Abstract
Background
Integration is an intermediate step in the HIV life cycle and is defined as the insertion of HIV-1 proviral DNA into the host chromosome. If integration does not occur when HIV-1 cDNA enters the nucleus, it circularizes upon itself and forms a 2-LTR circle. Monitoring the level of integrated HIV-1 cDNA in different primary cell subsets is very important, particularly regarding the effect of HAART in HIV-1 infected individuals. Because of limitations of prior HIV-1 integration assays, there is limited data on the level of integration and 2-LTR circle formation in primary cell subsets, particularly in human monocyte-derived macrophages and peripheral blood lymphocytes (PBL).
Results
In this study, we utilized a well-defined, sensitive two-step quantitative real-time PCR method to detect HIV-1 integration as well as conventional real-time PCR to detect 2-LTR circle formation in human macrophages and PBL isolated from six different healthy donors, as well as U373 CD4+ cells by infecting with HIV-1SX (R5) or dual-tropic isolate HIV-189.6 (R5/X4) virus strains. We used the FDA-approved integrase inhibitor, raltegravir, to determine quantitative differences of integrated HIV viral cDNA in HIV-1 infected cells with and without raltegravir treatment. Our results show that integration and 2-LTR circle formation can be assessed in primary macrophages, PBL, and a CD4+ cell line by this method. Specifically, our results demonstrate that this two-step real-time PCR method can distinguish between HIV-1 integrated viral cDNA and non-integrated nuclear HIV-1 2-LTR circles caused by impaired integration with raltegravir-treatment. This further confirms that only integrated HIV-1 cDNA can be specifically amplified and quantified by two-step PCR without non-specifically detecting non-integrated viral cDNA.
Conclusion
These results consistently demonstrate that the well-established real-time PCR assays used are robust, sensitive and quantitative for the detection of HIV-1 integration and 2-LTR circle formation in physiologically relevant human macrophages and PBL using lab-adapted virus strains, instead of pseudovirus. With two-step real-time PCR, we show that unintegrated, nuclear HIV-1 cDNA is not detected in raltegravir-treated cells, while specific for only integrated HIV-1 cDNA in non-treated cells. These methods could be applied as a useful tool in further monitoring specific therapy in HIV-1 infected individuals.
Background
Human immunodeficiency virus type 1 (HIV-1) is known to infect several primary cell types, predominantly CD4+ T lymphocytes and macrophages. HIV-1 infection results in a gradual decline in the number of CD4+ T cells, leading to the development of AIDS. Macrophages are also of particular importance for the pathogenesis of HIV-1, as the cells are likely to be the major cell type involved in mucosal transmission of HIV-1 [1-3]. In addition, macrophages appear to be more resistant to the cytopathic effects of HIV-1 infection, so they are thought to play a crucial role in viral persistence, latency, and dissemination [4,5].
Early steps of HIV-1 infection include viral entry by binding to the main receptor CD4 and either of two co-receptors CCR5 or CXCR4. Upon membrane fusion, the viral core is released into the cytoplasm. Once inside the cell, reverse transcriptase converts viral RNA into DNA which is then transported into the nucleus and integrates into the host chromosome. Integration, the intermediate step of the HIV-1 lifecycle, is dependent on viral integrase activity for efficiently spreading infection [6-10]. If HIV-1 cDNA enters the nucleus but does not integrate into the host cell chromosome, then the viral cDNA circularizes to form a 2-LTR circle [11,12]. Advent of more sensitive assays for HIV-1 integration can enhance our knowledge of how cellular factors play a role in HIV-1 integration [13,14]. Previous methods to quantify integrated viral DNA include one-step amplification [15], nested linker primer PCR (LP-PCR) [16], virus-specific primer with tag sequence [17], and real-time nested PCR using Alu-specific primers [18,19].
Liszewski et al. described the limitations of each assay and recently showed that this two-step Alu-gag PCR method has high sensitivity as well as robust quantitation [18]. Since this two-step Alu-gag PCR assay is well-defined and highly sensitive and specific, we used this assay for detecting and quantifying integration in our cell subsets. Additionally, while the previous studies utilized pseudotyped virus in their assays, we used clinical, lab-adapted HIV-1 strains to measure the level of integrated DNA in human macrophages, peripheral blood lymphocytes (PBL) and U373 CD4+ cell lines. We also employed the use of the FDA-approved integrase inhibitor, raltegravir. Because raltegravir prevents HIV-1 integration and causes formation of HIV-1 2-LTR circles, this allowed us to quantitatively assess the differences between integrated HIV-1 proviral DNA and unintegrated HIV-1 cDNA in HIV-1 infected cells.
