Abstract
Introduction
Thirty million men in the United States suffer from erectile dysfunction (ED) and this number is expected to double by 2025. Considered a major public health problem, which seriously affects the quality of life of patients and their partners, ED becomes increasingly prevalent with age and chronic smoking is a major risk factor in the development of ED.
Aim
To review available evidence concerning the effects of cigarette smoking on vascular changes associated with decreased nitric oxide (NO) bioavailability and increased reactive oxygen species (ROS) generation.
Methods
We examined epidemiological and clinical data linking cigarette smoking and ED, and the effects of smoking on vascular NO bioavailability and ROS generation.
Main Outcome Measures
There are strong parallels between smoking and ED and considerable evidence supporting the concept that smoking-related ED is associated with reduced bioavailability of NO because of increased ROS.
Results
Cigarette smoking-induced ED in human and animal models is associated with impaired arterial flow to the penis or acute vasospasm of the penile arteries. Long-term smoking produces detrimental effects on the vascular endothelium and peripheral nerves and also causes ultrastructural damage to the corporal tissue, all considered to play a role in chronic smoking-induced ED. Clinical and basic science studies provide strong indirect evidence that smoking may affect penile erection by the impairment of endothelium-dependent smooth muscle relaxation or more specifically by affecting NO production via increased ROS generation. Whether nicotine or other products of cigarette smoke mediate all effects related to vascular damage is still unknown.
Conclusions
Smoking prevention represents an important approach for reducing the risk of ED. The characterization of the components of cigarette smoke leading to ED and the mechanisms by which these components alter signaling pathways activated in erectile responses are necessary for a complete comprehension of cigarette smoking-associated ED.
Keywords: Erectile Dysfunction, Cigarette Smoking, Passive Smoking, Nitric Oxide, Nitric Oxide Synthase, Reactive Oxygen Species
Physiology of Erection
Penile erection is determined by pressure changes in the cavernosal sinuses. The vasculature of the erectile tissue differs from most vascular beds as it is composed of arterioles and hollow blood-filled sinuses, both of which are lined with smooth muscle and endothelial cells [1,2].
In the absence of arousal stimuli, cavernosal vasoconstriction maintains the penis in the nonerect state. Contraction of the cavernosal smooth muscle, mainly in response to norepinephrine released from the sympathetic innervation of the penis, narrows the arteriolar lumen and sinusoidal cavities, restricting blood flow to maintain low intracavernosal pressure (ICP) and a nonerect (flaccid) penis [1,2]. During sexual arousal or nocturnal tumescence, the release of nitric oxide (NO) (predominantly through the activation of nitric oxide synthase [NOS] in nonadrenergic noncholinergic [NANC] nerves and local endothelial cells) stimulates smooth muscle relaxation [1–3]. The resulting dilation of the cavernosal arterioles and sinuses results in increased blood flow (driven by the force of the arterial blood pressure) and a subsequent rise in ICP. This initial rise in ICP activates a veno-occlusive mechanism to limit the outflow of blood and further increase the pressure inside the cavernosum. The erectile response ensues as the force of the elevated pressure expands the outer tunica albuginea of the penis, resulting in the increased penile length and diameter characteristic of erection.
Although various vasodilators, such as acetylcholine and vasoactive intestinal peptide, have been implicated in the erectile response, NO is thought to be the principal stimulator of cavernosal vasodilation and penile erection. [3,4]. NO is formed from the precursor amino acid, L-arginine, by enzymatic action of NOS, which exists as three main isoforms: neuronal NOS (nNOS), inducible (iNOS), and endothelial NOS (eNOS). All three isoforms have been detected in the penis, although nNOS and eNOS are the main constitutively active NOS enzymes expressed in penile tissues. nNOS is found in the cavernous nerves and their terminal endings within the corpora cavernosa, as well as in the branches of the dorsal penile nerves and nerve plexuses in the adventitia of the deep cavernous arteries [4,5]. eNOS is largely found in the endothelial cells covering the cavernous spaces and helicine arteries, but not in the trabecular smooth muscle cells [6]. Endothelium-derived NO, largely generated in response to strain forces brought on by increased blood flow and pressure in the penis, is believed to sustain the erectile process and, in some specific conditions, may even guarantee erectile function in the absence of nNOS [7]. Upon its release, NO diffuses locally into adjacent smooth muscle cells of the corpus cavernosum and binds to soluble guanylyl cyclase, which catalyzes the conversion of guanosine trisphosphate to 3′,5′-cyclic guanosine monophosphate (cGMP). This cyclic nucleotide then activates protein kinase G, also known as cGMP-dependent protein kinase I, which decreases cytosolic calcium (Ca2+) by various mechanisms. The decay in cytosolic Ca2+ concentration induces relaxation of the vascular and cavernosal smooth muscle cells, leading to dilation of arterial vessels, increased blood flow into the sinuses of the corpora cavernosa, and penile erection [7,8].
Erectile Dysfunction and Smoking
Epidemiological and Clinical Data
Erectile dysfunction (ED), defined as the inability to attain and/or maintain penile erection [9], is a multifactorial condition that is estimated to affect more than 150 million men worldwide, with this number expected to double by 2025 [10,11]. Considered a major public health problem, which seriously affects the quality of life of patients and their partners, ED becomes increasingly prevalent with age. In addition, the presence of chronic illness (e.g., heart disease, hypertension, and diabetes mellitus), alcohol or drug abuse, sedentary lifestyle, and smoking are major risk factors for ED [10,11].
Data from the 2003 National Health Interview Survey (NHIS) indicated that approximately 21.6% of adults in the United States are current smokers [12]. Although the percentage is much lower than that reported in other countries (e.g., in China, in 2000, 60.2% of men and 6.9% of women aged 35–74 years are current smokers [13]), and the prevalence is lower than that reported in 2001 and 2002, the rate of decline is not sufficient to meet the National Health Service objective for 2010 (12%). In addition, an increase in smoking among adolescents was reported [14].
While it is clear that ED is multifactorial, the direct and negative effects of smoking on erectile function are well documented. Several epidemiological studies have shown that cigarette smoking not only increases the risk of ED, but also amplifies the risk of ED associated with other risk factors (e.g., hypertension, diabetes, and dyslipidaemia) or with aging [10,15–25].
Epidemiological data linking cigarette smoking and ED has been collected worldwide. In the United States, the Massachusetts Male Aging Study, which evaluated ED in men aged 40–70 years, indicated that cigarette smoking almost doubled the likelihood of moderate or complete ED at up to 10 years of follow-up [26]. In addition, a study of veterans from the Vietnam-era, aged 31–49 years, showed that a higher percentage of smokers reported ED problems in comparison to nonsmokers [15]. It has also been shown that the magnitude of the association between smoking and ED decreases across increasingly older age groups, suggesting that smoking may have a more apparent impact on erectile function in young male smokers than it does in older male smokers [27]. The greater magnitude of association between smoking and ED in young men is quite interesting because other traditional causes of ED are not as prevalent in this group as compared with older age groups. Importantly, this study also showed that pack-years of smoking is significantly associated with ED in former and current smokers [27]. Similar findings have been reported by studies conducted in other populations: Turkey [20], Brazil [21], Italy [21,22], Japan [21], Malaysia [21], Australia [24], Belgium [28], Netherlands [29], China [30], Canada [31], Finland [32], and the United Kingdom [33].
Many of these studies were conducted among patients with established cardiovascular diseases (atherosclerosis, diabetes, and hypertension). However, a study including 7,684 Chinese men, aged 35–74 years, showed that smoking is also associated with ED in men without clinical vascular disease [30]. The increased odds ratio of ED was directly correlated with the number of cigarettes smoked per day and the association between smoking and ED was even stronger in participants with diabetes [30].
A very recent report, the Boston Area Community Health (BACH) survey, has shown that although the association between passive smoking and ED is not statistically significant, the magnitude of the effect of passive smoking is comparable to 10–19 pack-years of smoking exposure [34]. A previous study showed that men exposed to passive smoking are at twice the risk of developing ED over a 9-year follow-up period [26]. These results suggest that although the increased risk in ED with passive smoking is small, long-term chronic exposure to passive smoking may have adverse effects on erectile function. Importantly, secondhand smoking or passive smoking also increases the risk of heart disease (by approximately 30%) and adversely affects vascular function [35]. Despite the fact that the dose of smoke delivered to active smokers is 100 times or more than that delivered to a passive smoker, the relative risk of cardiovascular disease for smokers is 1.78 compared with 1.31 for passive smokers. In many cases, the effects of even brief (minutes to hours) passive smoking are nearly as large as those from chronic active smoking. Passive smoking leads to 68–86% of the risk of light smoking, depending on the level of secondhand smoke exposure [35].
Cigarette smoking-induced ED in human and animal models has been associated with impaired arterial flow to the penis or acute vasospasm of the penile arteries. The relative risk of developing atherosclerosis in the penis and subsequent ED is 1.31 for each 10 pack-years smoked [17] and 86% of smokers have an abnormal penile vascular evaluation [16]. Furthermore, penile rigidity during nocturnal erection inversely correlates with the number of cigarettes smoked per day [18]. The effects of smoking on the vascular endothelium [36–39] and peripheral nerves [40,41] have been considered to play a role in chronic smoking-induced ED. Furthermore, long-term smoking causes ultrastructural damage to the corporal tissue and increases vascular stiffness [42].
Smoking, NO, and Oxidative Stress
As NO is thought to be the principal stimulator of cavernosal relaxation and penile erection [3,4], smoking- or cigarette-induced decreases in the synthesis or availability of NO may lead to ED. An insufficient synthesis of NO, leading to impaired corporal smooth muscle relaxation, occurs in either of two ways: (i) endothelial damage leading to reduced activity and/or down-regulation of eNOS; or (ii) decrease in the activity or levels of the penile nNOS. Accordingly, studies from several laboratories and clinical trials have demonstrated that both acute and chronic smoking cause impairment of NO production [36–38,43–48]. Most of these studies have addressed the effects of smoking on NO produced by eNOS activation or NO derived from the endothelial cells.
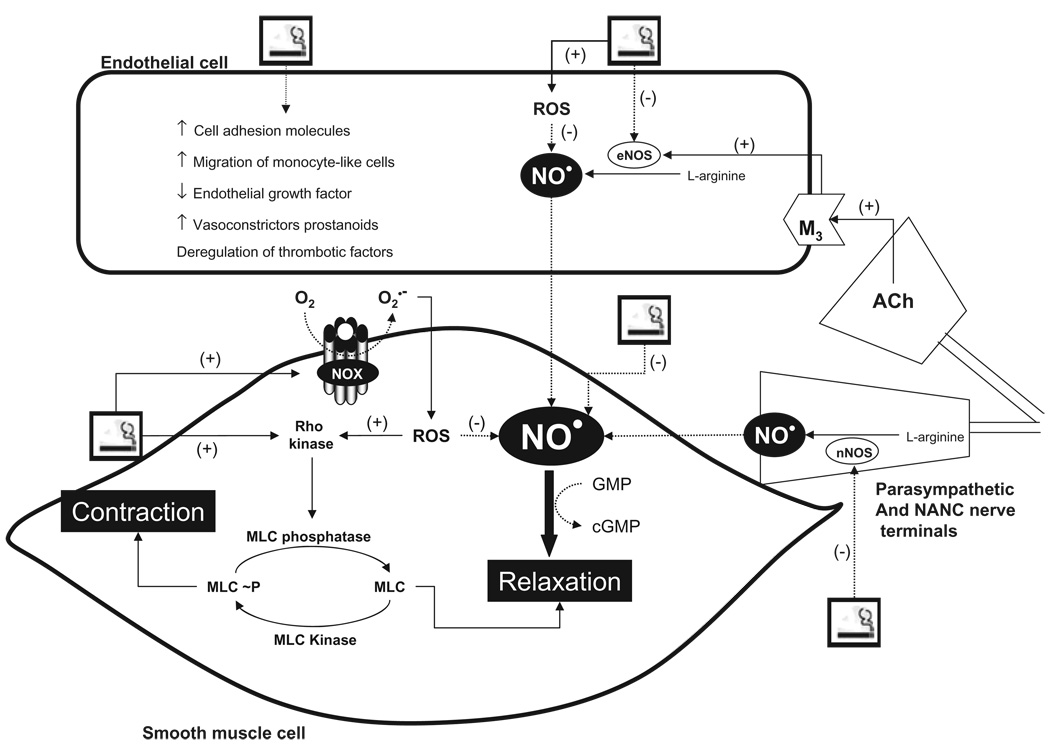
It is known that tobacco smoke has direct detrimental effects on endothelial cells, causing architectural and functional changes (Figure 1). These include decreased eNOS activity, impaired endothelium-dependent vasorelaxation, increased expression of cell adhesion molecules and transendothelial migration of monocyte-like cells, reduced response to vascular endothelial growth factor, and impaired regulation of important thrombotic factors [37,43–53]. In humans and animal models, cigarette smoke or products of cigarette smoke produce diffuse vascular injury in many organ systems and impair eNOS-dependent dilatation of large peripheral arteries and resistance arterioles. Furthermore, chronic smoking also modifies the functional properties of medial elastic fibers by promoting changes in the viscoelastic properties of the extracellular matrix and by inducing calcification of the medial elastic fibers [54,55].
Figure 1.
Mechanisms involved in cigarette-induced vascular injury.
NO is thought to be the principal stimulator of cavernosal relaxation and penile erection. There is considerable evidence supporting the concept that smoking-related ED is associated with reduced bioavailability of NO because of increased ROS. For details, see text.
Ach =acetylcholine; cGMP = 3′,5′-cyclic guanosine monophosphate eNOS, endothelial nitric oxide synthase; M3 = type 3 muscarinic receptor; MLC = myosin light chain; nNOS = neuronal nitric oxide synthase; NO = nitric oxide; ROCK = Rho-associated kinase; ROS = reactive oxygen species.
Although the effects of smoking on endothelial cells/eNOS are well established, much less information exists regarding the effects of smoking on nNOS-mediated penile erection. However, in rats, both at the age of 5 or 20 months, prolonged exposure to cigarette smoke reduces neuronal (not endothelial) NOS activity and content in the penis, but not in the cerebellum [56]. Interestingly, prenatal exposure to cigarette smoke induces loss of nNOS protein in the brainstem of neonatal rats [57]. In addition, a very recent report has shown that acute exposure to nicotine impairs nNOS-dependent reactivity of cerebral arterioles by a mechanism that appears to be related to the formation of superoxide anion [58].
Contradictory results have been shown regarding the effects of cigarette smoking on iNOS activity and expression. Whereas cigarette smoke condensates attenuate cytokine induction of iNOS protein expression and endogenous enzymatic nitrite production in glioma cells [59], and in murine and human lung epithelial cell lines (LA-4 and A549, respectively) [60], cigarette smoke increases iNOS expression, as well as nuclear factor kappa B and c-fos, in rat lung tissue [61]. Similarly, nicotine has been shown to potentiate lipopolysaccharide/interferon-gamma-induced cytotoxic effects by enhancing NO production and iNOS gene expression, suggesting a cross-talk between inflammation and smoking [62].
As cigarette smoke contains superoxide and other reactive oxygen species (ROS), it has been hypothesized that some of the adverse effects of smoking may result from oxidative damage to endothelial cells, which results in NO shortage [63] (Figure 1). ROS, which are oxygen-derived small molecules, are generated by many enzymes, but mainly by NOX enzymes (NADPH oxidases; nicotinamide adenine dinucleotide phosphate-oxidase) [64,65]. ROS avidly interact with a large number of molecules including other small inorganic molecules as well as proteins, lipids, carbohydrates, and nucleic acids. Through such interactions, ROS may irreversibly destroy or alter the function of the target molecule. Consequently, ROS have been increasingly identified as major contributors to damage in biological organisms. The antagonistic effect of ROS on the NO pathway is partially attributed to the fast reaction of superoxide with NO to produce peroxynitrite, thereby decreasing NO levels. Other mechanisms, including NOS uncoupling and inhibition of dimethylarginine dimethylaminohydrolase (DDAH), may also contribute to ROS-mediated shortage of NO levels [63–65]. Accordingly, increased inactivation of NO by superoxide results in impaired penile NO transmission and smooth muscle relaxation [66–68].
Smoking disrupts the dynamic balance between oxidation and antioxidation reactions and clearly exacerbates oxidative stress [52,69–76] (Figure 1). Smoking (or specific compounds of cigarette smoke) increases superoxide generation by both endothelial and smooth muscle cells, impairs acetylcholine-induced relaxation of arteries, increases mRNA expression of pro-inflammatory cytokines, such as interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha, all effects prevented by inhibition of NAD(P)H oxidase or by treatment with antioxidants [74–76]. As tetrahydrobiopterin (BH4) supplementation improves endothelial dysfunction in chronic smokers, it is accepted that, in addition to the free radical burden of cigarette smoke, a dysfunctional eNOS because of BH4 depletion contributes to endothelial dysfunction in chronic smokers [77–80]. In addition, cigarette smoking causes increased production of cyclooxygenase dependent and independent vasoconstrictor eicosanoids [79].
Interestingly, smoking activates Rho-associated kinase (ROCK) in forearm vascular smooth muscle cells,[81] and increased oxidative stress has also been associated with increased ROCK activity and vascular stiffness in humans [82]. In healthy male subjects, age and number of pack-years smoked are independent predictors of ROCK activity, suggesting that aging and accumulating smoking habit, which might induce excessive oxidative stress, are involved in ROCK activity in the vasculature [82]. The importance of ROCK in the Ca2+ sensitization of corpus cavernosum smooth muscle cells and maintenance of penile flaccidity is well recognized [8,83,84].
Further evidence that oxidative stress, mediated through the superoxide radical (superoxide) and other ROS, may be central to impaired cavernosal function in ED, include: (i) cavernosal vascular smooth muscle cells generate superoxide in response to a variety of stimuli, including nicotine, and mechanical injury [66–68]; (ii) oxidative stress, induced by inhibition of endogenous Cu/Zn superoxide dismutase (SOD) with diethyldithiocarbamate and a superoxide anion generator, in bovine retractor penis muscle strips, results in almost complete inhibition of nitrergic relaxation [85]; (iii) ED associated with pathological conditions, such as hypercholesterolaemia and diabetes, is accompanied by augmentation of NADPH oxidase-derived superoxide in the cavernosal tissue [86–88]; and (iv) treatment of diabetic animals with antioxidants or oxygen free radical scavengers, improves erectile function, enhances levels of circulating NO, increases nNOS expression and also enhances the therapeutic effect of the phosphodiesterase-5 (PDE-5) inhibitor sildenafil [88,89].
High concentrations of ROS, NO, peroxynitrite, peroxynitrate, and free radicals of organic compounds are found in the two phases of cigarette smoke: tar and gas-phase smoke [90]. In addition to these short-lived, highly reactive substances, the gas phase of cigarette smoke contains varying amounts of more stable substances that also have the potential to increase intracellular production of ROS [91]. These include a series of α, β-unsaturated aldehydes, such as acrolein and crotonaldehyde, α, β-unsaturated ketones, and a number of saturated aldehydes [91]. These stable compounds have been shown to react nonenzymatically with thiol groups that may be involved in the regulation of enzymes, including NADPH oxidase [92,93]. Furthermore, because of their stability, these compounds could induce ROS production in vascular fields remote from the primary site of exposure [92].
Another interesting aspect that should be considered is the association between smoking and testosterone. Testosterone plays a major role in men’s sexual function. Testosterone regulates not only sexual activity and libido, but also cGMP formation, through NOS stimulation, and its catabolism, through PDE-5 activity [94,95]. Decreased plasma testosterone levels are associated with ED and studies in castrated animals have proven that addition of testosterone, and its conversion to dihydrotestosterone, restores erectile function [96]. In addition, late onset hypogonadism (LOH), or testosterone deficiency syndrome, is often associated with reduced sexual desire and reduced nocturnal penile erections [97]. Patients with LOH are less responsive to treatment with PDE-5 inhibitors, but testosterone replacement in these subjects restores responsiveness to PDE-5 inhibitors [97]. Interestingly, several studies have shown that testosterone levels are significantly increased in current smokers [98–101]. Corona and colleagues have recently confirmed an association between vascular ED and smoking, and also the presence of higher testosterone levels in current smokers [101]. Most of these reports have found a positive correlation between testosterone levels and the number of cigarettes smoked daily [98–100]. In addition, in a longitudinal study with an 11-year follow-up, men who quit smoking have an increased risk of hypogonadism [99]. It is possible that increased testosterone levels, which at a first glance would suggest a more sexual-favorable hormonal milieu, represent a compensatory mechanism to the ultrastructural and functional damage generated by smoking. Additional studies are needed to clarify the relationship between smoking, ED and testosterone.
Components Involved in the Effects of Cigarette Smoking
Many of the vascular effects of chronic smoking are attributed to nicotine, a tertiary amine composed of a pyridine ring. Nicotine induces endothelial dysfunction [102–104], which in dogs depends on duration and dose of nicotine treatment [104]. Nicotine-induced endothelial dysfunction has been associated with increased plasma levels of asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor and also a risk factor for cardiovascular disease. Nicotine-induced ADMA accumulation is related to a decrease in activity of DDAH, a major hydrolase of ADMA [102]. Whether nicotine or other products of cigarette smoke mediates all effects related to vascular damage is still unknown.
Harten and Meston have recently reported that nicotine significantly attenuates physiological sexual arousal in healthy nonsmoking men [105]. However, the only report on the effects of smoking on nNOS activity/expression has shown that nicotine is not responsible for the reduced nNOS activity and content induced by passive cigarette smoking [56]. Incubation of penile slices or rat penile smooth muscle cells with nicotine or conitine (the major nicotine metabolite) does not change nitrite levels or nNOS activity [56]. Dr. Osawa’s group has shown that aqueous extracts of cigarette and cigarette smoke cause inactivation of eNOS and nNOS [80,106–108]. By using an in vitro system containing purified NOS isoforms, they observed that aqueous extract of cigarette or cigarette smoke inactivates nNOS in a time- and metabolism-based manner. The compound responsible for such effect is not nicotine and seems to be a low molecular weight, nonvolatile, cationic, organic molecule, found in an alkaloid-containing fraction from tobacco (Nicotiana tabacum) [106].
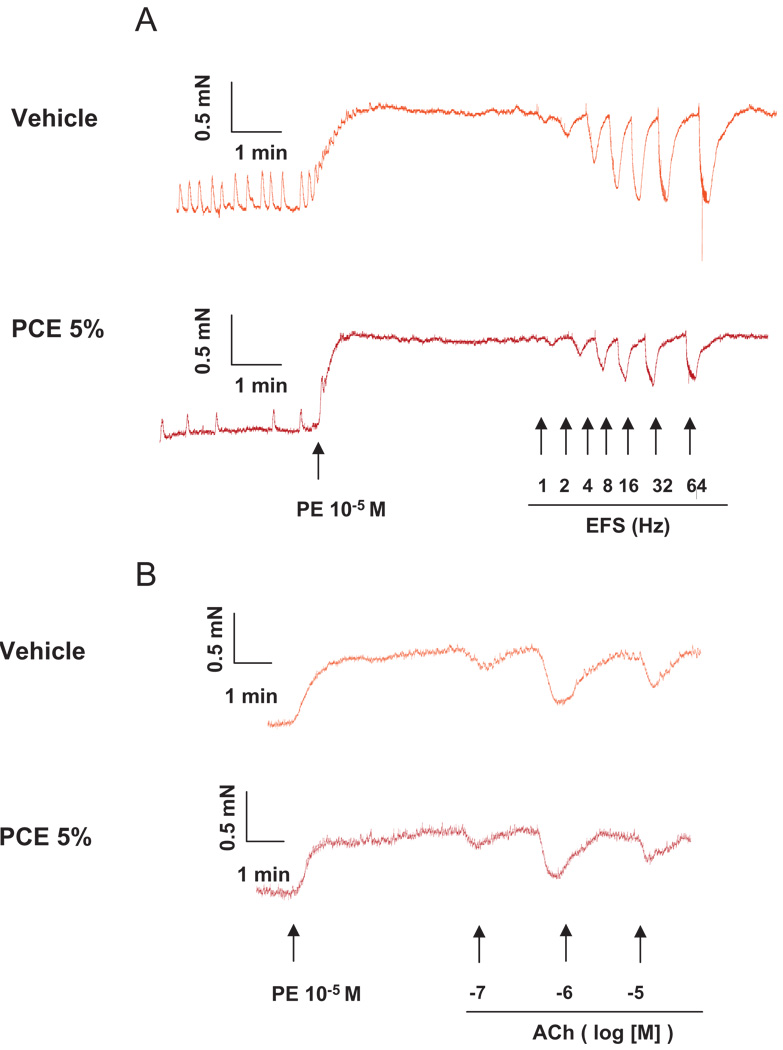
We have tested the effects of a water-soluble extract made from cigarettes and an HPLC-purified fraction of nonburned cigarettes on isolated mouse cavernosal strips. Functional responses that are mediated by activation of eNOS and nNOS were addressed. The purified fraction of cigarette extract (5%) inhibits electrical field stimulation (EFS)-induced relaxation, which relies on nNOS activation, but it does not interfere with acetylcholine-induced relaxation, which relies on eNOS-derived NO (Figure 2). To determine relaxant responses to NANC nerve stimulation (nNOS activation), strips were treated with the sympathetic nerve-blocking agent bretylium tosylate (3 × 10−5 M) and atropine (a muscarinic receptor antagonist; 10−6 M) for 45 minutes, contracted with phenylephrine (PE 10−5 M), and then EFS (conducted at 50 V, 1-ms pulse width and trains of stimuli lasting 10 seconds at varying frequencies [1 to 32 Hz]) was performed.
Figure 2.
Effects of cigarette extract on EFS-induced relaxation of murine cavernosal strips.
Representative tracing showing that the HPLC-purified fraction of non-burned cigarettes (5%) inhibits (A) EFS-induced relaxation, which relies on nNOS activation, but it did not interfere with (B) acetylcholine-induced relaxation, which relies on eNOS-derived NO.
Ach = acetylcholine; EFS = electrical field stimulation; PCE =purified fraction of nonburned cigarettes; PE = phenylephrine.
On the other hand, in vitro analyses have shown that out of 4,800 different compounds in cigarette smoke, the mixture of metals and oxidants is crucial to produce endothelium damage [108]. Cigarette smoke contains a number of different metals, including aluminum, cadmium, chromium, copper, lead, mercury, nickel, and zinc. The combination of metals and antioxidants leads to a chain reaction of protein oxidation, functional impairment of the microtubule system, contraction of endothelial cells, endothelial dysfunction, and to denudation of the inner vascular surface [108]. A recent report has shown that smokers smoking more than three cigarettes per day had significantly increased serum levels of cadmium and strontium compared with nonsmokers [109]. Although not significant, passive smokers and study subjects smoking less than three cigarettes per day also showed increases in the concentration of both metals [109].
As increased serum cadmium levels have been shown to contribute to smoking-induced peripheral arterial disease [110], the deleterious effects of cigarette smoking may be also related to changes in body’s metal homeostasis.
Perspectives
Although cessation of cigarette smoking can improve ED in a considerable proportion of smokers, age and severity of ED before cessation are inversely related to the chance of improvement. Smoking prevention represents a very important approach for reducing the risk of ED. The characterization of the components of cigarette smoke leading to ED, as well as a better understanding of how these components alter signaling pathways activated in erectile responses, are necessary for a complete comprehension of cigarette smoking-associated ED.
Acknowledgments
This study was supported by grants from the National Institutes of Health (HL71138 and HL74167), National Institute on Drug Abuse, NIH (DA22354-01), Fundacao de Amparo a Pesquisa do Estado de Sao Paulo—FAPESP and Coordenaçao de Aperfeiçoamento de Pessoal de Nivel Superior—CAPES, Brazil.
Footnotes
Conflict of Interest: None declared.
Statement of Authorship
-
Conception and DesignRita C. Tostes; Fernando S. Carneiro; Fernanda R.C. Giachini
-
Acquisition of DataFernando S. Carneiro; Anthony J. Lee; Romulo Leite
-
Analysis and Interpretation of DataFernando S. Carneiro; Rita C. Tostes
-
Drafting the ArticleFernando S. Carneiro; Fernanda R.C. Giachini; Rita C. Tostes
-
Revising It for Intellectual ContentYoichi Osawa; R. Clinton Webb
-
Final Approval of the Completed ArticleRita C. Tostes; Yoichi Osawa; R. Clinton Webb
Copyright of Journal of Sexual Medicine is the property of Blackwell Publishing Limited and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- 1.Andersson KE. Pharmacology of penile erection. Pharmacol Rev. 2001;53:417–450. [PubMed] [Google Scholar]
- 2.Andersson KE, Steif CG. Neurotransmission and the contraction and relaxation of penile erectile tissues. World J Urol. 1997;15:14–20. doi: 10.1007/BF01275151. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AL. Nitric oxide in the penis-science and therapeutic implications from erectile dysfunction to priapism. J Sex Med. 2006;3:578–582. doi: 10.1111/j.1743-6109.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 4.Magee TR, Kovanecz I, Davila HH, Ferrini MG, Cantini L, Vernet D, Zuniga FI, Rajfer J, Gonzalez-Cadavid NF. Antisense and short hairpin RNA (shRNA) constructs targeting PIN (Protein Inhibitor of NOS) ameliorate aging-related erectile dysfunction in the rat. J Sex Med. 2007;4:633–643. doi: 10.1111/j.1743-6109.2007.00459.x. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AL, Tillman SL, Chang TS, Epstein JI, Lowenstein CJ, Bredt DS, Snyder SH, Walsh PC. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- 6.Dail WG, Barba V, Leyba L, Galindo R. Neural and endothelial nitric oxide synthase activity in rat penile erectile tissue. Cell Tissue Res. 1995;282:109–116. doi: 10.1007/BF00319137. [DOI] [PubMed] [Google Scholar]
- 7.Burnett AL, Nelson RJ, Calvin DC, Liu JX, Demas GE, Klein SL, Kriegsfeld LJ, Dawson VL, Dawson TM, Snyder SH. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
- 8.Leite R, Giachini FRC, Carneiro FS, Nunes KP, Tostes RC, Webb RC. Targets for the treatment of erectile dysfunction: Is NO still the answer? Rec Pat Cardiovasc Drug Disc. 2007;2:119–132. doi: 10.2174/157489007780832579. [DOI] [PubMed] [Google Scholar]
- 9.NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA. 1993;270:83–90. [PubMed] [Google Scholar]
- 10.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: Results of the Massachusetts Male Aging Study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 11.Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–56. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Cigarette smoking among adults—United States, 2003. [accessed January 31, 2008];Morb Mortal Wkly Rep. 2005 54:509–513. Available at: http://www.cdc.gov/mmwR/preview/mmwrhtml/mm5420a3.htm. [PubMed]
- 13.Gu D, Wu X, Reynolds K, Duan X, Xin X, Reynolds RF, Whelton PK, He J Interasia collaborative group. Cigarette smoking and exposure to environmental tobacco smoke in China. Am J Public Health. 2004;94:1972–1976. doi: 10.2105/ajph.94.11.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Healthy people 2010: Understanding and improving health. [accessed January 31, 2008];Washington, DC: 2000 Available at: http://www.healthypeople.gov/Document/html/uih/uih_bw/uih_4.htm#tobaccouse.
- 15.Mannino DM, Klevens RM, Flanders WD. Cigarette smoking: An independent risk factor for impotence? Am J Epidemiol. 1994;140:1003–1008. doi: 10.1093/oxfordjournals.aje.a117189. [DOI] [PubMed] [Google Scholar]
- 16.Shabsigh R, Fishman IJ, Schum C, Dunn JK. Cigarette smoking and other vascular risk factors in vasculogenic impotence. Urology. 1991;38:227–231. doi: 10.1016/s0090-4295(91)80350-g. [DOI] [PubMed] [Google Scholar]
- 17.Rosen MP, Greenfield AJ, Walker TG, Grant P, Dubrow J, Bettman MA, Fried LE, Goldstein I. Cigarette smoking: an independent risk factor for atherosclerosis in the hypogastric-cavernous arterial bed of men with arteriogenic impotence. J Urol. 1991;145:759–763. doi: 10.1016/s0022-5347(17)38444-6. [DOI] [PubMed] [Google Scholar]
- 18.Hirshkowitz M, Karacan I, Howell JW, Arcasoy MO, Williams RL. Nocturnal penile tumescence in cigarette smokers with erectile dysfunction. Urology. 1992;34:101–107. doi: 10.1016/0090-4295(92)90263-v. [DOI] [PubMed] [Google Scholar]
- 19.Austoni E, Mirone V, Parazzini F, Fasolo CB, Turchi C, Pescatori ES, Ricci E, Gentile V. Andrology Prevention Week Centre, Italian Society of Andrology. Smoking as a risk factor for erectile dysfunction: Data from the Andrology Prevention Weeks 2001–2002. A study of the Italian Society of Andrology (S.I.A.) Eur Urol. 2005;48:810–817. doi: 10.1016/j.eururo.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 20.Oksuz E, Malhan S. The prevalence of male sexual dysfunction and potential risk factors in Turkish men: A web-based survey. Int J Impot Res. 2005;17:539–545. doi: 10.1038/sj.ijir.3901357. [DOI] [PubMed] [Google Scholar]
- 21.Nicolosi A, Moreira ED, Jr, Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: Cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61:201–206. doi: 10.1016/s0090-4295(02)02102-7. [DOI] [PubMed] [Google Scholar]
- 22.Mirone V, Imbimbo C, Bortolotti A, Di Cintio E, Colli E, Landoni M, Lavezzari M, Parazzini F. Cigarette smoking as risk factor for erectile dysfunction: Results from an Italian epidemiological study. Eur Urol. 2002;41:294–297. doi: 10.1016/s0302-2838(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 23.Bortolotti A, Fedele D, Chatenoud L, Colli E, Coscelli C, Landoni M, Lavezzari M, Santeusanio F, Parazzini F. Cigarette smoking: A risk factor for erectile dysfunction in diabetics. Eur Urol. 2001;40:392–396. doi: 10.1159/000049805. [DOI] [PubMed] [Google Scholar]
- 24.Millett C, Wen LM, Rissel C, Smith A, Richters J, Grulich A, de Visser R . Smoking and erectile dysfunction: Findings from a representative sample of Australian men. Tob Control. 2006;15:136–139. doi: 10.1136/tc.2005.015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Urologic Diseases in America Project. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166:207–212. doi: 10.1001/archinte.166.2.207. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HA, Johannes CB, Derby CA, Kleinman KP, Mohr BA, Araujo AB, Mckinlay JB. Erectile dysfunction and coronary risk factors: Prospective results from the Massachusetts Male Aging Study. Prev Med. 2000;30:328–338. doi: 10.1006/pmed.2000.0643. [DOI] [PubMed] [Google Scholar]
- 27.Gades NM, Nehra A, Jacobson DJ, McGree ME, Girman CJ, Rhodes T, Roberts RO, Lieber MM, Jacobsen SJ. Association between smoking and erectile dysfunction: A population-based study. Am. J Epidemiol. 2005;161:346–351. doi: 10.1093/aje/kwi052. [DOI] [PubMed] [Google Scholar]
- 28.Mak R, De Backer G, Kornitzer M, De Meyer JM. Prevalence and correlates of erectile dysfunction in a population-based study in. Belgium. Eur Urol. 2002;41:132–138. doi: 10.1016/s0302-2838(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 29.Blanker MH, Bohnen AM, Groeneveld FP, Bersen RM, Prins A, Thomas S, Bosch JL. Correlates for erectile and ejaculatory dysfunction in older Dutch men: A community-based study. J Am Geriatr Soc. 2001;49:436–442. doi: 10.1046/j.1532-5415.2001.49088.x. [DOI] [PubMed] [Google Scholar]
- 30.He J, Reynolds K, Chen J, Chen CS, Wu X, Duan X, Reynolds R, Bazzano LA, Whelton PK, Gu D. Cigarette smoking and erectile dysfunction among Chinese men without Clinical Vascular Disease. Am J Epidemiol. 2007;166:803–809. doi: 10.1093/aje/kwm154. [DOI] [PubMed] [Google Scholar]
- 31.Polsky JY, Aronson KJ, Heaton JP, Adams MA. Smoking and other lifestyle factors in relation to erectile dysfunction. BJU Int. 2005;96:1355–1359. doi: 10.1111/j.1464-410X.2005.05820.x. [DOI] [PubMed] [Google Scholar]
- 32.Shiri R, Häkkinen J, Koskimäki J, Tammela TL, Auvinen A, Hakama M. Smoking causes erectile dysfunction through vascular disease. Urology. 2006;68:1318–1322. doi: 10.1016/j.urology.2006.08.1088. [DOI] [PubMed] [Google Scholar]
- 33.Green JS, Holden ST, Ingram P, Bose P, St George DP, Bowsher WG. An investigation of erectile dysfunction in Gwent. Wales. BJU Int. 2001;88:551–553. doi: 10.1046/j.1464-4096.2001.01274.x. [DOI] [PubMed] [Google Scholar]
- 34.Kupelian V, Link CL, McKinlay JB. Association between smoking, passive smoking, and erectile dysfunction: Results from the Boston Area Community Health (BACH) Survey. Eur Urol. 2007;52:416–422. doi: 10.1016/j.eururo.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: Nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 36.Pittilo RM. Cigarette smoking and endothelial injury: A review. In: Dana JN, editor. Tobacco smoking and atherosclerosis. New York: Plenum Press; 1990. pp. 61–78. [DOI] [PubMed] [Google Scholar]
- 37.Celermajer DS, Sorensen KE, Georgakopoulos D, Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 38.Butler R, Morris AD, Struthers AD. Cigarette smoking in men and vascular responsiveness. Br J Clin Pharmacol. 2001;52:145–149. doi: 10.1046/j.0306-5251.2001.01434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesmann F, Petersen SE, Leeson PM, Francis JM, Robson MD, Wang Q, Choudhury R, Channon KM, Neubauer S. Global impairment of brachial, carotid, and aortic vascular function in young smokers: Direct quantification by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;44:2056–2064. doi: 10.1016/j.jacc.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 40.Chung SM, Gay CA, McCrary JA. Nonarteric ischemic optic neuropathy. The impact of tobacco use. Ophthalmology. 1994;101:779–782. doi: 10.1016/s0161-6420(94)31266-8. [DOI] [PubMed] [Google Scholar]
- 41.Ishii DN. Implication of insulin-like growth factors in the pathogenesis of diabetic neuropathy. Brain Res Rev. 1995;20:47–67. doi: 10.1016/0165-0173(94)00005-a. [DOI] [PubMed] [Google Scholar]
- 42.Rehill N, Beck CR, Yeo KR, Yeo WW. The effect of chronic tobacco smoking on arterial stiffness. Br J Clin Pharmacol. 2006;61:767–773. doi: 10.1111/j.1365-2125.2006.02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andersson KE. Erectile physiological and patho-physiological pathways involved in erectile dysfunction. J Urol. 2003;170:S6–S13. doi: 10.1097/01.ju.0000075362.08363.a4. [DOI] [PubMed] [Google Scholar]
- 44.Brunner H, Crockoft JR, Deanfield J, Donald A, Ferannini E, Halcox J, Kiowski W, Lüscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J Hypertens. 2005;23:233–246. doi: 10.1097/00004872-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Zeiher AM, Schachinger VMinners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 46.Shen Y, Rattan V, Sultana CKalra VK. Cigarette smoke condensate-induced adhesion molecule expression and transendothelial migration of monocytes. Am J Physiol. 1996;270:H1624–H1633. doi: 10.1152/ajpheart.1996.270.5.H1624. [DOI] [PubMed] [Google Scholar]
- 47.Adams MR, Jessup W, Celermajer DS. Cigarette smoking is associated with increased human monocyte adhesion to endothelial cells: Reversibility with oral L-arginine but not vitamin C. J Am Coll Cardiol. 1997;29:491–497. doi: 10.1016/s0735-1097(96)00537-2. [DOI] [PubMed] [Google Scholar]
- 48.Matetzky S, Tani S, Kangavari S, Dimayuga P, Yano J, Xu H, Chyu KY, Fishbein MC, Shah PK, Cercek B. Smoking increases tissue factor expression in atherosclerotic plaques: Implications for plaque thrombogenicity. Circulation. 2000;102:602–604. doi: 10.1161/01.cir.102.6.602. [DOI] [PubMed] [Google Scholar]
- 49.Shimasaki Y, Saito Y, Yoshimura M, Kamitani S, Miyamoto Y, Masuda I, Nakayama M, Mizuno Y, Ogawa H, Yasue HNakao K. The effects of long-term smoking on endothelial nitric oxide synthase mRNA expression in human platelets as detected with real-time quantitative RT-PCR. Clin Appl Thromb Hemost. 2007;13:43–51. doi: 10.1177/1076029606296402. [DOI] [PubMed] [Google Scholar]
- 50.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: Role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–284. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Cui Z, Han Z, Li Z, Hu H, Patel JM, Antony V, Block ER, Su Y. Involvement of calpain-calpastatin in cigarette smoke-induced inhibition of lung endothelial nitric oxide synthase. Am J Respir Cell Mol Biol. 2005;33:513–520. doi: 10.1165/rcmb.2005-0046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barua RS, Ambrose JA, Srivastava S, DeVoe MC, Eales-Reynolds LJ. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107:2342–2347. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 53.Barua RS, Ambrose JA, Eales-Reynolds LJ, DeVoe MC, Zervas JG, Saha DC. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104:1905–1910. doi: 10.1161/hc4101.097525. [DOI] [PubMed] [Google Scholar]
- 54.Guo X, Oldham MJ, Kleinman MT, Phalen RF, Kassab GS. Effect of cigarette smoking on nitric oxide, structural, and mechanical properties of mouse arteries. Am J Physiol Heart Circ Physiol. 2006;291:H2354–H2361. doi: 10.1152/ajpheart.00376.2006. [DOI] [PubMed] [Google Scholar]
- 55.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J Am Coll Cardiol. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 56.Xie Y, Garban H, Ng C, Rajfer J, Gonzalez-Cadavid NF. Effect of long-term passive smoking on erectile function and penile nitric oxide synthase in the rat. J Urol. 1997;157:1121–1126. [PubMed] [Google Scholar]
- 57.Hasan SU, Simakajornboon N, MacKinnon Y, Gozal D. Prenatal cigarette smoke exposure selectively alters protein kinase C and nitric oxide synthase expression within the neonatal rat brainstem. Neurosci Lett. 2001;301:135–138. doi: 10.1016/s0304-3940(01)01624-x. [DOI] [PubMed] [Google Scholar]
- 58.Arrick DM, Mayhan WG. Acute infusion of nicotine impairs nNOS-dependent reactivity of cerebral arterioles via an increase in oxidative stress. J Appl Physiol. 2007;103:2062–2067. doi: 10.1152/japplphysiol.00411.2007. [DOI] [PubMed] [Google Scholar]
- 59.Mazzio EA, Kolta MG, Reams RR, Soliman KF. Inhibitory effects of cigarette smoke on glial inducible nitric oxide synthase and lack of protective properties against oxidative neurotoxins in vitro. Neurotoxicology. 2005;26:49–62. doi: 10.1016/j.neuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Hoyt JC, Robbins RA, Habib M, Springall DR, Buttery LD, Polak JM, Barnes PJ. Cigarette smoke decreases inducible nitric oxide synthase in lung epithelial cells. Exp Lung Res. 2003;29:17–28. doi: 10.1080/01902140303759. [DOI] [PubMed] [Google Scholar]
- 61.Chang WC, Lee YC, Liu CL, Hsu JD, Wang HC, Chen CC, Wang CJ. Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol. 2001;75:28–35. doi: 10.1007/s002040000168. [DOI] [PubMed] [Google Scholar]
- 62.Chen YC, Shen SC, Lin HY, Tsai SH, Lee TJ. Nicotine enhancement of lipopolysaccharide/interferon-gamma-induced cytotoxicity with elevating nitric oxide production. Toxicol Lett. 2004;153:191–200. doi: 10.1016/j.toxlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Zhang WZ, Venardos K, Chin-Dusting J, Kaye DM. Adverse effects of cigarette smoke on NO bioavailability: Role of arginine metabolism and oxidative stress. Hypertension. 2006;48:278–285. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 64.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 65.Bedard K, Krause KH. Oxidases: Physiology and pathophysiology. The NOX Family of ROS-Generating NADPH. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 66.Koupparis AJ, Jeremy JY, Muzaffar S, Persad R, Shukla N. Sildenafil inhibits the formation of superoxide and the expression of gp47 NAD[P]H oxidase induced by the thromboxane A2 mimetic, U46619, in corpus cavernosal smooth muscle cells. BJU Int. 2005;96:423–427. doi: 10.1111/j.1464-410X.2005.05643.x. [DOI] [PubMed] [Google Scholar]
- 67.Hotston MR, Jeremy JY, Bloor J, Koupparis A, Persad R, Shukla N. Sildenafil inhibits the up-regulation of phosphodiesterase type 5 elicited with nicotine and tumour necrosis factor-alpha in cavernosal vascular smooth muscle cells: Mediation by superoxide. BJU Int. 2007;99:612–618. doi: 10.1111/j.1464-410X.2006.06618.x. [DOI] [PubMed] [Google Scholar]
- 68.Ozkara H, Alan C, Atukeren P, Uyaner I, Demirci C, Gumustas MK, Alici B. Changes of nitric oxide synthase-containing nerve fibers and parameters for oxidative stress after unilateral cavernous nerve resection or manipulation in rat penis. Chin J Physiol. 2006;49:160–166. [PubMed] [Google Scholar]
- 69.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105:1155–1157. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 70.Burke A, Fitzgerald GA. Oxidative stress and smoking-induced vascular injury. Prog Cardiovasc Dis. 2003;46:79–90. doi: 10.1016/s0033-0620(03)00076-8. [DOI] [PubMed] [Google Scholar]
- 71.Culcasi M, Muller A, Mercier A, Clement JL, Payet O, Rockenbauer A, Marchand V, Pietri S. Early specific free radical-related cytotoxicity of gas phase cigarette smoke and its paradoxical temporary inhibition by tar: An electron paramagnetic resonance study with the spin trap DEPMPO. Chem Biol Interact. 2006;164:215–231. doi: 10.1016/j.cbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 72.Michaud SE, Dussault S, Groleau J, Haddad P, Rivard A. Cigarette smoke exposure impairs VEGF-induced endothelial cell migration: Role of NO and reactive oxygen species. J Mol Cell Cardiol. 2006;41:275–284. doi: 10.1016/j.yjmcc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Ozyurt H, Pekmez H, Parlaktas BS, Kus I, Ozyurt B, Sarsilmaz M. Oxidative stress in testicular tissues of rats exposed to cigarette smoke and protective effects of caffeic acid phenethyl ester. Asian J androl. 2006;8:189–193. doi: 10.1111/j.1745-7262.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 74.Orosz Z, Csiszar A, Labinskyy N, Smith K, Kaminski PM, Ferdinandy P, Wolin MS, Rivera A, Ungvari Z. Cigarette smoke-induced proinflammatory alterations in the endothelial phenotype: Role of NAD(P)H oxidase activation. Am J Physiol Heart Circ Physiol. 2007;292:H130–H139. doi: 10.1152/ajpheart.00599.2006. [DOI] [PubMed] [Google Scholar]
- 75.Jaimes EA, DeMaster EG, Tian RX, Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler Thromb Vasc Biol. 2004;24:1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 76.Wannamethee SG, Lowe GD, Shaper AG, Rumley A, Lennon L, Whincup PH. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur Heart J. 2005;26:1765–1773. doi: 10.1093/eurheartj/ehi183. [DOI] [PubMed] [Google Scholar]
- 77.Raij L, DeMaster EG, Jaimes EA. Cigarette smoke-induced endothelium dysfunction: Role of superoxide anion. J Hypertens. 2001;19:891–897. doi: 10.1097/00004872-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 78.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, Meinertz T, Münzel T. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86:E36–E41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 79.Higman DJ, Strachan AM, Buttery L, Hicks RC, Springall DR, Greenhalgh RM, Powell JT. Smoking impairs the activity of endothelial nitric oxide synthase in saphenous vein. Arterioscler Thromb Vasc Biol. 1996;16:546–552. doi: 10.1161/01.atv.16.4.546. [DOI] [PubMed] [Google Scholar]
- 80.Lowe ER, Everett AC, Lee AJ, Lau M, Dunbar AY, Berka V, Tsai AL, Osawa Y. Time-dependent inhibition and tetrahydrobiopterin depletion of endothelial nitric-oxide synthase caused by cigarettes. Drug Metab Dispos. 2005;33:131–138. doi: 10.1124/dmd.104.001891. [DOI] [PubMed] [Google Scholar]
- 81.Noma K, Higashi Y, Jitsuiki D, Hara K, Kimura M, Nakagawa K, Goto C, Oshima T, Yoshizumi M, Chayama K. Smoking activates Rho-kinase in smooth muscle cells of forearm vasculature in humans. Hypertension. 2003;41:1102–1105. doi: 10.1161/01.HYP.0000067062.92836.9E. [DOI] [PubMed] [Google Scholar]
- 82.Noma K, Goto C, Nishioka K, Jitsuiki D, Umemura T, Ueda K, Kimura M, Nakagawa K, Oshima T, Chayama K, Yoshizumi M, Liao JK, Higashi Y. Roles of Rho-associated kinase and oxidative stress in the pathogenesis of aortic stiffness. J Am Coll Cardiol. 2007;49:698–705. doi: 10.1016/j.jacc.2006.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: A mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mills TM, Chitaley K, Wingard CJ, Lewis RW, Webb RC. Effect of Rho-kinase inhibition on vasoconstriction in the penile circulation. J Appl Physiol. 2001;91:1269–1273. doi: 10.1152/jappl.2001.91.3.1269. [DOI] [PubMed] [Google Scholar]
- 85.Mok JS, Paisley K, Martin W. Inhibition of nitrergic neurotransmission in the bovine retractor penis muscle by an oxidant stress: effects of superoxide dismutase mimetics. Br J Pharmacol. 1998;124:111–118. doi: 10.1038/sj.bjp.0701809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shukla N, Jones R, Persad R, Angelini GD, Jeremy JY. Effect of sildenafil citrate and a nitric oxide donating sildenafil derivative, NCX 911, on cavernosal relaxation and superoxide formation in hypercholesterolaemic rabbits. Eur J Pharmacol. 2005;517:224–231. doi: 10.1016/j.ejphar.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 87.Kovanecz I, Ferrini MG, Vernet D, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Pioglitazone prevents corporal veno-occlusive dysfunction in a rat model of type 2 diabetes mellitus. BJU Int. 2006;98:116–124. doi: 10.1111/j.1464-410X.2006.06268.x. [DOI] [PubMed] [Google Scholar]
- 88.Musicki B, Burnett AL. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19:129–138. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 89.De Young L, Yu D, Bateman RM, Brock GB. Oxidative stress and antioxidant therapy: Their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830–836. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 90.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686:12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 91.Stedman RL. The chemical composition of tobacco and tobacco smoke. Chem Rev. 1968;68:153–207. doi: 10.1021/cr60252a002. [DOI] [PubMed] [Google Scholar]
- 92.Cooper KO, Witz G, Witmer C. The effects of alpha, beta-unsaturated aldehydes on hepatic thiols and thiol-containing enzymes. Fundam Appl Toxicol. 1992;19:343–349. doi: 10.1016/0272-0590(92)90172-e. [DOI] [PubMed] [Google Scholar]
- 93.Corte ED, Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972;126:739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Traish A, Kim N, Traish AM, Kim N. The physiological role of androgens in penile erection: Regulation of corpus cavernosum structure and function. J Sex Med. 2005;2:759–770. doi: 10.1111/j.1743-6109.2005.00094.x. [DOI] [PubMed] [Google Scholar]
- 95.Vignozzi L, Corona G, Petrone L, Filippi S, Morelli AM, Forti G, Maggi M. Testosterone and sexual activity. J Endocrinol Invest. 2005;28:39–44. [PubMed] [Google Scholar]
- 96.Guay AT. Testosterone and erectile physiology. Aging Male. 2006;9:201–206. doi: 10.1080/13685530601051155. [DOI] [PubMed] [Google Scholar]
- 97.Morelli A, Corona G, Filippi S, Ambrosini S, Forti G, Vignozzi L, Maggi M. Which patients with sexual dysfunction are suitable for testosterone replacement therapy? J Endocrinol Invest. 2007;30:880–888. doi: 10.1007/BF03349232. [DOI] [PubMed] [Google Scholar]
- 98.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men. The fifth Tromsø study. Int J Androl. 2007;30:137–143. doi: 10.1111/j.1365-2605.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 99.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, Salonen JK. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: A prospective cohort study. J Clin Endocrinol Metab. 2005;90:712–719. doi: 10.1210/jc.2004-0970. [DOI] [PubMed] [Google Scholar]
- 100.Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol. 1988;128:796–805. doi: 10.1093/oxfordjournals.aje.a115033. [DOI] [PubMed] [Google Scholar]
- 101.Corona G, Mannucci E, Petrone L, Ricca V, Mansani R, Cilotti A, Balercia G, Chiarini V, Giommi R, Forti G, Maggi M. Psychobiological correlates of smoking in patients with erectile dysfunction. Int J Androl. 2007;30:137–143. doi: 10.1038/sj.ijir.3901351. [DOI] [PubMed] [Google Scholar]
- 102.Jiang DJ, Jia SJ, Yan J, Zhou Z, Yuan Q, Li YJ. Involvement of DDAH/ADMA/NOS pathway in nicotine-induced endothelial dysfunction. Biochem Biophys Res Commun. 2006;349:683–693. doi: 10.1016/j.bbrc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 103.Xiao D, Huang X, Yang S, Zhang L. Direct effects of nicotine on contractility of the uterine artery in pregnancy. J Pharmacol Exp Ther. 2007;322:180–185. doi: 10.1124/jpet.107.119354. [DOI] [PubMed] [Google Scholar]
- 104.Miller VM, Clouse WD, Tonnessen BH, Boston US, Severson SR, Bonde S, Rud KS, Hurt RD. Time and dose effect of transdermal nicotine on endothelial function. Am J Physiol Heart Circ Physiol. 2000;279:H1913–H1921. doi: 10.1152/ajpheart.2000.279.4.H1913. [DOI] [PubMed] [Google Scholar]
- 105.Harte CB, Meston CM. Acute effects of nicotine on physiological and subjective sexual arousal in nonsmoking men: A randomized, double-blind, placebo-controlled trial. J Sex Med. 2008;5:110–121. doi: 10.1111/j.1743-6109.2007.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Demady DR, Lowe ER, Everett AC, Billecke SS, Kamada Y, Dunbar AY, Osawa Y. Metabolism-based inactivation of neuronal nitric-oxide synthase by components of cigarette and cigarette smoke. Drug Metab Dispos. 2003;31:932–937. doi: 10.1124/dmd.31.7.932. [DOI] [PubMed] [Google Scholar]
- 107.Lowe ER, Everett AC, Lau M, Dunbar AY, Michener D, Osawa Y. Naturally occurring neuronal NO-synthase inactivators found in Nicotiana tabacum (Solanaceae) and other plants. Phytomedicine. 2006;14:344–352. doi: 10.1016/j.phymed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 108.Bernhard D, Csordas A, Henderson B, Rossman A, Kind M, Wick G. Cigarette smoke metal catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J. 2005;19:1096–1107. doi: 10.1096/fj.04-3192com. [DOI] [PubMed] [Google Scholar]
- 109.Bernhard D, Rossmann A, Henderson B, Kind M, Seubert A, Wick G. Increased serum cadmium and strontium levels in young smokers: Effects on arterial endothelial cell gene transcription. Arterioscler Thromb Vasc Biol. 2006;26:833–838. doi: 10.1161/01.ATV.0000205616.70614.e5. [DOI] [PubMed] [Google Scholar]
- 110.Navas-Acien A, Selvin E, Sharrett AR, Calderon-Aranda E, Silbergeld E, Guallar E. Lead, cadmium, smoking, and increased risk of peripheral arterial disease. Circulation. 2004;109:3196–3201. doi: 10.1161/01.CIR.0000130848.18636.B2. [DOI] [PubMed] [Google Scholar]