Abstract
Background
Macrophages contribute to the progression and acute complications of atherosclerosis. Macrophage imaging may serve as a biomarker to identify subclinical inflamed lesions, to predict future risk, and to aid in the assessment of novel therapies.
Methods and Results
To test the hypothesis that nanoparticle-enhanced, high-resolution magnetic resonance imaging (MRI) can measure plaque macrophage accumulation, we used 3-T MRI with a macrophage-targeted superparamagnetic nanoparticle preparation (monocrystalline iron oxide nanoparticles-47 [MION-47]) in cholesterol-fed New Zealand White rabbits 6 months after balloon injury. In vivo MRI visualized thickened abdominal aortas on both T1- and T2-weighted spin-echo images (T1 spin echo, 20 axial slices per animal; T2 spin echo, 28 slices per animal). Seventy-two hours after MION-47 injection, aortas exhibited lower T2 signal intensity compared with before contrast imaging (signal intensity ratio, aortic wall/muscle: before, 1.44±0.26 versus after, 0.95±0.22; 164 slices; P<0.01), whereas T1 spin echo images showed no significant change. MRI on ex vivo specimens provided similar results. Histological studies colocalized iron accumulation with immunoreactive macrophages in atheromata. The magnitude of signal intensity reduction on T2 spin echo in vivo images further correlated with macrophage areas in situ (150 slices; r=0.73). Treatment with rosuvastatin for 3 months yielded diminished macrophage content (P<0.05) and reversed T2 signal intensity changes (P<0.005). Signal changes in rosuvastatin-treated rabbits correlated with reduced macrophage burden (r=0.73). In vitro validation studies showed concentration-dependent MION-47 uptake by human primary macrophages.
Conclusion
The magnitude of T2 signal intensity reduction in high-resolution MRI after administration of superparamagnetic phagocytosable nanoparticles can assess macrophage burden in atheromata, providing a clinically translatable tool to identify inflamed plaques and to monitor therapy-mediated changes in plaque inflammation.
Keywords: atherosclerosis, inflammation, macrophages, magnetic resonance imaging, nanoparticles
Accumulation of activated macrophages influences clinical outcomes of cardiovascular diseases such as atherosclerosis and its acute complications, cardiac remodeling after acute infarction, and arterial and valvular calcification. In atherosclerosis, macrophage accumulation initiates lesion development and triggers clinical events by elaborating various molecules that promote inflammation, plaque disruption, and subsequent thrombus formation.1–4 However, conventional anatomic measures of advanced lesions (eg, plaque size or luminal stenoses) do not necessarily correlate with macrophage content. Imaging of macrophages should therefore offer a much-needed strategy for identifying inflamed plaques, often precursors of acute thrombotic events, enabling pathophysiological investigations, and assessing the effects of interventions.
Molecular imaging visualizes specific cell types or biological processes.5–9 Magnetic resonance imaging (MRI) noninvasively produces tomographic images with high soft-tissue contrast and excellent spatial resolution. Previous studies, including ours, reported that MRI with a 1.5-T scanner detects the progression and regression of aortic atherosclerosis in rabbits,10,11 characterizes human plaque components,12 and monitors changes in plaques in patients during drug interventions.13 The introduction of macrophage-targeted superparamagnetic nanoparticles improves the capabilities of MRI to image vascular targets specifically.14,15 Macrophages internalize such nanoparticles, altering the local magnetic field and thus producing the T2 shortening effect as visualized by signal reduction.16–19 The present study used a 3-T clinical MRI scanner in combination with recently developed biocompatible monocrystalline iron oxide nanoparticles-47 (MION-47) to test the specific hypothesis that high-resolution MRI enhanced with superparamagnetic nanoparticles can measure macrophage accumulation in atherosclerotic plaques and monitor changes in inflammatory burden during statin treatment.
Methods
See the online-only Data Supplement for detailed methods.
Animal Protocol
Aortic atherosclerosis was induced in 9 male New Zealand White rabbits through consumption of an atherogenic diet and mechanical injury, as previously described.11,20 In 3 rabbits, the atherogenic diet was halted at 3 months, and rosuvastatin (1 mg · kg−1 · d−1, Astra-Zeneca, London, UK) was administered mixed in a regular diet; the 6 other rabbits continued the atherogenic diet.
Superparamagnetic Nanoparticles
MION-47 has a ≈5-nm diameter core of superparamagnetic iron oxide coated with a ≈10-nm-thick dextran layer and has a long blood half-life, which facilitates its accumulation in macrophages of atherosclerotic plaques. The characteristics of MION have been described previously.17,21
A previous study rigorously evaluated toxicity of a similar class of nanoparticles.22 Researchers in this study infused rats and dogs with up to 168 mg Fe/kg AMI-25. Peak concentrations of Fe were found in the liver after 2 hours and in the spleen after 4 hours. Fe slowly cleared from the liver (half-life, 3 days) and spleen (half-life, 4 days) and was incorporated into the hemoglobin of erythrocytes in a time-dependent fashion. Histological and serological studies detected no acute or subacute toxic effects. In addition, MION-47 has similar size and biological properties as ferumoxtran-10, a Food and Drug Administration–approved agent.23
MION-47 was infused via an ear vein (10 mg Fe/kg, ≈40 mg Fe per rabbit). This dose was chosen on the basis of a preliminary in vivo MRI study comparing the effects of 2.6, 10, and 30 mg Fe/kg on changes in T2 signal intensity (SI) on spin-echo (SE) images. The recommended dose of ferumoxtran is 2.6 mg Fe/kg and of ferumoxytol is 7 mg Fe/kg, with higher doses possible in severely anemic patients. Although 2.6 mg Fe/kg produced no significant T2 effect on the aorta, 30 mg Fe/kg resulted in overwhelming signal reduction in various tissues surrounding the aorta, eg, back muscles. Seventy-two hours after injections of 10 mg Fe/kg MION-47, the signal loss in back muscles had already returned to baseline while reliably producing T2 signal reduction of the atherosclerotic aorta in these preliminary experiments.
MRI Procedure
Rabbits were sedated and imaged supine in a GE 3-T MRI system using a 4-channel knee coil. Twenty-eight axial slices on T2-weighted SE sequence (echo time, 34 ms; repetition time, 1800 ms) and 20 axial slices on T1-weighted fast SE (echo time, 13 ms; repetition time, 800 ms) of the abdominal aorta were acquired. Other general conditions included the following: number of excitations, 6; field of view, 6×6 cm; slice/gap, 2.0/1.0 mm; data matrix, 256×192; and in-plane resolution, 234×312 μm, plus spatial/spectral saturation pulses to null blood and peri-adventitial fat signal.
Results
Human Primary Macrophages Internalize MION-47 In Vitro
Initial validation studies examined in vitro whether macrophages internalize MION-47. After incubation with MION-47, human primary macrophages differentiated in culture media containing 5% human serum for 14 days tended to accumulate iron particles in a concentration-dependent manner at greater levels than did day 1 monocytes (Figure 1A and 1B). Iron nanoparticle uptake, if any, in cultured human smooth muscle and endothelial cells fell below the threshold of detection of the assay.
Figure 1.

Kinetics of superparamagnetic nanoparticles (MION-47). A, In vitro macrophage uptake of MION-47 increased during differentiation from day 1 to 14. The cultured human primary macrophages contained MION-47 in a concentration-dependent manner (0, 30, 100, and 300 μg/mL for 72 hours) as determined by the quantitative colorimetric iron assay. The data represent 3 different donors that provided similar results. B, DAB-enhanced Perl Prussian blue staining demonstrating iron uptake in human macrophages coincubated with MION-47.
3-T MRI Detects Progression of Atherosclerosis
Normocholesterolemic rabbits usually have an abdominal aortic wall thinner than 200 to 300 μm. The 3-T MRI system used in the present study, which afforded resolutions of 234×312 μm per pixel, barely visualized the normal aortic wall (Figure 2A). After mechanical injury and cholesterol supplementation for 3 or 6 months, however, this instrumentation readily detected the thickened wall (Figure 2B). The aortic wall area on T2SE correlated well with the histomorphometric measurements (Figure 2C). The vessel wall appeared isointense to muscle on T1SE, whereas it generally appeared brighter than muscle on T2SE (Figure 2D).
Figure 2.
MRI (3 T) detects atherosclerotic plaque burden in hypercholesterolemic rabbits. A, MRI (3 T) barely visualized normal rabbit abdominal aorta in vivo. B, MRI (3 T) detected thickened aortic wall on a T2-weighted sequence (T2SE) in vivo 3 or 6 months after the initiation of cholesterol feeding and balloon injury. MRIs further exhibited the progression of maximal wall thickness from 0.47 mm at 3 months to 0.94 mm at 6 months in the same rabbit. C, Quantitative analysis closely associated T2SE MRI and the histological measurements of aortic wall area (164 slices in 6 rabbits). D, Vessel wall was isointense to muscle on T1SE, whereas it generally appeared brighter than muscle on T2SE at 6 months after initiation of cholesterol feeding and balloon injury.
MION-47 Produces T2 Signal Reduction in Atherosclerotic Aortic Wall MRIs
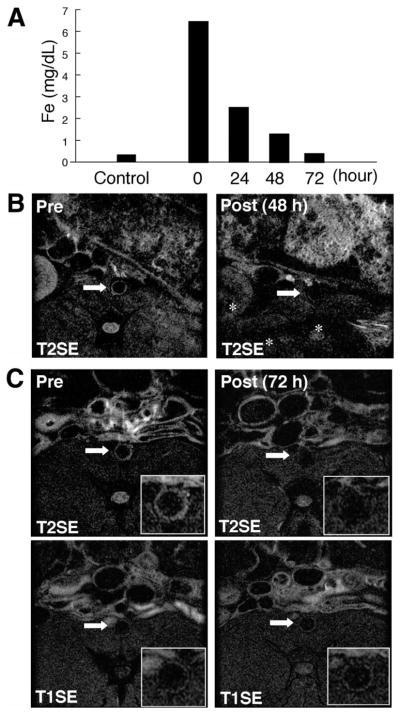
Plasma iron concentrations in rabbits increased dramatically immediately after MION-47 injection as expected (10 mg Fe/kg), and returned to the levels of control rabbits within 72 hours (Figure 3A). MRI showed vessel wall signal reduction on T2SE in vivo at 24 hours (not shown) and 48 hours after MION-47 injection (Figure 3B). T2-weighted signal also declined in other tissues, including back muscle (Figure 3B). This SI decline in atherosclerotic aortas persisted at 72 hours; however, other organs, including back muscles, rebounded to their initial SIs (Figure 3C). Iron nanoparticles did not appear to alter the aortic wall SI on T1SE (Figure 3C). Superimposing subtraction pseudocolor images (Figure 4A, top) illustrates the localization and magnitude of T2 signal reduction. Reconstructing the axial MRIs 3-dimensionally into the coronal views demonstrated that T2 signal reduction occurred in the entire abdominal aorta, although its magnitude varied (Figure 4A, bottom).
Figure 3.
Superparamagnetic iron nanoparticles (MION-47) produced T2-signal reduction in atherosclerotic plaques. A, In vivo kinetics of MION-47 in rabbits. Plasma concentrations of iron after MION-47 injection were determined by the quantitative colorimetric iron detection assay. Plasma iron concentrations decreased at 72 hours after MION-47 injection to the level of control rabbits. The data represent 3 independent experiments that provided similar results. B, MION-47 decreased T2-SI in the aortic wall of hypercholesterolemic rabbits 48 hours after injection. Other organs (eg, kidney, back muscle, and bone marrow, indicated by asterisk) also showed the signal reduction. C, top, MION-47–mediated T2-SI reduction in aortic wall lasted 72 hours after the injection with minimal reduction of SI in other organs. Back muscles had similar SI in preinjection and postinjection MRIs. Bottom, MION-47 produced no substantial change in the SI on T1SE.
Figure 4.
Superparamagnetic nanoparticles (MION-47) reduced signal on T2SE consistently in atherosclerotic aortas. A, top, Axial MRIs (left and middle) showed the T2 signal reduction in the abdominal aorta of a cholesterol-fed rabbit 72 hours after MION-47 injection (Pre vs Post). Top right, T2-SI reduction was further demonstrated as superimposed pseudocolor of image subtraction. Bottom, Three-dimensionally reconstructed coronal view of the same aorta showed that T2-SI reduction occurred throughout the aortic wall, although its magnitude varied. Arrows indicate the level of the axial views shown in the top row. B, MION-47 administration produced statistically significant reduction in T2-SI at 164 levels of the aortic wall of cholesterol-fed rabbits 72 hours after injection (P<0.001). Notably, T2-SI in back muscles did not differ substantially. C, The average T2-SI ratio (aortic wall T2-SI/back muscle T2-SI) in all in vivo axial slices of a representative rabbit decreased after MION-47 injection, although the magnitude varied. D, Quantitative analysis of 164 axial slices from 6 cholesterol-fed rabbits further indicated that MION-47 administration produced a statistically significant reduction in the T2-SI ratio in the aortic wall (P<0.01). E, MION-47 injection did not substantially affect the T1-SI ratio (120 slices, 6 rabbits).
Quantitative analysis of 164 MRI slices from 6 hypercholesterolemic rabbits on atherogenic diet without rosuvastatin treatment demonstrated that MION-47 administration (10 mg Fe/kg) reduced the T2-SI in the aortic wall 72 hours after injection, whereas T2-SI in back muscles did not differ substantially between preadministration and postadministration images (Figure 4B). These quantitative data agree with representative images shown in Figures 3C and 4A. Despite the lack of apparent change in muscle T2-SI, further normalizing the aortic wall SI by the muscle SI on each MRI (SI ratio=aortic wall SI/back muscle SI) enabled the measurement of net T2-SI reduction in the aorta and compensated for the superior-inferior inhomogeneity of the MRI receiver coil. The data demonstrated that MION-47 produced significant T2-SI changes in all 28 slices of an individual rabbit (Figure 4C). Notably, all 6 rabbits studied showed similar results (164 slices; Figure 4D), which correlates with the data on the aortic wall T2-SI (Figure 4B). In contrast, MION-47 injection did not substantially alter the SI ratio on T1SE (Figure 4E). Ex vivo MRI on excised aortas further validated the MION-47–mediated T2-SI changes in vivo (Figure 5). Fixatives such as paraformaldehyde may affect MR signal characteristics24; in our past work, however, it did not cause appreciable alterations in SI.12 Moreover, in the present study, fixation does not appear to affect the colocalization of iron and macrophages on histological examination, which correlates with regions of T2-SI loss on MRI. Arteries from rabbits injected with MION-47 show T2-SI suppression even after paraformaldehyde fixation.
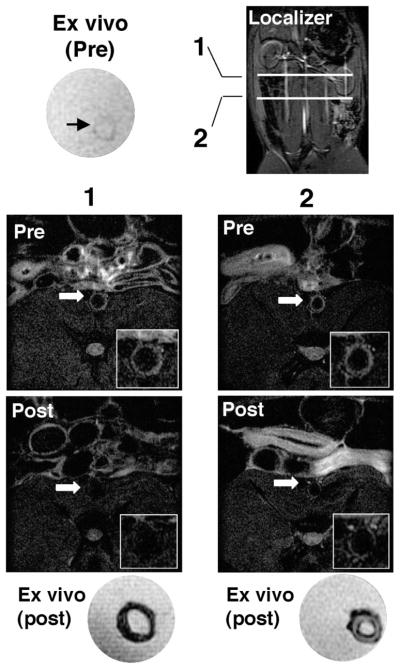
Figure 5.
MRI validated ex vivo the T2 signal reduction effect of MION-47 administration. Before MION-47 injection, the ex vivo atherosclerotic wall showed high T2-SI (top left; ex vivo, pre). The image localizer indicates levels of slices 1 and 2 (bottom). Both in vivo and ex vivo images of slice 1 exhibited the T2-SI reduction in the whole layer of aortic wall. Slice 2 showed in vivo and ex vivo T2-SI reduction in the intimal layer close to the luminal surface.
T2-SI Changes In Vivo Correlate With Macrophage Accumulation In Situ
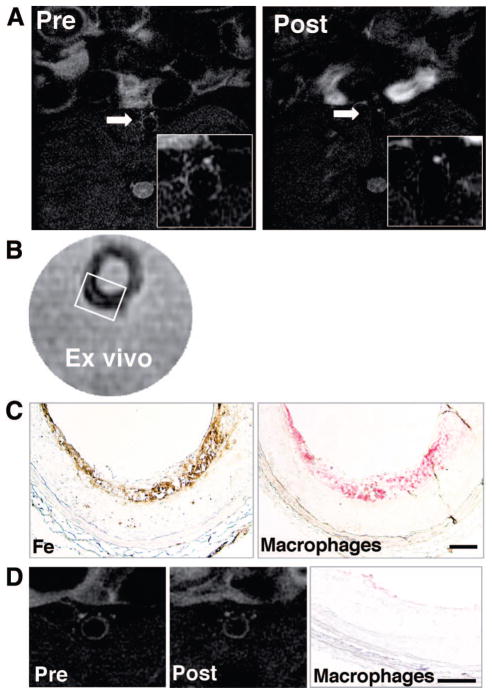
Macrophage immunostaining and iron staining corroborated the mechanisms of T2-SI loss in the atherosclerotic aorta of hypercholesterolemic rabbits in vivo and ex vivo (Figure 6A through 6C). Conversely, segments of the aorta that exhibited little (if any) T2-SI reduction contained sparse macrophages or iron (Figure 6D).
Figure 6.
The magnitude of MION-47–mediated T2-SI reduction is associated with the extent of macrophage accumulation in rabbit aortic atherosclerosis. A and B, MRIs show similar patterns of T2-SI reduction in vivo and ex vivo. C, Histological analyses of colocalized iron accumulation (DAB-enhanced Perl Prussian blue) in macrophages (RAM-11) in the luminal surface of atherosclerotic aorta at the level shown in the in vivo and ex vivo MRIs. The rectangle in the ex vivo MRI (B) indicates the area shown in C. D, A representative MRI that exhibited less T2-SI reduction associated with few, if any, macrophages identified by immunostaining. Bars=100 μm.
The Magnitude of T2-SI Reduction Quantitatively Reflects Plaque Macrophage Burden
The present study tested the specific hypothesis that MRI can quantify macrophage burden in atherosclerosis by examining whether T2-SI changes correlate with macrophage burden. Macrophage content in atherosclerotic plaques represented by percent immunoreactive area in situ (absolute positive area/absolute aortic wall area) correlated closely with the magnitude of T2-SI reduction in vivo (Figure 7A). These data agree with the representative images shown in Figure 6. In contrast, the magnitude of T2-SI reduction correlated poorly with the wall area (Figure 7B). Collectively, these results suggest that nanoparticle-enhanced MRI can measure the extent of macrophage accumulation and does not merely reflect anatomic plaque burden, affirming our study hypothesis.
Figure 7.
Quantitative analyses correlate T2-SI changes and precontrast T2-SI levels with macrophage burden. A, Quantitative analysis demonstrated that in vivo magnitude of T2-SI reduction correlated with macrophage content in situ (percent macrophage-positive area in total aortic wall area) (r=0.73; 150 MRI slices in 6 rabbits). B, The in vivo T2-SI reduction correlated poorly with plaque area measured histologically (r=0.26, n=164). C, T2 changes correlated with precontrast T2-SI in vivo (r=0.67; n=164). D, Correlations between precontrast T2-SI and percent macrophage-positive area (r=0.53; n=164).
T2-SI Reduction Correlates With Pre–MION-47 T2 SI Levels
Accumulating evidence suggests that hyperintensity on T2-weighted MRI is associated with inflamed lesions that contain increased free water content (water not bound by macromolecules or organelles).25 Aortic wall with higher SI on T2SE before MION-47 administration produced greater T2-SI changes after injection (Figure 7C). Similarly, precontrast T2-SI levels correlated with macrophage accumulation (Figure 7D), indicating that precontrast T2-SI may also reflect, indirectly via water content, macrophage burden in atherosclerosis.
Statin Administration Decreases Macrophage Accumulation and T2-SI Reduction in Atherosclerosis
We further tested whether an intervention could alter this imaging index of macrophage content. Rosuvastatin administration was started in rabbits 3 months after mechanical injury and initiation of a high-cholesterol diet. The 3 rabbits treated with rosuvastatin added to a standard diet for 3 months had lower total cholesterol levels than the 6 untreated rabbits (control group, 1122±89 mg/dL; rosuvastatin group, 63±18 mg/dL; P<0.01). Rosuvastatin-treated rabbits exhibited reversal of T2-SI changes in atherosclerosis in vivo and ex vivo, validated by histological analyses exhibiting less macrophage burden and iron accumulation within the intima (Figure 8A and 8B) compared with rabbits that did not receive rosuvastatin (Figure 6). MION-47 did not significantly reduce T2-SI in rosuvastatin-treated rabbits (Figure 8C). The magnitude of T2-SI reduction in rabbits treated with rosuvastatin was significantly lower than that of controlled rabbits that continued on the atherogenic diet (Figure 8D). Furthermore, these 2 factors, T2-SI changes and macrophage burden, were closely correlated (Figure 8E; see Figure 7A for the untreated rabbits). Although precontrast T2-SI was not substantially decreased in rosuvastatin-treated rabbits (Figure 8F), this metric remained positively correlated with T2-SI reduction (Figure 8G).
Figure 8.
Cholesterol lowering with rosuvastatin reduced macrophage accumulation and T2-SI reduction. A, MION-47 administration produced few, if any, T2-SI changes in vivo and ex vivo in the aortic wall of a rabbit treated for 3 months with rosuvastatin. Histological analysis detected less macrophage and iron accumulation compared with untreated rabbits, shown in Figure 6.B, Rosuvastatin treatment reduced plaque macrophages (P<0.05) in 6 control rabbits (n=150 slices) compared with 3 rosuvastatin-treated rabbits (n=81 slices). C, In the rabbits treated with rosuvastatin for 3 months (n=81 slices), T2-SI changes induced by MION-47 (Pre vs Post) in the aortic wall were not significant (P=0.079), unlike changes noted in control rabbits (Figure 4D; P<0.001). D, The T2-SI reduction resulting from MION-47 administration was significantly smaller (P<0.005) than the reduction in control rabbits that remained hypercholesterolemic for the entire study period. E, Greater macrophage content was correlated with greater T2-SI reduction in the treated animals (r=0.73; n=78 slices). See Figure 7A for comparison with hypercholesterolemic rabbits. F, Cholesterol lowering with rosuvastatin did not significantly reduce precontrast T2-SI levels (n=164 slices) compared with hypercholesterolemic controls (n=81 slices). G, The positive correlations (r=0.74) between precontrast T2-SI levels and T2 changes in rosuvastatin-treated rabbits (n=81 slices) remained similar to those in hypercholesterolemic rabbits (also see Figure 7D).
Discussion
Macrophage burden influences clinical outcomes of various inflammatory diseases, including atherosclerosis.1,3,4 Therefore, development of novel circulating or imaging biomarkers targeting macrophages should help identify patients with subclinical inflamed lesions and provide new and important insights into preventive cardiovascular medicine. The present study demonstrated in vivo and validated ex vivo and in situ that the magnitude of T2-SI reduction, resulting from the presence of superparamagnetic iron nanoparticles (MION-47), reflects the extent of macrophage accumulation in the aorta of hypercholesterolemic rabbits, supporting the ability of this technique to measure inflammatory burden in atherosclerosis. Such a noninvasive imaging approach might also monitor changes in inflammation during therapeutic interventions.
Previous studies, including our own, proved MRI useful in detecting plaque size changes during the progression or regression of experimental atherosclerosis.10,11,13 Yet, anatomic assessments of atherosclerosis may not reflect the pathophysiology underlying acute clinical events. We and others established the role of macrophage products in the onset of acute thrombotic complications of atherosclerosis.2–4,20,26 Recent contributions have linked clinical evidence, macrophage biology, and molecular imaging research to establish the feasibility of visualizing macrophage burden or activation in cardiovascular diseases.8,9,18,19,27,28 The present results affirm that an MR approach combined with ultrasmall superparamagnetic iron oxide particles can quantitatively assess inflammatory burden within the plaque. This imaging approach, when translated to humans, should aid the study of patients with subclinical high-risk lesions.
Clinical evidence established that cholesterol lowering reduces acute coronary events.29,30 Preclinical studies, including our own, further demonstrated that dietary cholesterol lowering and statin treatment can limit inflammation, improve endothelial activation/dysfunction, and limit monocyte/macrophage infiltration in arteries.1,2,20,26,31 Diminished macrophage burden should limit proteolytic and thrombogenic activities in atheromata, reducing cardiovascular risk. Therefore, establishing methods that can noninvasively monitor changes in plaque macrophage content or activity during interventions will likely provide new insights into preventive cardiovascular medicine and offer powerful tools for choosing doses and assessing efficacy in pilot clinical trials on novel antiinflammatory therapies. Indeed, the present results shed mechanistic light on recent clinical studies that indirectly suggested that an antiinflammatory effect of statins may contribute to their clinical benefit.32,33
Accumulating evidence suggests that macrophages internalize superparamagnetic iron nanoparticles to a greater extent than do endothelial and smooth muscle cells.16,34 The present study shows in vitro that MION-47 uptake by macrophages depends on their state of differentiation. The underlying mechanisms of macrophage uptake of such MRI contrast agents remain incompletely understood and may involve multiple mechanisms (eg, fluid-phase versus receptor-mediated endocytosis).16,35 Recent studies suggested the role of several molecules in this process, including macrophage-scavenger receptor A and integrin Mac-1 (CD11b/CD18).36,37 Activation of macrophages may also enhance the uptake of superparamagnetic nanoparticles.37 Although the mechanisms of endocytosis remain uncertain, the T2-SI reduction caused by iron accumulation in macrophages reflects metabolic activity of these proinflammatory phagocytes, providing direct relevance to complications of atherosclerosis.
Increased iron accumulation in inflamed plaques may involve other factors. Enhanced vascular permeability resulting from injured endothelium or leaky microvessels may promote nano-particle penetration into atherosclerotic lesions, augmenting access of the iron oxide to macrophages. The long half-life of the latest class of ultrasmall superparamagnetic iron oxide particles, including MION-47 and ferumoxtran-10, also favors efficient accumulation within plaques. In addition, hyperintense appearance on T2-weighted MRI without any enhancing agents likely results from increased free water.25,38 Thus, the association of inflammation in atherosclerosis with local edema may lead indirectly to its detection. Oxidative stress mediated by reactive oxygen species may also affect T2-SI.39
In support, a previous study correlated T2-SI with serum levels of biomarkers of inflammation in humans.40 Extensive quantitative analyses in the present study positively correlated precontrast T2-SI and plaque macrophage content (Figure 7C). Precontrast T2-SI further associated with the magnitude of T2-SI reduction in atherosclerotic aortas (Figure 7D). Intervention with rosuvastatin, an agent with lipid-lowering and likely antiinflammatory actions, further supported these findings. As discussed above, cholesterol lowering improves aspects of endothelial cell dysfunction and reduces macrophage accumulation.20,26,31 The present study demonstrates that statin treatment reduced total cholesterol levels in hypercholesterolemic rabbits and substantially reduced T2-SI in both precontrast and postcontrast images (Figure 8).
This investigation used a 3-T high-resolution clinical MRI scanner and consistently produced satisfactory in vivo plaque images. The spatial resolution produced in this study (234×312 μm) should suffice for imaging macrophage burden in human carotid or proximal coronary arteries. MRI combined with the superparamagnetic iron nanoparticles documented here presents a favorable method for screening and monitoring arteries in patients because of its noninvasive nature and the absence of iodine-based contrast or radioactive agents. Although recent developments now enable positive contrast with superparamagnetic iron nanoparticles,41 the techniques used here permit clinical translation without modification of the software on the MRI scanner.
Recent efforts have also attempted to establish noninvasive MRI as a tool for detecting other aspects of plaque inflammation, including cell growth or apoptosis,42 thin fibrous caps,43 intraplaque microvessels or hemorrhage,44,45 thrombus formation,46 and lipids.47,48 Ongoing studies are further exploring the feasibility of visualizing plaque macrophages in a manner more specific to the activated state using small-molecule conjugates49 or detecting specific molecules associated with acute complications of atherosclerosis such as myeloperoxidase, a product of macrophages and other inflammatory leukocytes, and vascular cell adhesion molecule 1.50
The present study expands more than a decade of fundamental research into the inflammatory mechanisms that prevail in atheromata and promote its clinical expression.1,2 Gauging plaque macrophage accumulation as a well-validated indicator of local inflammatory status has several clinical applications, including the assessment of the inflammatory burden, rather than the mere anatomy, of atherosclerotic plaques in individual patients before clinical complication occurs. Visualizing macrophage accumulation or function as a biomarker thus offers a potentially powerful strategy in personalized preventive medicine. This method will also provide a research tool to further dissect the role of vascular inflammation in clinical outcomes. Finally, noninvasive imaging of macrophage burden directly implicated in plaque pathobiology may furnish an important functional biomarker for the evaluation of novel antiinflammatory therapies in humans.
CLINICAL PERSPECTIVE.
Accumulation of macrophages influences clinical outcomes of various inflammatory diseases, including atherosclerosis. Particularly, these proinflammatory phagocytes promote not only development of atherosclerosis but also its acute thrombotic complications. Development of novel circulating or imaging biomarkers targeting macrophages should thus help to identify patients with subclinical inflamed lesions and to provide new and important insights into preventive cardiovascular medicine. Molecular or functional imaging, a rapidly emerging technology, visualizes biological or pathological processes in specific organs or disease contexts, in addition to providing anatomic information. The present study represents modern molecular imaging that can assess vascular inflammation. We demonstrate that high-resolution 3-T MRI enhanced with superparamagnetic iron nanoparticles can measure inflammatory burden in atherosclerosis of hypercholesterolemic rabbits. Macrophages internalize such iron particles, most likely via phagocytosis, thus changing the magnetic field in inflamed tissues. The present study demonstrates that the magnitude of T2-weighted signal intensity loss, reflecting phagocytic activity of macrophages, is associated positively with the extent of accumulation of these immune cells in atherosclerotic plaques. Such a noninvasive imaging approach might also offer a powerful tool to monitor the effects of antiinflammatory therapies in clinical practice or during clinical trials for new drugs. Our study indeed reports that the magnitude of T2-weighted signal intensity loss significantly decreased after statin treatment and correlated well with the reduction of lesional macrophage accumulation. Furthermore, such macrophage-targeted molecular imaging should provide novel insight into the mechanisms of atherosclerosis and its acute complications.
Acknowledgments
We thank Dr Robert Glynn for statistical analysis; Drs Hirokazu Fujiwara, Raymond Kwong, and Eric Larose for their help with protocol development; and Joan Edgett and Sara Karwacki for their editorial assistance.
Sources of Funding
This work was supported in part by grants from the Reynolds Foundation (Drs Libby and Weissleder), the National Institutes of Health (HL56895 to Drs Libby, Aikawa, and Ganz; HL66086 to Dr Aikawa; HL80472 to Dr Libby; HL078641 to Dr Weissleder; TPEN grant U01-HL080731 to Drs Weissleder and Libby; U41RR19703 to D.F. Kacher), the AstraZeneca Investigator-Sponsored Study Program (Dr Aikawa), the Japan Heart Foundation/Bayer (Dr Morishige), and the Uehara Memorial Foundation (Dr Morishige).
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.891804/DC1.
Disclosures
Dr Aikawa has received a research grant from AstraZeneca. Dr Ganz has received honoraria from Pfizer for his speakers’ bureau appointments. Dr Libby has served as an unpaid consultant to and member of the steering committees of investigator-initiated clinical trials for Astra-Zeneca, including A Study to Evaluate the Effect of Rosuvastatin on Intravascular Ultrasound-Derived Coronary Atheroma Burden (ASTEROID), Justification for the Use of statins in Primary prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), and Study of Coronary Atheroma by Intravascular Ultrasound: Effect of Rosuvastatin Versus Atorvastatin (SATURN). The other authors report no conflicts.
References
- 1.Aikawa M, Libby P. The vulnerable atherosclerotic plaque: pathogenesis and therapeutic approach. Cardiovasc Pathol. 2004;13:125–138. doi: 10.1016/S1054-8807(04)00004-3. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 3.Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41:15S–22S. doi: 10.1016/s0735-1097(02)02834-6. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47:C13–C18. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury RP, Fuster V, Fayad ZA. Molecular, cellular and functional imaging of atherothrombosis. Nat Rev Drug Discov. 2004;3:913–925. doi: 10.1038/nrd1548. [DOI] [PubMed] [Google Scholar]
- 7.Sosnovik DE, Nahrendorf M, Weissleder R. Molecular magnetic resonance imaging in cardiovascular medicine. Circulation. 2007;115:2076–2086. doi: 10.1161/CIRCULATIONAHA.106.658930. [DOI] [PubMed] [Google Scholar]
- 8.Deguchi J, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 9.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115:377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 10.Skinner MP, Yuan C, Mitsumori L, Hayes CE, Raines EW, Nelson JA, Ross R. Serial magnetic resonance imaging of experimental atherosclerosis detects lesion fine structure, progression and complications in vivo. Nat Med. 1995;1:69–73. doi: 10.1038/nm0195-69. [DOI] [PubMed] [Google Scholar]
- 11.McConnell MV, Aikawa M, Maier SE, Ganz P, Libby P, Lee RT. MRI of rabbit atherosclerosis in response to dietary cholesterol lowering. Arterioscler Thromb Vasc Biol. 1999;19:1956–1959. doi: 10.1161/01.atv.19.8.1956. [DOI] [PubMed] [Google Scholar]
- 12.Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, Aikawa E, Kinlay S, Schoen FJ, Selwyn AP, Ganz P. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation. 2005;112:2324–2331. doi: 10.1161/CIRCULATIONAHA.105.538942. [DOI] [PubMed] [Google Scholar]
- 13.Corti R, Osende JI, Fallon JT, Fuster V, Mizsei G, Jneid H, Wright SD, Chaplin WF, Badimon JJ. The selective peroxisomal proliferator-activated receptor-gamma agonist has an additive effect on plaque regression in combination with simvastatin in experimental atherosclerosis: in vivo study by high-resolution magnetic resonance imaging. J Am Coll Cardiol. 2004;43:464–473. doi: 10.1016/j.jacc.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 14.Weissleder R, Elizondo G, Stark DD, Hahn PF, Marfil J, Gonzalez JF, Saini S, Todd LE, Ferrucci JT. The diagnosis of splenic lymphoma by MR imaging: value of superparamagnetic iron oxide. AJR Am J Roentgenol. 1989;152:175–180. doi: 10.2214/ajr.152.1.175. [DOI] [PubMed] [Google Scholar]
- 15.Josephson L, Groman E, Weissleder R. Contrast agents for magnetic resonance imaging of the liver. Targeted Diagn Ther. 1991;4:163–187. [PubMed] [Google Scholar]
- 16.Schulze E, Ferrucci JT, Jr, Poss K, Lapointe L, Bogdanova A, Weissleder R. Cellular uptake and trafficking of a prototypical magnetic iron oxide label in vitro. Invest Radiol. 1995;30:604–610. doi: 10.1097/00004424-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Shen T, Weissleder R, Papisov M, Bogdanov A, Jr, Brady TJ. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 18.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 19.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 20.Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content of rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds F, O’Loughlin T, Weissleder R, Josephson L. Method of determining nanoparticle core weight. Anal Chem. 2005;77:814–817. doi: 10.1021/ac049307x. [DOI] [PubMed] [Google Scholar]
- 22.Weissleder R, Stark DD, Engelstad BL, Bacon BR, Compton CC, White DL, Jacobs P, Lewis J. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am J Roentgenol. 1989;152:167–173. doi: 10.2214/ajr.152.1.167. [DOI] [PubMed] [Google Scholar]
- 23.Anzai Y, Piccoli CW, Outwater EK, Stanford W, Bluemke DA, Nurenberg P, Saini S, Maravilla KR, Feldman DE, Schmiedl UP, Brunberg JA, Francis IR, Harms SE, Som PM, Tempany CM. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–788. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 24.Dalager-Pedersen S, Falk E, Ringgaard S, Kristensen IB, Pedersen EM. Effects of temperature and histopathologic preparation on the size and morphology of atherosclerotic carotid arteries as imaged by MRI. J Magn Reson Imaging. 1999;10:876–885. doi: 10.1002/(sici)1522-2586(199911)10:5<876::aid-jmri37>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 25.Toussaint JF, Southern JF, Fuster V, Kantor HL. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1533–1542. [PubMed] [Google Scholar]
- 26.Aikawa M, Sugiyama S, Hill CC, Voglic SJ, Rabkin E, Fukumoto Y, Schoen FJ, Witztum JL, Libby P. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- 27.Sosnovik DE, Nahrendorf M, Deliolanis N, Novikov M, Aikawa E, Josephson L, Rosenzweig A, Weissleder R, Ntziachristos V. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation. 2007;115:1384–1391. doi: 10.1161/CIRCULATIONAHA.106.663351. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi RA, Mallawarachi C, U-King-Im JM, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirkpatrick PJ, Brown J, Gillard JH. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 29.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 30.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 31.Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 32.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Macfadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 34.Moore A, Marecos E, Bogdanov A, Jr, Weissleder R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214:568–574. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 35.Moore A, Weissleder R, Bogdanov A., Jr Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging. 1997;7:1140–1145. doi: 10.1002/jmri.1880070629. [DOI] [PubMed] [Google Scholar]
- 36.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 37.von Zur Muhlen C, von Elverfeldt D, Bassler N, Neudorfer I, Steitz B, Petri-Fink A, Hofmann H, Bode C, Peter K. Superparamagnetic iron oxide binding and uptake as imaged by magnetic resonance is mediated by the integrin receptor Mac-1 (CD11b/CD18): implications on imaging of atherosclerotic plaques. Atherosclerosis. 2007;193:102–111. doi: 10.1016/j.atherosclerosis.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 38.Denis MC, Mahmood U, Benoist C, Mathis D, Weissleder R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc Natl Acad Sci U S A. 2004;101:12634–12639. doi: 10.1073/pnas.0404307101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noseworthy MD, Bray TM. Effect of oxidative stress on brain damage detected by MRI and in vivo 31P-NMR. Free Radic Biol Med. 1998;24:942–951. doi: 10.1016/s0891-5849(97)00383-3. [DOI] [PubMed] [Google Scholar]
- 40.Weiss CR, Arai AE, Bui MN, Agyeman KO, Waclawiw MA, Balaban RS, Cannon RO., III Arterial wall MRI characteristics are associated with elevated serum markers of inflammation in humans. J Magn Reson Imaging. 2001;14:698–704. doi: 10.1002/jmri.10023. [DOI] [PubMed] [Google Scholar]
- 41.Korosoglou G, Weiss RG, Kedziorek DA, Walczak P, Gilson WD, Schar M, Sosnovik DE, Kraitchman DL, Boston RC, Bulte JW, Weissleder R, Stuber M. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J Am Coll Cardiol. 2008;52:483–491. doi: 10.1016/j.jacc.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadeghi MM, Krassilnikova S, Zhang J, Gharaei AA, Fassaei HR, Esmailzadeh L, Kooshkabadi A, Edwards S, Yalamanchili P, Harris TD, Sinusas AJ, Zaret BL, Bender JR. Detection of injury-induced vascular remodeling by targeting activated alphavbeta3 integrin in vivo. Circulation. 2004;110:84–90. doi: 10.1161/01.CIR.0000133319.84326.70. [DOI] [PubMed] [Google Scholar]
- 43.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 44.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, Wickline SA. Molecular imaging of angiogenesis in early-stage atherosclerosis with alpha(v)beta3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 45.Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 46.Johnstone MT, Botnar RM, Perez AS, Stewart R, Quist WC, Hamilton JA, Manning WJ. In vivo magnetic resonance imaging of experimental thrombosis in a rabbit model. Arterioscler Thromb Vasc Biol. 2001;21:1556–1560. doi: 10.1161/hq0901.094242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirol M, Itskovich VV, Mani V, Aguinaldo JG, Fallon JT, Misselwitz B, Weinmann HJ, Fuster V, Toussaint JF, Fayad ZA. Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation. 2004;109:2890–2896. doi: 10.1161/01.CIR.0000129310.17277.E7. [DOI] [PubMed] [Google Scholar]
- 48.Underhill HR, Yuan C, Zhao XQ, Kraiss LW, Parker DL, Saam T, Chu B, Takaya N, Liu F, Polissar NL, Neradilek B, Raichlen JS, Cain VA, Waterton JC, Hamar W, Hatsukami TS. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial [erratum appears. Am Heart J. 2008;2155:1127. doi: 10.1016/j.ahj.2007.11.018. [DOI] [PubMed] [Google Scholar]; Am Heart J. 2008;155:584.e581–e588. [Google Scholar]
- 49.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 50.Shepherd J, Hilderbrand SA, Waterman P, Heinecke JW, Weissleder R, Libby P. A fluorescent probe for the detection of myeloperoxidase activity in atherosclerosis-associated macrophages. Chem Biol. 2007;14:1221–1231. doi: 10.1016/j.chembiol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]