Results
HIV-1 Integration in U373 cells
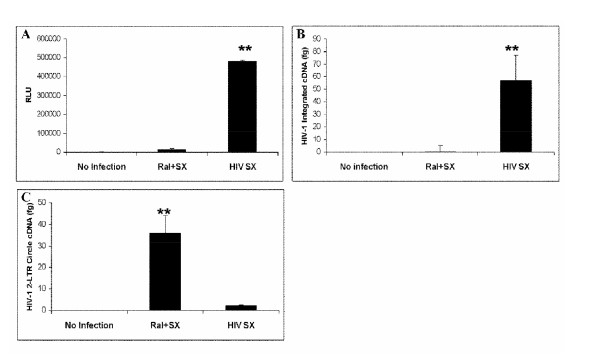
To verify Alu-gag two-step PCR could be used to detect HIV-1 integration system, HIV-1SX, a CCR5-tropic virus strain, was used to infect U373-MAGI-CCR5 cells (MOI = 0.1) with or without raltegravir treatment (Merck & CO. Inc., Whitehouse Station, NJ). FDA-approved raltegravir blocks HIV-1 integration by preventing strand transfer, and thus preventing HIV-1 from successfully inserting its viral cDNA into the host chromosome [20,21]. Forty-eight hours post-infection, cellular genomic DNA was isolated from U373 cells for detection of HIV-1 integration; meanwhile, β-galactosidase activity was analyzed for determination of HIV-1 infection. As shown in Figure 1A β-galactosidase activity in raltegravir treated cells with HIV-1SX infection was not seen, similar to control condition (non-infected/non-treated cells). However, there was a 6-fold increase in HIV-1SX infected cells without raltegravir treatment. In Figure 1B, HIV-1 integration was shown to be significantly different between infected cells with and without raltegravir treatment (P < 0.01), indicating detection of integration in HIV-infected cells in the absence of raltegravir treatment. Figure 1C shows that raltegravir-treated cells prevent integration and is the only treatment causing formation of 2LTR circles. This also confirms specificity for HIV-1 integration because non-integrated HIV-1 cDNA is not amplified by these real-time PCR probes. Three independent experiments were performed, and the data were consistent each time, proving to be a reproducible and reliable method for detection of integration in U373 cells.
Figure 1.
Quantitation of HIV-1 integration and 2-LTR circle formation in CD4+ U373 cells. U373-MAGI-CCR5 cells were plated in 6-well plates with or without raltegravir treatment 24 h prior to infection and during infection (MOI = 0.1). Two days after infection, (A) β-galactosidase activity (expressed as RLU = Relative Light Units) was analyzed for determination of HIV-1SX infection; (B) cellular genomic DNA was extracted from U373 cells 48 h after infection and HIV-1SX integration was detected using two-step quantitative PCR, and (C) 2-LTR circle formation was measured by real-time PCR. (**p < 0.01)
HIV-1 Integration in human PBL
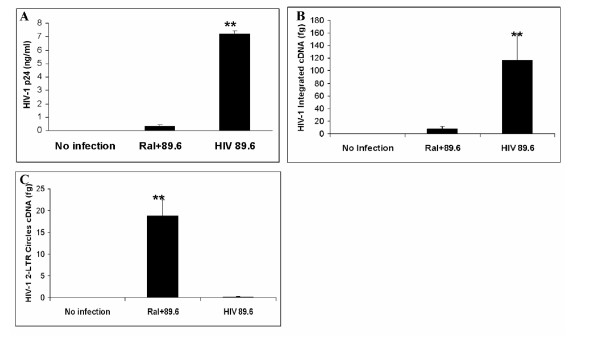
In order to assess integration in primary cell subsets, PBL were isolated from human blood, and infected with dual-tropic virus strain HIV-189.6. As shown in Figure 2A, virus production (HIV-1 p24 measured by ELISA) in infected PBL was significantly lower (more than 7-fold) with raltegravir treatment compared to those without raltegravir (P < 0.01). The integration data (Figure 2B) was highly consistent with p24 data, showing HIV-189.6 integration as significantly higher (more than 6-fold) in infected cells without raltegravir compared to raltegravir-treated cells (P < 0.01). In addition, Figure 2C shows that raltegravir treatment does increase 2LTR circle formation. These data are representative of six experiments in PBL.
Figure 2.
Quantitation of HIV-1 integration and 2-LTR circle formation in human PBL. PBL were plated in 6-well plates with or without raltegravir treatment 24 h prior to infection with HIV-189.6, during infection and 48 h after infection (MOI = 0.1). (A) Seven days after infection, supernatant was assessed for p24 level of each group by p24 capture ELISA; (B) Six days after infection, cellular genomic DNA was extracted from PBLs and HIV-1 integration was measured by two-step quantitative PCR, and (C) 2-LTR circle formation was measured by real-time PCR. (**p < 0.01)
HIV-1 Integration in human macrophages
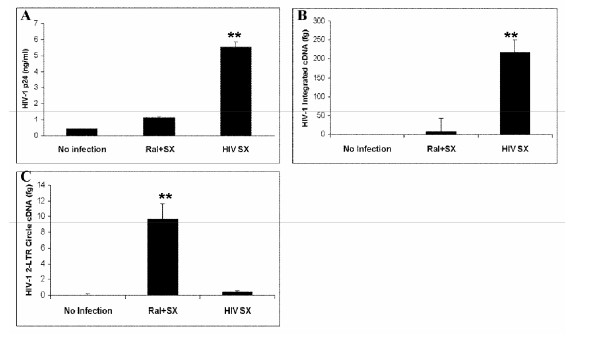
Human monocyte-derived macrophages were isolated from human blood, and infected with HIV-1SX. As shown in Figure 3A, virus production in infected macrophages was approximately 5-fold higher in cells without raltegravir treatment as compared to those with raltegravir treatment (P < 0.01). Similarly to other cell types, macrophages treated with raltegravir show a significant decrease in viral cDNA integration into the genome when compared with the cells without raltegravir treatment (P < 0.01), as shown in Figure 3B. Figure 3C shows that raltegravir-treatment increases 2LTR circle formation. For all cell systems used in this study, there was no cytotoxicity observed in raltegravir-treated cells (data not shown).
Figure 3.
Quantitation of HIV-1 integration and 2-LTR circle formation in human macrophages. Macrophages were plated in 6-well plates with or without raltegravir treatment 24 h prior to infection, during infection and 48 h after infection (MOI = 0.1). (A) Seven days after infection, supernatant was assessed for p24 level of each group by p24 capture ELISA; (B) Six days after infection, cellular genomic DNA was extracted from macrophages, and HIV-1SX integration was measured by two-step quantitative PCR, and (C) 2-LTR circle formation was measured by real-time PCR. (**p < 0.01)
Taken together, these results suggest that this two-step quantitative PCR method can be used effectively to quantitate HIV-1 integration in primary human macrophages and PBL, as well as our CD4+ U373 cell line.
Conclusions
We used the antiretroviral integrase inhibitor, raltegravir, to distinguish between integrated and non-integrated HIV-1 cDNA in infected primary PBL, macrophages, and a human CD4+ cell line. We detected HIV-1 integration by utilizing a well-defined two-step quantitative PCR method [19], which has proven to be a specific and sensitive approach in different cell subsets based on our reproducible results. In both raltegravir-treated and non-treated cells, viral RNA is reverse transcribed into viral cDNA and transported into the nucleus. In non-treated cells, viral cDNA integrates into the host chromosome, as detected by two-step real-time PCR; whereas in raltegravir-treated cells, viral cDNA forms 2-LTR circles preventing it from integrating into the host chromosome, as shown by conventional real-time PCR. Yu et al. have used this method to show that patients on HAART have decreased levels of integrated HIV-1 proviral DNA as compared to patients off HAART [22]. Thus, this method may be considered for the routine analysis of HIV-1 DNA integration to evaluate the integration efficiency of retroviral vectors in different cell subsets.
Our study extends the previous work performed by others [18,19] to detect integration in primary human cell subsets - PBL and macrophages using this two-step PCR technique. This is important because macrophages and PBL are crucial for HIV-1 infection, latency, and persistence [4,5]. As such, we infect human macrophages or PBLs derived from six different healthy donors, as well as infect a CD4+ cell line, and consistently demonstrate similar results using two different virus strains. By using these primary cell subsets, we show that this method can be useful in precisely monitoring the level of integration in laboratory settings and perhaps in HIV-infected patients to conclusively determine if it is affected by specific antiretroviral therapy. Thus, by using raltegravir as a control, we demonstrate that two-step PCR is specific in detecting only integrated HIV-1 cDNA and not other HIV-1 cDNA in the nucleus or cell. Additionally, we utilized lab-adapted R5- and dual-tropic strains of HIV-1 instead of pseudovirus to more closely mimic natural infection. Furthermore, this approach could reveal if HIV-1 integration persists within specific cellular subsets in patients on highly active antiretroviral therapy (HAART).
Methods
U373 cells
U373-MAGI-CCR5 cells (contributed by Drs. Michael Emerman and Adam Geballe), are modified U373 glioblastoma cells that are used for HIV infection experiments. U373-MAGI-CCR5 cells express β-galactosidase under the control of HIV LTR, which is trans-activated by HIV Tat protein in relation to the level of virus replication [23,24]. In addition, these cells express CD4 and human chemokine receptor CCR5 on its surface, which allow infection by primary HIV R5 strains [24]. U373 cells were propagated in 90% DMEM supplemented with 10% fetal bovine serum, 0.2 mg/ml G418, 0.1 mg/ml hygromycin B, and 1.0 μg/ml puromycin. For infection experiments, U373 cells were maintained in 90% DMEM, 10% fetal bovine serum, and 1% penicillin/streptomycin.
Preparation of human PBL
PBL were isolated from PBMC obtained from six different healthy human buffy coats prepared by the University of Texas Medical Branch (UTMB) Blood Bank in Galveston, TX. After the initial 24 h incubation of PBMC on 10 cm petri dishes, supernatant (containing PBL) was transferred to 50 ml tube and cells were isolated by centrifugation. Cells then were resuspended in stimulation media (RPMI 1640 media with 20% Fetal calf serum (FCS); 1% Penicillin/Streptomycin; 5 μg/ml phytohemagglutinin) and incubated at 37°C with 5% CO2 for 72 h. PBL were then collected by centrifugation and resuspended in growth media (RPMI 1640 with 1% L-glutamine; 1% Penicillin/Streptomycin; 20% FCS; 20 units/ml IL-2).
Preparation of human macrophages
Primary human macrophages were purified from healthy human PBMC (from the same six blood donors as human PBL isolation) by adherence to plastic tissue culture dishes as described previously [25]. Briefly, PBMC were purified by Ficoll-Hypaque centrifugation from buffy coats of healthy HIV-negative blood donors prepared by the UTMB Blood Bank. Primary monocyte-derived macrophages were obtained by adherence for 7 days to plastic petri dishes initially coated with human AB serum [26]. During differentiation, macrophages were cultured in Iscove's modified Dulbecco's medium supplemented with 20% FCS; 1% L-glutamine and 1% Penicillin/Streptomycin.
Viruses and infection
HIV-1SX, which is a chimeric M-tropic virus (R5) encoding the majority of the HIV-1JRFL envelope protein in an HIV-1NL4-3 backbone, and dual-tropic (R5/X4) HIV-189.6, which is a HIV-1 laboratory adapted strain originally isolated from infected individuals, were purchased from the Virology Core Facility, Center for AIDS Research at Baylor College of Medicine, Houston, TX. HIV-1SX stock containing 69.681 ng/ml of HIV p24 with 65,325 TCID50/ml was used to infect macrophages and U373 cells. HIV-189.6 stock containing 49.977 ng/ml of HIV p24 with 261,300 TCID50/ml was used to infect PBL. HIV-1 stocks were titrated, and for all experiments, the inoculum was 7 ng of p24 per 1.5 × 105 cells (MOI 0.1). Raltegravir (Merck & Co., Inc., Whitehouse Station, NJ) is a well-characterized, FDA-approved HIV-1 integrase inhibitor. It had been previously tested in our lab and showed no visual cytopathic effects or any cytotoxicity at 20 μM (data not shown). U373-MAGI cells, primary macrophages, and PBL were plated in 6-well plates at 1.5 × 105 cells per well 24 h prior to infection. Each of these cell subsets was plated into three 6-well plates. In the first plate, cells were infected with HIV-1 only; the second plate was treated with raltegravir (20 μM) 24 h prior to HIV-1 infection and during infection; the third plate contained non-infected/non-treated cells serving as a negative control. After 4 h incubation of virus inoculum (0.5 ml/well) at 37°C, fresh medium (1.5 ml) was added to each well. For macrophages and PBL, genomic DNA was extracted 6 days post-infection using DNeasy Blood and Tissue Kit (QIAGEN, Alameda, CA) according to the manufacturer's instructions. To assess infection, supernatant was harvested for HIV p24 levels in each group by a p24 capture ELISA kit (Immuno Diagnostics, Inc, Woburn, MA) according to the manufacturer's instructions. Since the HIV replication kinetics are more rapid in U373-MAGI cells than in primary macrophages and PBL, genomic DNA was extracted from U373 cells 48 h post-infection. To assess infection of HIV-1SX in U373 cells, the cells were lysed and analyzed for β-galactosidase activity using the Beta-Glo Assay System (Promega, Madison, WI) and a Dynex MLX Luminometer.
PCR
For the pre-amplification of genomic DNA from macrophages, PBL, and U373 cells the following primers were used: Alu forward, 5'-GCC TCC CAA AGT GCT GGG ATT ACA G-3'; and HIV-1 gag reverse, 5'-GCT CTC GCA CCC ATC TCT CTC C-3' [18,19]. The PCR solution contained 1× TaqMan Universal Master Mix, No AmpErase UNG (Applied Biosystems, Carlsbad, CA), 100 nM Alu forward primer, and 600 nM gag reverse primer, and 5 μl of DNA for every 15 μl of PCR solution. The Thermocycler (Applied Biosystems GeneAmp PCR system 2700) was programmed to perform a 2 min hot start at 94°C, followed by 30 steps of denaturation at 93°C for 30 seconds, annealing at 50°C for 1 minute, and extension at 70°C for 1 minute 40 seconds.
Quantitative real-time PCR
For quantitation of HIV-1 integration, a second round real-time quantitative PCR was performed using 7 μl of the material from the pre-amplification step. These samples were run along with known dilutions of HIV-1SX plasmid cDNA used for a standard curve. This standard curve was used to quantify the amplified DNA. The sequences of the primers used are as follows: LTR forward, 5'-GCC TCA ATA AAG CTT GCC TTG A-3'; and LTR reverse, 5'-TCC ACA CTG ACT AAA AGG GTC TGA-3' [19]. The LTR molecular beacon probe, labeled on the 5' terminus with the reporter fluorophore 6-carboxyfluorescein (FAM) and on its 3' terminus with Black Hole Quencher 1 (DBH1), had the following sequence: 5'-FAM-GCG AGT GCC CGT CTG TTG TGT GAC TCT GGT AAC TAG CTC GC-DBH1-3' [19]. For quantitation of HIV-1 2-LTR circles, small non-genomic DNA was isolated from cells using a Qiagen Miniprep kit. To identify 2-LTR circle formation, primers MH535 (5'-AAC TAG GGA ACC CAC TGC TTA AG-3') and MH536 (5'-TCC ACA GAT CAA GGA TAT CTT GTC-3') were used with the MH603 probe (5'-(FAM)-ACA CTA CTT GAA GCA CTC AAG GCA AGC TTT-(TAMRA)-3') [27]. All reactions were performed in a volume of 20 μl containing 1× TaqMan Universal Master Mix, No AmpErase UNG, and 200 nM of forward primer, reverse primer, and molecular probe. All reactions were performed using Applied Biosystems TaqMan Universal Master Mix and run using an Applied Biosystems 7500 Fast Real-time PCR system and 7500 Fast System Software. The thermal program started with 2 min at 50°C, followed by a 10 minute hot start at 95°C. This was followed by 40 cycles of 95°C for 15 seconds and 60°C for 60 seconds. GAPDH was used as an internal control to normalize total DNA.
Statistical analysis
To evaluate the sensitivity and specificity of this method, we detected the quantity of integration in three different cells, and compared them by student's t-test to determine differences between raltegravir treated groups and virus only infection groups. P < 0.05 was considered as significant difference.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BF and GL performed all experiments and drafted the manuscript. ND participated in the design of the study and contributed to drafting the manuscript. MRF conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Brian Friedrich, Email: bmfriedr@utmb.edu.
Guangyu Li, Email: guli@utmb.edu.
Natallia Dziuba, Email: nadziuba@utmb.edu.
Monique R Ferguson, Email: mrfergus@utmb.edu.
Acknowledgements
This work was supported by Public Health Service grant HL088999 from the National Heart, Lung, and Blood Institute. We thank the NIH AIDS Research and Reference Reagent Program for providing the U373-MAGI-CCR5 cells. We thank Edward Siwak, Ph.D., Associate Director of Virology Core Facility, Center for AIDS Research at Baylor College of Medicine, Houston, TX for providing HIV-1SX and HIV-189.6. Also, we greatly appreciate Merck & CO., Inc. for generously providing raltegravir used in our studies; Dr. Michael Miller for experimental advice; Dr. William A. O'Brien for his excellent editorial suggestions.
References
- von Lindern JJ, Rojo D, Grovit-Ferbas K, