and NeuroD induce monkey iris-derived cells to adopt photoreceptor-specific phenotypes. The new cells display rod photoreceptor-specific electrophysiological response to light stimuli. [DOI] [PubMed] [Google Scholar]
- 60•.Sun G, Asami M, Ohta H, et al. Retinal stem/progenitor properties of iris pigment epithelial cells. Dev Biol. 2006;289:243–52. doi: 10.1016/j.ydbio.2005.10.035. The authors report that iris pigment epithelial (IPE)-derived cells from post-hatching chicken form spheres of cells expressing retinal progenitor markers in non-adherent culture, express neural phenotypes when cultured on the laminin-coated substratum, and display the phenotypes of photoreceptor neurons and Müller glia when co-cultured with embryonic retinal cells or grafted into embryonic retina. [DOI] [PubMed] [Google Scholar]
- 61•.MacNeil A, Pearson RA, MacLaren RE, et al. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007;25:2430–8. doi: 10.1634/stemcells.2007-0035. This study shows the presence, in the pars plana and iris regions of adult porcine eye, of progenitor cells that proliferate readily, form neurospheres comprised of cells expressing retinal progenitor markers, and on laminin-coated substratum they differentiate into neurons and glia. [DOI] [PubMed] [Google Scholar]
- 62•.Asami M, Sun G, Yamaguchi M, Kosaka M. Multipotent cells from mammalian iris pigment epithelium. Dev Biol. 2007;304:433–46. doi: 10.1016/j.ydbio.2006.12.047. This study characterizes multipotent cells within the iris pigment epithelium (IPE) of postnatal and adult rodents and finds discrete populations of highly-pigmented cells in IPE display a pronounced capacity to form neurospheres and differentiate into photoreceptor cells. [DOI] [PubMed] [Google Scholar]
- 63.Tropepe V, Coles BLK, Chaisson BJ, et al. Retinal stem cells in the adult mouse eye. Science. 2000;287:2032–6. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 64.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–21. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 65•.Gu P, Harwood LJ, Zhang X, et al. Isolation of retinal progenitor and stem cells from the porcine eye. Mol Vis. 2007;13:1045–57. The authors report the isolation of retinal progenitor cells from the ciliary epithelium (CE) of porcine eyes, and find a decrease with age in CE’s capacity to produce primary nestin- and Pax6-immunoreactive neurosphere colonies. [PMC free article] [PubMed] [Google Scholar]
- 66•.Zhao X, Das AV, Bhattacharya S, et al. Derivation of neurons with functional properties from adult limbal epithelium: implications in autologous cell therapy for photoreceptor degeneration. Stem Cells. 2008;26:939–49. doi: 10.1634/stemcells.2007-0727. The authors report that the limbal epithelium (LE)-derived neural progenitors generate neurons with electrophysiological properties typical of functional neurons and can be directly differentiated along rod photoreceptor lineage in vitro and in vivo. [DOI] [PubMed] [Google Scholar]
- 67.Liang L, Ma W, Yan R-T, et al. Exploring RPE as a source of photoreceptors: differentiation and integration of transdifferentiating cells grafted into embryonic chick eyes. Invest Ophthalmol Vis Sci. 2006;47:5066–74. doi: 10.1167/iovs.06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68••.Cicero SA, Johnson D, Reyntjens S, et al. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci USA. 2009;106:6685–90. doi: 10.1073/pnas.0901596106. By showing that CE-derived spheres consist of proliferating pigmented CE cells rather than retinal stem cells, this report presents evidence disputing early reports of the CE in the mammalian eye containing retinal stem cells. In human and mouse CE-derived spheres, all cells display molecular, cellular, and morphological features of differentiated pigmented CE cells, they aberrantly express nestin when exposed to growth factors and low levels of pan-neuronal markers, but retain their pigmented CE cell morphology and fail to differentiate into retinal neurons in vitro or in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi M, Palmer TD, Takahashi J, Gage FH. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol Cell Neurosci. 1998;12:340–8. doi: 10.1006/mcne.1998.0721. [DOI] [PubMed] [Google Scholar]
- 70.Kicic A, Shen WY, Wilson AS, et al. Differentiation of marrow stromal cells into photoreceptors in the rat eye. J Neurosci. 2003;23:7742–9. doi: 10.1523/JNEUROSCI.23-21-07742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Y, Teng FY, Tang BL. Coaxing bone marrow stromal mesenchymal stem cells towards neuronal differentiation: progress and uncertainties. Cell Mol Life Sci. 2006;63:1649–57. doi: 10.1007/s00018-006-6019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–83. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Banin E, Obolensky A, Idelson M, et al. Retinal incorporation and differentiation of neural precursors derived from human embryonic stem cells. Stem Cells. 2006;24:246–57. doi: 10.1634/stemcells.2005-0009. [DOI] [PubMed] [Google Scholar]
- 74•.Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–74. doi: 10.1073/pnas.0601990103. This report describes efficient generation from human embryonic stem cells of retinal progenitor cells, which primarily differentiate into inner retinal neurons (ganglion and amacrine cells), with functional glutamate receptors in culture, but with increased expression of photoreceptor markers when co-cultured with retinas from a mouse with photoreceptor degeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75•.Osakada F, Ikeda H, Mandai M, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol. 2008;26:215–24. doi: 10.1038/nbt1384. The author describe a stepwise protocol that direct mouse, monkey and human embryonic stem cells to differentiate into cells expression genes characteristic of retinal pigment epithelial cells and photoreceptors (cones and rods) [DOI] [PubMed] [Google Scholar]
- 76••.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;9:73–9. doi: 10.1016/j.stem.2008.10.015. This study found that human embryonic stem cells-derived retinal cells differentiate into functional photoreceptors and restore light responses to animals, after transplantation into the eyes of a mouse model of Leber’s congenital amaurosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osakada F, Ikeda H, Sasai Y, Takahashi M. Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc. 2009;4:811–24. doi: 10.1038/nprot.2009.51. [DOI] [PubMed] [Google Scholar]
- 78•.Hirami Y, Osakada F, Takahashi K, et al. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126–31. doi: 10.1016/j.neulet.2009.04.035. Following a stepwise treatment, mouse and human induced pluripotent stem cells give rise to cells that express markers of retinal progenitor cells, retinal pigment epithelial cells, and photoreceptors. [DOI] [PubMed] [Google Scholar]
- 79•.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci USA. 2009;106:16698–166703. doi: 10.1073/pnas.0905245106. This report describes how, following a targeted and stepwise differentiation process, human embryonic stem cells and induced pluripotent stem cells undertake a sequence of stages that mimic retinogenesis during vertebrate embryonic development to eventually differentiating into retinal pigment epithelial cells and cones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lamba DA, McUsic A, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5:e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S-Z. Tales of retinogenesis told by human stem cells. Proc Natl Acad Sci USA. 2009;106:16543–4. doi: 10.1073/pnas.0908643106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otani A, Dorrell MI, Kinder K, et al. Rescue of retinal degeneration by intravitreally injected adult bone marrow-derived lineage-negative hematopoietic stem cells. J Clin Inves. 2004;114:765–74. doi: 10.1172/JCI21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carr AJ, Vugler AA, Hikita ST, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buchholz DE, Hikita ST, Rowland TJ, et al. Derivation of functional retinal pigmented epithelium from induced pluripotent sstem cells. Stem Cells. 2009;27:2427–34. doi: 10.1002/stem.189. [DOI] [PubMed] [Google Scholar]