Abstract
We have previously shown that group V secretory phospholipase A2 (sPLA2) regulates phagocytosis of zymosan and Candida albicans by a mechanism that depends on fusion of phagosomes with late endosomes in macrophages. Here we report that group V sPLA2 (Pla2g5)-null mice exposed to an extract of house dust mite Dermatophagoides farinae (Df) had markedly reduced pulmonary inflammation and goblet cell metaplasia compared to wild-type (WT) mice. Pla2g5-null mice had also impaired Th2-type adaptive immune responses to Df compared to WT mice. Pla2g5-null bone marrow-derived dendritic cells (BMDCs) activated by Df had delayed intracellular processing of allergen and impaired allergen-dependent maturation, a pattern recapitulated by the native lung DCs of Df-challenged mice. Adoptively transferred Df-loaded Pla2g5-null BMDCs were less able than Df-loaded WT BMDCs to induce pulmonary inflammation and Th2 polarization in WT mice. However, Pla2g5-null recipients transferred with WT or Pla2g5-null Df-loaded BMDCs exhibited significantly reduced local inflammatory responses to Df, even though the transfer of WT BMDCs still induced an intact Th2 cytokine response in regional lymph nodes. Thus, the expression of group V sPLA2 in APC regulates Ag processing and maturation of dendritic cells, and contributes to pulmonary inflammation and immune response against Df. Furthermore, an additional yet to be identified resident cell type is essential for the development of pulmonary inflammation, likely a cell in which group V sPLA2 is upregulated by Df and whose function is also regulated by group V sPLA2.
Introduction
Phospholipases A2 (PLA2s) are a family of enzymes that cleave the ester-bound fatty acids from membrane phospholipids (1, 2). PLA2-liberated arachidonic acid is the substrate for prostaglandin and leukotriene generation, a function that absolutely requires cytosolic PLA2α (cPLA2α) (3). Although members of the PLA2 family share common functions such as generation of eicosanoids and lysophospholipids, some PLA2s have additional cell type-specific functions dictated by their subcellular localization. We have previously reported that group V secretory PLA2 (sPLA2) is expressed in the trans-Golgi network and recycling endosomes of mouse peritoneal macrophages (4, 5). Furthermore, group V sPLA2, but not cPLA2α, regulates phagocytosis of zymosan by peritoneal macrophages (5, 6). We have shown that mouse peritoneal macrophages lacking group V sPLA2 have delayed fusion of phagosomes with late endosomes and lysosomes, leading to defective phagocytosis and killing of Candida albicans and increased susceptibility to Candida infection (7).
Phagocytes [neutrophils, macrophages, and dendritic cells (DCs)] are a heterogeneous population of cells that can ingest particles. The fate of the ingested particles depends on the characteristics of the phagocytic cell and of the particles ingested (pathogens, infected cells, apoptotic cells). Phagocytes derived from the monocyte lineage, such as macrophages, effectively clear pathogens and have modest antigen presenting function. In contrast, DCs, also derived from the monocyte lineage, are potent APCs. Immature DCs reside proximal to mucosal surfaces such as those lining the airways (8). When immature DCs encounter Ags in the context of a pathogen-associated molecular pattern (PAMP), they undergo maturation, migrate to the lymph nodes, and present the Ag to T cells (9, 10). This process initiates the adaptive immune response. The PAMP-induced activation step is absolutely required for DCs to undergo maturation and drive adaptive immune responses (8, 10, 11). Allergens are ingested by DCs through endocytosis, processed, and presented on MHC-II to CD4+ Th cells. Ovalbumin (OVA), the most commonly used Ag in studies of allergen-induced pulmonary inflammation, activates DCs only in the context of exogenous adjuvants. Instead, natural allergens often activate DCs by carrying endogenous adjuvants that mimic PAMPs, thereby driving DC maturation and the subsequent adaptive immune response. In particular, allergens derived from house dust mites can directly activate DCs through protein and carbohydrate structures that mimic PAMPs and stimulate pattern recognition receptors on DCs and other cells (12-15).
Previously, group V sPLA2 was shown to be necessary for the development of airway hyperresponsiveness in an OVA-induced mouse model of airway inflammation (16). The mechanisms and cell targets by which group V sPLA2 contributes to pulmonary inflammation were yet to be defined. Because we had previously demonstrated that group V sPLA2 is essential for phagocytic function of peritoneal macrophages, we postulated that it may also have a role in the processing of Ags by DCs. In the present study, we used an extract of the house dust mite Dermatophagoides farinae (Df), which can drive maturation of endogenous lung DCs and induces pulmonary inflammation without need for systemic immunization, to investigate the role of group V sPLA2 in the maturation of DCs, Ag processing, and in the induction of the Df-specific immune response and consequent pulmonary inflammation.
Materials and Methods
Df-induced pulmonary inflammation
Groups of 7- to 9- week-old C57BL/6 wild type (WT) and Pla2g5-null (4) mice received Df extract (3 μg) (Greer Laboratories, Lenoir, NC) in 20 μl of NaCl 0.9% (containing <0.005 EU/ml) (Sigma, St. Louis, MO) or saline alone intranasally on days 0, 4, 7, 11, 14, and 18 (14).
Twenty-four h after the last treatment, mice were euthanized and exsanguinated, and bronchoalveolar lavage (BAL) was performed. BAL fluid cells were cytocentrifuged onto slides, stained with Diff-quick (Fisher Diagnostic, Middletown, VA), and differentially counted. Cell-free BAL fluid was assayed for the content of cysteinyl leukotrienes (cys-LTs; LTC4/LTD4/LTE4) (GE Healthcare BioSciences, Buckinghamshire, UK), prostaglandin (PG) D2, and PGE2 (Cayman Chemical, Ann Arbor, MI) by EIA.
All animal studies described here were approved by the Animal Care and Use Committee of Dana Farber Cancer Institute (Boston, MA).
Histologic assessment of pulmonary inflammation
Left lungs were collected from the mice at the time of euthanasia, fixed for at least 8 h in 4% paraformaldehyde, washed twice with PBS containing 2% DMSO, suspended in 50 mM NH4Cl overnight at 4°C and finally embedded in glycolmethacrylate or paraffin. Two-micrometer-thick sections were stained by the chloroacetate esterase (CAE) reaction to assess inflammatory cell infiltrates. For histological study of the mucus-secreting cells of the airway epithelia (goblet cells), lung sections were stained with Periodic acid-Shiff (PAS). Congo red dye was used to highlight eosinophil infiltrates. The extent of cellular infiltration of the tissue was evaluated on fifteen bronchovascular bundles (BVBs) of comparable large-caliber preterminal bronchi (diameter 200-200 μM) by a pathologist without knowledge of the particular mouse genotype or procedure. The goblet cells positively stained for the presence of mucus at the PAS reaction were enumerated in at least four independent BVBs of each lung and data were expressed as the average of goblet cell counts stained in the bronchi of each section per millimeter of bronchial basal lamina, as measured by Image J (National Institutes of Health image analysis software [http://rsbweb.nih.gov/ij]) (14). For immunofluorescence, lung sections were deparaffinized and rehydrated. Antigen retrieval was performed with Target Retrieval Solution (Dako, Denmark) at 97°C for 30 minutes. After blocking with 10% chicken serum (Santa Cruz Biotechnology, Inc., CA), the rabbit anti-mouse group V sPLA2 antibody (4) was added and incubated at 37°C for 1 hour. Samples were washed and incubated at 37°C for 1 hours with the secondary antibody Alexa Fluor® 594 chicken anti-rabbit IgG (1:1000, Invitrogen Crop., Carlsbad, CA) and nuclear staining reagent Hoechst 33342 (Invitrogen Crop., Carlsbad, CA). The sections were washed and covered with Fluoroscield mounting media (Electron Microscopy Sciences, Hatfield, PA). Lung sections were imaged with an Eclipse 80i microscope (Nikon Instruments Inc., Melville, NY) and pictures were acquired by Hamamatsu C10800 digital camera and HCImage sofeware 1.1.3.1 (Hamamatsu Corp., Bridgewater, NJ).
Real-time quantitative PCR (qPCR) of mRNA transcripts in the lung
Right lings were collected at time of euthanasia and snap frozen. Total RNA was isolated from tissue homogenates with Tri-Reagent (Sigma), reverse transcribed into cDNA (RT2 First Strand kit, SABiosciences, Frederick, MD), and assayed by real-time qPCR for IL-5, IL-13, IL-17A, IFN-γ, Muc5ac, Clca3/Gob-5, and GAPDH on a Mx3005P thermal cycler (Stratagene Santa Clara, CA) with the use of SYBR®Green/ROX® master mix (SABiosciences). The ratio of each mRNA relative to the GAPDH mRNA was calculated with the ΔΔCt method. Primers (Supplemental Table I) were designed with PrimerQuestSM software (IDT, Coralville, IA).
In vitro restimulation of parabronchial lymph node cells
The parabronchial lymph node (PLN) was collected from the upper-right chest of each mouse and homogenized in complete medium [RPMI, 10% FBS, 1% non-essential amino acids, 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 50 μM 2-ME (Sigma)], plus 25 mM HEPES, and 1 mM sodium pyruvate. The red cells were lysed, and 4×106 nucleated cells were incubated for 72 h with medium alone or containing 20 μg/ml Df (14). Cytokine release in the supernatants was measured by ELISA (eBioscience, San Diego, CA).
Measurement of serum Igs
Total IgE levels were measured by ELISA (BD Biosciences, San Jose, CA). To measure Df-specific IgE, mouse serum was incubated at 4°C overnight in a 96-well plate coated with anti-mouse IgE (BD Biosciences). Bound Df-IgE was detected by adding biotinylated Df, streptavidin-horseradish peroxidase conjugate (BD Biosciences), and TMB substrate solution (Invitrogen, Carlsbad, CA). Absorbance was read at 450 nm - 570 nm, and the results were expressed as net O.D. (sample O.D. – blank O.D.). Df-specific IgG1 was measured by ELISA with plate-bound Df, an alkaline phosphatase-conjugated anti-mouse IgG1 (Southern Biotech, Birmingham, AL) and substrate solution (p-nitrophenyl phosphate, Sigma). Absorbance was read at 405 nm, and the results were expressed as net O.D.
Bone marrow-derived DC (BMDC) generation and adoptive transfer
BMDCs were obtained as described (17). Briefly bone marrow from femurs and tibiae of mice was collected, disaggregated, and resuspended at a concentration of 4×105/ml non-RBC in complete medium containing 20 ng/ml recombinant murine GM-CSF (PeproTech, Rocky Hill, NJ). On days 3, 6 and 8 half of the culture supernatant was replaced by equal volume of fresh medium containing GM-CSF. On day 9, the floating cells were harvested; an aliquot was cultured in complete medium with 100 μg/ml Df (Df-BMDCs) (18). After 24 h, cells were analyzed by flow cytometry or instilled intratracheally (5×104/mouse) into anesthetized mice (19). Mice that received Df-BMDC received saline or 3 μg Df intranasally on days 11 and 14 and were euthanized on day 15.
Flow cytometry
BMDCs or Df-BMDCs were fixed in 2% paraformaldehyde, blocked, and incubated (1h, 4°C) with the following Abs: FITC-MHC-II (clone M5/114.15.2), PE-Cy7-CD11c (N418), APC-CD40 (1C10), PE-CD80 (16-10A1), PE-CD86 (GL1) (eBioscience), PE-CD252 (RM134L; BioLegend, San Diego, CA), or with corresponding isotypes as controls. The acquisition was performed on a FACSCanto flow cytometer with FACSDiva software (BD Biosciences), and data were analyzed with FlowJo (Tree Star, Ashland, OR).
Processing of Df by BMDCs
BMDCs were seeded on glass-bottomed 35-mm dishes (World Precision Instruments, Sarasota, FL) and labeled with Cell Tracker™ Orange CMTMR (Invitrogen) according to the manufacturer protocol. After 4 h, 100 μg/ml of Df labeled with an Alexa Fluor-488 labeling kit (Invitrogen) was gently pelleted onto BMDCs. The dishes were incubated for 45 min on ice, washed to remove unbound Ag and transferred to a heated Nikon C1 confocal system (7) equipped with a 40X Planapo lens (NA= 1.3). Each image was volume rendered from 10 to 12 Z-stacks of 0.5 μm with Nikon EZ-C Gold version 3.40 build 691 (7). Time series were acquired at a rate of 1 min per frame: frames were 3 min apart. Videos were made using Photoshop CS3 to animate the volume rendered TIF images.
Statistics
To compare three or more groups we used the Kruskal-Wallis non parametric test with Dunn's post test to correct for multiple comparisons, using Prism software (GraphPad, La Jolla, CA).Variation between two groups with unequal variance was determined using the Mann-Whitney test. All the other comparisons used a two-tailed Student`s t-test. Values of p < 0.05 were considered significant.
Results
Effects of group V sPLA2 deficiency on Df-induced pulmonary inflammation
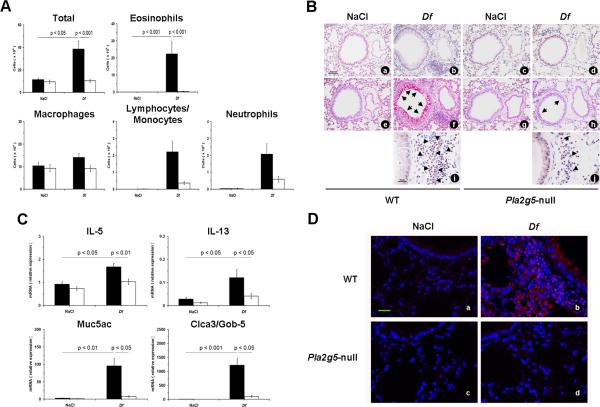
We used a mouse model of allergic pulmonary inflammation induced by Df to elucidate the role of group V sPLA2 in regulating the local innate and adaptive immune responses. The intranasal administration of 3 μg of Df twice weekly for 3 wk induced in WT mice significant increases in total BAL fluid cell numbers and eosinophils (Fig. 1A). In contrast, the total number of cells in the BAL fluid of Df-treated Pla2g5-null mice was no different than the levels of the saline controls (Fig. 1A). Compared to Df-treated WT controls, Pla2g5-null mice had significantly reduced numbers of total BAL fluid cells and eosinophils (Fig. 1A).
Figure 1.
Df-induced pulmonary inflammation in WT and Pla2g5-null mice. A, Total and differential cell counts from BAL fluid of WT (filled bars; n= 6 and 16 in NaCl-and Df-treated groups, respectively) and Pla2g5-null (open bars; n= 7 and 18, respectively) mice. B, Tissue sections of lung showing BVBs from WT (NaCl: a, e; Df: b, f, i) and Pla2g5-null (NaCl: c, g; Df: d, h, j) mice treated with NaCl or Df intranasally were stained by the CAE reation (a-d) for assessing inflammatory cell infiltrates or by PAS reaction (e-h), for depicting mucus-secreting cells (arrows) and by CR dye (i and j) (arrow heads) to demonstrate accumulation of eosinophils. Original magnifications, x20 (CAE and PAS) and x40 (CR). C, Expression of mRNA transcripts for IL-5, IL-13, Muc5ac, and Clca3/Gob-5 in the lung of NaCl- and Df-treated WT (filled bars) and Pla2g5-null (open bars) mice, measured by real-time qPCR. D. Lung sections of WT and Pla2g5-null mice treated with NaCl (a, c) or Df (b, d) intranasally showing staining for group V sPLA2 (red) and nuclei (blu). Original magnification 40X. Scale bar 25 um. (B, D) Pictures are from one representative mouse per group from one of two experiments. Data are expressed as ratio of mRNA expression relative to GAPDH. Values are mean ± SEM from two independent experiments.
Histologic evaluation of the lung tissue (Fig. 1B) revealed that BVBs were invested with inflammatory infiltrates consisting of mononuclear cells along with eosinophils, neutrophils, lymphocytes and plasma cells, and mucus-producing goblet cells in the WT mice treated with Df. Compared to the Df-treated WT mice, the lung tissue of Df-treated Pla2g5-null mice showed markedly reduced BVB inflammation and goblet cell metaplasia. Morphometric analysis of lung preparations revealed that compared to WT controls, Pla2g5-null mice had significant fewer BVBs invested with cellular infiltrates (14.06 ± 0.27 vs. 8.28 ± 0.67 /15 BVBs, p < 0.01) and markedly fewer mucus-producing goblet cells (47.35 ± 2.48 vs. 9.57 ± 1.64 /mm of bronchial basal lamina, p < 0.01).
Because group V sPLA2 has been reported to contribute to eicosanoid generation in certain circumstances (4, 20-22) and eicosanoids have been reported to play a pivotal role in promoting the development of a proallergic Th2 type immune response (23-25) we measured the concentration of eicosanoids in the BAL fluids of WT and Pla2g5-null Df-treated mice. There were not markedly differences between the two genotypes (data not shown).
We then evaluated the effect of the loss of group V sPLA2 on the expression by the lung of cytokines and other inducible transcripts associated with the inflammatory response to Df by real-time qPCR. The lungs of Df-treated mice from both genotypes showed induced expression of transcripts encoding the Th2 cytokines IL-5 and IL-13 (Fig. 1C), as well as IL-17A, and IFN-γ mRNA relative to their respective naïve control mice groups. However, the levels of IL-5 and IL-13 mRNAs were significantly lower in Df-treated Pla2g5-null mice than in WT mice (Fig. 1C). There were similar levels of IL-17A and IFN-γ in the lungs of Pla2g5-null mice and WT mice (data not shown). Transcripts encoding the IL-13-inducible mucus-associated proteins Muc5ac and Clca3/Gob-5 (26, 27) were strongly expressed in the lungs of the WT mice treated with Df, and were much lower in the lungs of Df-treated Pla2g5-null mice (Fig. 1C).
To determine whether group V sPLA2 protein was constitutively expressed in the lung and upregulated in response to Df, we performed immunohistochemistry using a polyconal antibody to group V sPLA2 (4, 5) on paraffin-embedded lung sections. Compared with the lungs of the naïve WT controls, the lungs of Df-treated WT mice showed strong staining for group V sPLA2 (Fig. 1D). The staining for group V sPLA2 localized to cells with the anatomic locations and morphologies typical of granulocytes, macrophages, and goblet cells. In contrast, there was no staining in the lungs of either naïve or Df-treated Pla2g5 mice.
Adaptive immune response to Df in WT and Pla2g5-null mice
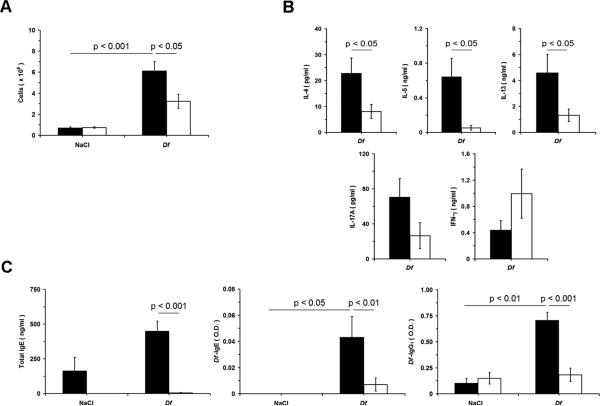
Next, we investigated the contribution group V sPLA2 to the adaptive immune response induced by Df Ags in LN. PLNs collected from saline or Df-treated WT and Pla2g5-null mice were isolated and cells were counted and stimulated with Df 20 μg/ml to evaluate the profile of cytokines released in the supernatant. The numbers of cells in the draining PLN were increased in both WT and Pla2g5-null mice receiving Df. However, the number of cells from the PLN of Df-treated Pla2g5-null mice was significantly lower than that from Df-treated WT mice (Fig. 2A). PLN cells (equalized for cell number) from WT and Pla2g5-null mice restimulated with the Df released IL-4, IL-5, IL-13, IL-17A, and IFN-γ; but the levels of IL-4, IL-5, and IL-13 from Pla2g5-null PLN cells were significantly lower than those from WT cells (Fig. 2B). The levels of IL-17A and IFN-γ in the supernatants of Pla2g5-null PLN cells compared to those from WT cells were not significantly different (Fig. 2B). The serum levels of Df-specific IgE, and Df-specific IgG1 significantly increased after treatment of WT mice with Df. In contrast, the levels of these Abs and the total IgE were dramatically lower in the serum of the Pla2g5-null mice than those in the WT controls (Fig. 2C).
Figure 2.
PLN cellularity, cytokine release from restimulated PLN cells, and serum Igs in NaCl- and Df-treated WT and Pla2g5-null mice. PLNs and serum were obtained from WT (filled bars; n= 6 and 16 in NaCl- and Df-treated groups, respectively) and Pla2g5-null (open bars; n= 7 and 18, respectively) mice. A, Number of cells obtained from PLNs. ELISA measurement of (B) cytokine release from restimulated PLN cells and (C) total serum IgE and Df-specific IgE and IgG1. Values are mean ± SEM from two independent experiments.
Processing of Df by WT and Pla2g5-null BMDCs
To determine whether DCs lacking group V sPLA2 could process Df Ags normally, we pulsed BMDCs from WT (Video 1) and Pla2g5-null mice (Video 2) with Alexa Fluor-488-labeled Df and followed the movement of the labeled Ag for 2 h to allow for the degradation of labeled Df. Fluorescence decreased progressively in WT BMDCs, but persisted in the Pla2g5-null BMDCs. At 2 h, the fluorophore was still visible in most of the Pla2g5-null BMDCs but was largely absent in the WT BMDCs.
Df-induced maturation of WT and Pla2g5-null BMDCs
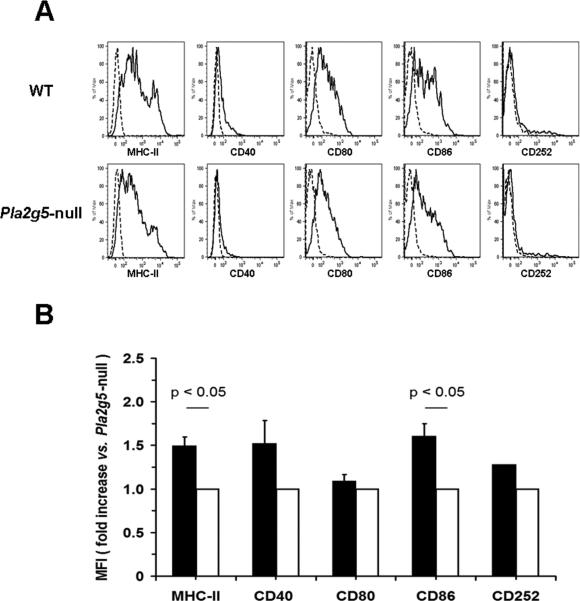
In another set of experiments we sought to evaluate the effects of the lack of group V sPLA2 on the maturation of BMDCs induced by Df. Naïve BMDCs from WT and Pla2g5-null mice expressed comparable levels of CD40, CD80, CD86, and CD252, whereas the expression of MHC-II was slightly diminished on Pla2g5-null BMDCs (data not shown). After ex vivo pulse with Df for 24 h (18), the surface expression of MHC-II, and CD86, increased in WT Df-BMDCs more than in Pla2g5-null Df-BMDCs (Fig. 3B, as shown for one experiment, Fig. 3A). The surface expressions of CD40, CD80 and CD252 were slightly reduced on Pla2g5-null Df-BMDCs than on WT Df-BMDCs.
Figure 3.
Df-induced maturation in WT and Pla2g5-null BMDCs. A, Expression of MHC-II, CD40, CD80, CD86, and CD252 (solid lines) vs. the isotype controls (dashed lines) on gated CD11c+ Df-BMDCs from one experiment representative of three. B, Net (negative control subtracted) mean fluorescence intensity (MFI) of MHC-II, CD40, CD80, CD86, and CD252 expression on WT (filled bars) and Pla2g5-null (open bars) Df-BMDCs evaluated by flow cytometry and expressed as fold increase vs. Pla2g5-null Df-BMDCs. Values are mean ± SEM from three independent experiments.
To determine whether the absence of group V sPLA2 altered DC maturation in vivo, we isolated CD11c+ cells from the lungs of WT and Pla2g5-null Df-treated mice by mechanical dispersion followed by a density gradient. The WT CD11c+ cells had ~1.5 fold increased expression of MHC-II compared to Pla2g5-null cells in two experiments (data not shown).
Role of group V sPLA2 expression by DCs and resident lung cells in pulmonary inflammation and adaptive immunologic responses induced by Df
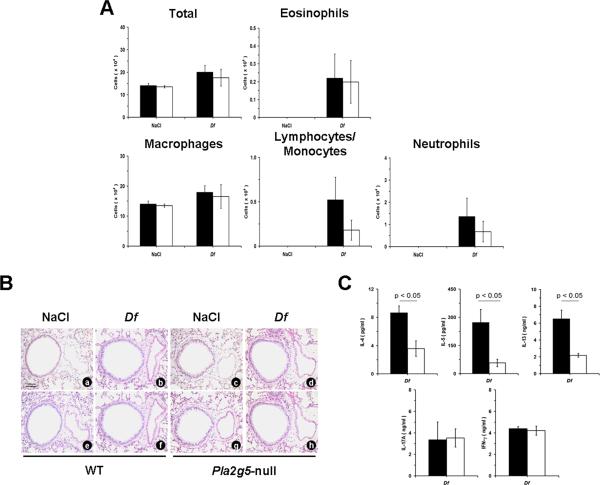
To understand whether the defective function of DCs lacking group V sPLA2 could contribute to the impaired local and acquired immune response to Df in vivo, WT naïve mice were treated intranasally with saline or 3 μg of Df after intratracheal engraftment with WT or Pla2g5-null BMDCs loaded in vitro with Df (28). The transfer of WT and Pla2g5-null Df-BMDCs did not induce any response in the airways of WT recipient mice in the absence of intranasal challenge with Df (Fig. 4A). However, the intranasal administration of Df to WT mice that were engrafted with WT Df-BMDCs elicited an increase in BAL fluid cellularity and eosinophils. In contrast, WT mice receiving Pla2g5-null Df-BMDCs and challenged with Df had substantially fewer total BAL fluid cells and eosinophils (Fig. 4A). These findings were mirrored in the histologic analysis of lung tissue, in which the transfer of WT Df-BMDCs into WT recipients tended to elicit more infiltration of BVBs and goblet cell metaplasia after Df challenge than did the transfer of Pla2g5-null Df-BMDCs (Fig. 4B). Likewise, the transfer of WT Df-BMDCs resulted in trends toward greater numbers of lymph node cells (data not shown), and restimulation of these cells with Df resulted in higher levels of IL-4, IL-5, and IL-13 than did samples from mice receiving Pla2g5-null Df-BMDCs (Fig 4C). IL-17A and IFN-γ levels were not significantly different between the WT mice receiving WT and Pla2g5-null Df-BMDCs (Fig. 4C).
Figure 4.
Pulmonary inflammation and cytokine release from restimulated PLNs in WT mice adoptively transferred with WT and Pla2g5-null Df-BMDCs. A, Total cell and differential counts from BAL fluid of WT mice receiving WT (filled bars; n= 2 and 13 in NaCl- and Df-treated groups, respectively) or Pla2g5-null (open bars; n= 2 and 11, respectively) Df-BMDCs. B, Sections of lung showing BVBs from WT mice receiving WT (NaCl: a, e; Df: b, f) or Pla2g5-null (NaCl: c, g; Df: d, h) Df-BMDCs were stained by CAE (a-d) reaction or by PAS reactions (e-h, arrows point to mucus-secreting cells). C, ELISA measurement of cytokine release from restimulated PLN cells of Df-treated WT mice receiving WT (filled bars; n= 12) or Pla2g5-null (open bars; n= 7) Df-BMDCs. Pictures in (B) are from one representative mouse per group from one of two experiments. Original magnifications, x20. Other data are mean ± SEM from two independent experiments.
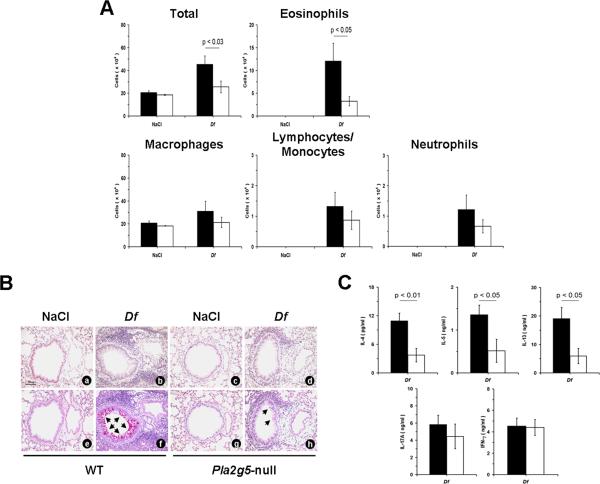
To determine whether expression of group V sPLA2 by resident cells in the host tissue also influenced the response to Df in the BMDC transfer protocol, Pla2g5-null mice received transfers of both WT Df-BMDCs and Pla2g5-null Df-BMDCs. In these experiments, Df treatment of the recipient mice resulted in very little cellular response in the BAL fluid, regardless of the BMDC genotype (Fig. 5A). The recipients of WT Df-BMDCs had similar number of monocytes/lymphocytes, neutrophils, and eosinophils compared with the recipients of Pla2g5-null Df-BMDCs (Fig. 5A). The infiltration of BVBs and the metaplasia of goblet cells were very mild and not evidently different in mice receiving WT Df-BMDCs and Pla2g5-null cells (Fig. 5B). The numbers of cells obtained from the PLNs of mice receiving WT Df-BMDCs were not significantly different from those of mice receiving Pla2g5-null Df-BMDCs (data not shown). However, the restimulated PLN cells from the Pla2g5-null mice receiving WT Df-BMDCs released significantly more IL-4, IL-5, and IL-13 than did the PLN cells from the recipients of Pla2g5-null Df-BMDCs. There were no differences in IL-17A or IFN-γ levels (Fig. 5C).
Figure 5.
Pulmonary inflammation and cytokine release from restimulated PLNs in Pla2g5-null mice adoptively transferred with WT and Pla2g5-null Df-BMDCs. A, Total cell and differential counts from BAL of Pla2g5-null mice transferred with WT (filled bars; n= 2 and 6 in NaCl- and Df-treated groups, respectively) or Pla2g5-null (open bars; n= 2 and 5, respectively) Df-BMDCs. B, Sections of lung showing BVBs from Pla2g5-null mice receiving with WT (NaCl: a, e; Df: b, f) or Pla2g5-null (NaCl: c, g; Df: d, h) Df-BMDCs and treated with NaCl or Df intranasally were stained by the CAE (a-d) or by the PAS (e-h) reactions. C, ELISA measurement of cytokine release from restimulated PLN cells of Df-treated Pla2g5-null mice receiving with WT (filled bars; n= 4) or Pla2g5-null (open bars; n= 4) Df-BMDCs. Pictures in (B) are from one representative mouse per group from one of two experiments. Original magnifications, x20. Other data are mean ± SEM from two independent experiments.
Discussion
We have previously shown that group V sPLA2 regulates phagocytosis through different receptors and that this function is related to ability of group V sPLA2 to regulate phagosome maturation in macrophages (5, 7). This mechanism also contributes to phagocytosis and killing of Candida albicans and to the in vivo innate immune response against the pathogen, which are greatly impaired in Pla2g5-null mice. (7). Df is an extract from a clinically relevant house dust mite that activates innate resident APCs as a prerequisite to the adaptive immune response and inflammation (8, 11). Group V sPLA2 contributes to the development of airway hyperresponsiveness in an OVA-induced model of pulmonary disease in which allergic sensitization is induced by intraperitoneal injection of OVA plus Alum as an adjuvant (16). We postulated that group V sPLA2 might play a prominent role in a Df-induced model due to its demonstrated importance in macrophages and the ability of Df to activate endogenous APCs in the lung. We first compared the cellular components of BAL fluids obtained from WT and Pla2g5-null mice treated with intranasal Df at a dose sufficient to induce moderate pulmonary inflammation in WT C57BL/6 mice. The importance of group V sPLA2 in this model was supported by the markedly reduced numbers of total cells and granulocytes (Fig. 1A) in the BAL fluid of the Df-treated Pla2g5-null mice relative to the Df-treated WT controls. This included a near absence (95% reduction) of eosinophils, a cellular surrogate of a Th2-type immune response. These findings were further supported by the reduced bronchovascular cellular infiltrates (Fig. 1B). The markedly decreased numbers of goblet cells (Fig. 1B) and associated goblet cell transcripts (Fig. 1C) in the lungs of the Df-treated Pla2g5-null mice compared to WT controls are also consistent with the defective generation of Th2 cytokines observed in these animals. In particular, the decreased expression of IL-5 is consistent with the reduced numbers of eosinophils, whereas the reduction in IL-13 correlated with the diminution in goblet cell metaplasia (Fig. 1C). Df contains ligands for C-type lectin receptors, protease activated receptors, and likely other pattern recognition receptors that contribute to breaking tolerance to the allergen by inducing expression of cytokines and enzymes in resident cells in the lung. We speculate that group V sPLA2 is one of these inducible products, since its expression is strikingly upregulated in the lung of Df-treated WT mice in several target cell types (Fig. 1D). These data indicate that group V sPLA2 plays a robust role in local inflammatory responses induced by a clinically relevant antigen, and its absence impairs local expression of Th2 cytokines and its downstream markers typical of Df-driven pulmonary inflammation (29).
To directly test whether group V sPLA2 was involved in the generation of a Th2 response to Df, we measured cytokine generation by restimulated parabronchial lymph node cells (mostly reflecting antigen-specific CD4+ T cells), as well as the levels of the Th2 signature antibodies (IgE and IgG1) in the serum (30, 31). Both the total numbers of cells in the PLNs (Fig. 2A) and the generation of IL-4, IL-5, and IL-13, but not IFN-γ (Fig. 2B) were significantly lower in the Pla2g5-null mice than in the samples from the WT controls, suggesting that group V sPLA2 is required for the local induction of a Th2 type immune response. Furthermore, the production of the Th2-associated Abs was virtually absent in Pla2g5-null mice (Fig. 2C). IL-5 is required for eosinophilia (32, 33), whereas IL-13 is needed to induce goblet cell metaplasia (34, 35), and IL-4 required for induction of IgE and IgG1 (33). Thus, the reduced inflammatory response induced by Df in the lungs of Pla2g5-null mice, along with the markedly diminished antibody responses, are consistent with the lack of pathogenetic Th2 cytokines. This suggests that group V sPLA2 regulates a proximal mechanism in the immune response to Df.
Next we tested the hypothesis that the defective Df-induced Th2 response in the Pla2g5-null mice reflected a requirement for this enzyme in antigen presentation by DCs, an essential proximal step in the pulmonary immune response to antigens. We have previously reported that group V sPLA2 localizes in the trans-Golgi network and in the recycling endosomes of peritoneal macrophages (5), and regulates phagocytosis by a mechanism dependent on delay in fusion of phagosomes with late endosomes and lysosomes (7). Ag presentation by DCs likewise requires intracellular trafficking and degradation of Ags to reach late endosomes and lysosomes where the peptides generated during this process are loaded onto MHC-II (36). We investigated the role of group V sPLA2 in processing of Alexa-488-labeled Df by BMDCs using live cell imaging. In WT Df-BMDCs, the fluorescence emitted by the labeled antigens progressively diminished and was almost lost after 2 h of observation (Video 1). In Df-BMDCs derived from Pla2g5-null mice, the labeled antigen was still visible after 2 h in most of the cells (Video 2). These data are consistent with defective or delayed degradation of the labeled Ag in Pla2g5-null BMDCs during the movement of Df Ags from the surface to the late endosomes and lysosomal compartment.
When DCs take up Ags in the context of PAMPs, they undergo maturation, as indicated by increased expression of MHC-II and costimulatory molecules (10, 37). Df mimic PAMPs and therefore can directly activate DCs and induce their maturation (12, 13). Whereas in vitro stimulation of WT and Pla2g5-null BMDCs with Df induced maturation and upregulation of costimulatory molecules in both genotypes (Fig. 3A), the Pla2g5-null Df-BMDCs expressed significant less MHC-II and CD86 (Fig. 3B). Thus, group V sPLA2 plays a critical role not only in Ag processing, but also in the maturation of BMDCs following Df loading. Furthermore the analysis of CD11c+ cells isolated from the lung of Df-treated mice confirmed that the expression of group V sPLA2 is important for maturation of APC. To our knowledge this is also the first time that a secretory PLA2 has been shown to regulate the function of an APC.
To address whether the defect in Ag processing in Pla2g5-null BMDCs can contribute to the development of pulmonary inflammation in vivo, we used an adoptive transfer model of BMDC-dependent sensitization to Df (28). WT and Pla2g5-null Df-BMDCs were transferred to WT recipient mice, which then were challenged twice with Df after Df-BMDC engraftment. The transfer of Pla2g5-null Df-BMDCs resulted in substantially less BAL fluid cellularity and granulocyte accumulation (Fig. 4A) after Df challenge than did transfers of WT Df-BMDCs. This pattern was recapitulated in the histologic assessments of inflammation and goblet cell metaplasia (Fig. 4B). The fact that the production of Th2 cytokines by restimulated PLN cells was selectively impaired in the WT recipients of Pla2g5-null Df-BMDCs is consistent with the hypothesis that the impaired APC function of BMDCs (Fig. 4C) lacking group V sPLA2 is a key determinant of the immunologic phenotype in this model.
Since group V sPLA2 is expressed by resident cells in the lung other than DCs (epithelium, smooth muscle and macrophages as well as infiltrating granulocytes, Fig. 1D) (16), we performed transfers of both WT Df-BMDCs and Pla2g5-null Df-BMDCs into Pla2g5-null recipients to determine the contributions of non-DCs to the phenotype. Regardless of the Df-BMDC genotype, Pla2g5-null recipients showed very little BAL cellularity, tissue infiltration and goblet cell metaplasia when challenged with Df (Fig. 5, A and B). Nevertheless, restimulation of the PLN cells isolated from Pla2g5-null mice transferred with WT Df-BMDCs elicited a significant production of IL-5 and IL-13 (Fig. 5C), with significantly diminished responses from the PLN cells isolated from the recipients of Pla2g5-null Df-BMDCs. While the latter data indicate that the BMDC-associated group V sPLA2 is the primary determinant of the Df-induced Th2 immune response in this model, the expression of group V sPLA2 in at least one other resident lung cell is critical to subsequent development of Df -driven pulmonary inflammation.
It is important to notice some limitations of our study. Further work is needed to define the other cell types in which group V sPLA2 plays an important role in this model, as well as the intracellular mechanisms by which group V sPLA2 regulates DC maturation and antigen processing. Although Df is an allergen clinically relevant to human asthma, the Df model of pulmonary inflammation only reproduce some of the features of the human asthma. Furthermore the function of group V sPLA2 could be different in other paradigms of allergic airway inflammation or in different strains of mice.
In summary, our study demonstrates for the first time that group V sPLA2 contributes to the pathogenesis of Df-induced pulmonary inflammation through a mechanism that is unique among the enzymes of the PLA2 family: the presence of group V sPLA2 in DCs is critical for Df processing and cell maturation, prerequisites to Df-induced adaptive immune response and inflammation. Using an adoptive transfer model of dendritic cell-dependent sensitization to Df, we demonstrate that the development of a pulmonary Th2 response to dust mite allergens depends on group V sPLA2 expression by DCs, but that expression of the same enzyme by a resident cell population is essential for subsequent pulmonary inflammation. Therefore, group V sPLA2 might represent a therapeutic target for pharmacologic intervention in the treatment of diverse types of human asthma.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health Grants AI064226 (to B.B.), HL070946 (to H.R.K.), AI52353, AI31599, AI07306, HL36110 and by generous contributions from the Vinik Family (to J.A.B). G.G. is supported in part by an educational grant from the Ph.D. Program in Clinical Pathophysiology and Experimental Medicine of the University of Naples “Federico II”, Naples, Italy.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy And Infectious Diseases or the National Institutes of Health.
Abbreviations used in this paper
- PLA2
phospholipase A2
- cPLA2
cytosolic PLA2
- sPLA2
secretory PLA2
- DC
dendritic cell
- PAMP
pathogen-associated molecular pattern
- Df
extract of house dust mite Dermatophagoides farinae
- Pla2g5
gene encoding for group V sPLA2
- BAL
bronchoalveolar lavage
- or cys-LT
cysteinyl leukotriene
- PG
prostaglandin
- CAE
chloroacetate esterase
- PAS
periodic acid Schiff
- BVB
bronchovascular bundle
- CR
Congo red
- PLN
parabronchial lymph node
- BMDC
bone marrow-derived DC
- CMTMR
5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine)
References
- 1.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 3.Gijon MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. 2000;275:20146–20156. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 4.Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP. Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J Biol Chem. 2004;279:16488–16494. doi: 10.1074/jbc.M313748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, Arm JP. Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis. J Biol Chem. 2006;281:6691–6698. doi: 10.1074/jbc.M508314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotti M, Evans JH, Burke D, Leslie CC. Cytosolic phospholipase A2 translocates to forming phagosomes during phagocytosis of zymosan in macrophages. J Biol Chem. 2004;279:19113–19121. doi: 10.1074/jbc.M313867200. [DOI] [PubMed] [Google Scholar]
- 7.Balestrieri B, Maekawa A, Xing W, Gelb MH, Katz HR, Arm JP. Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans. J Immunol. 2009;182:4891–4898. doi: 10.4049/jimmunol.0803776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 11.Willart MA, Hammad H. Alarming Dendritic Cells for Allergic Sensitization. Allergol Int. 59 doi: 10.2332/allergolint.09-RAI-0162. [DOI] [PubMed] [Google Scholar]
- 12.Barrett NA, Maekawa A, Rahman OM, Austen KF, Kanaoka Y. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J Immunol. 2009;182:1119–1128. doi: 10.4049/jimmunol.182.2.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundequist A, Nallamshetty SN, Xing W, Feng C, Laidlaw TM, Uematsu S, Akira S, Boyce JA. Prostaglandin E2 exerts homeostatic regulation of pulmonary vascular remodeling in allergic airway inflammation. J Immunol. 2010;184:433–441. doi: 10.4049/jimmunol.0902835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, Karp CL. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz NM, Meliton AY, Arm JP, Bonventre JV, Cho W, Leff AR. Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice. J Immunol. 2007;179:4800–4807. doi: 10.4049/jimmunol.179.7.4800. [DOI] [PubMed] [Google Scholar]
- 17.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 18.Machida I, Matsuse H, Kondo Y, Kawano T, Saeki S, Tomari S, Obase Y, Fukushima C, Kohno S. Cysteinyl leukotrienes regulate dendritic cell functions in a murine model of asthma. J Immunol. 2004;172:1833–1838. doi: 10.4049/jimmunol.172.3.1833. [DOI] [PubMed] [Google Scholar]
- 19.Brown RH, Walters DM, Greenberg RS, Mitzner W. A method of endotracheal intubation and pulmonary functional assessment for repeated studies in mice. J Appl Physiol. 1999;87:2362–2365. doi: 10.1152/jappl.1999.87.6.2362. [DOI] [PubMed] [Google Scholar]
- 20.Munoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O'Leary E, Myou S, Zhu X, Bonventre JV, Leff AR, Cho W. Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils. J Biol Chem. 2003;278:38813–38820. doi: 10.1074/jbc.M302476200. [DOI] [PubMed] [Google Scholar]
- 21.Wijewickrama GT, Kim JH, Kim YJ, Abraham A, Oh Y, Ananthanarayanan B, Kwatia M, Ackerman SJ, Cho W. Systematic evaluation of transcellular activities of secretory phospholipases A2. High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells. J Biol Chem. 2006;281:10935–10944. doi: 10.1074/jbc.M512657200. [DOI] [PubMed] [Google Scholar]
- 22.Balestrieri B, Arm JP. Group V sPLA2: classical and novel functions. Biochim Biophys Acta. 2006;1761:1280–1288. doi: 10.1016/j.bbalip.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Henderson WR, Jr., Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, Rudensky AY, Gelb MH. Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J Exp Med. 2007;204:865–877. doi: 10.1084/jem.20070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell-dependent pulmonary inflammation. J Immunol. 2006;176:4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- 25.Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206:2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakanishi A, Morita S, Iwashita H, Sagiya Y, Ashida Y, Shirafuji H, Fujisawa Y, Nishimura O, Fujino M. Role of gob-5 in mucus overproduction and airway hyperresponsiveness in asthma. Proc Natl Acad Sci U S A. 2001;98:5175–5180. doi: 10.1073/pnas.081510898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 28.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173:6384–6392. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 30.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production By T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–1747. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gavett SH, Chen X, Finkelman F, Wills-Karp M. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 32.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA, Sheppard D, Erle DJ. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- 35.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 36.van Niel G, Wubbolts R, Stoorvogel W. Endosomal sorting of MHC class II determines antigen presentation by dendritic cells. Curr Opin Cell Biol. 2008;20:437–444. doi: 10.1016/j.ceb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Pierre P, Turley SJ, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman RM, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]; Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res. 2009;50(Suppl):S237–242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.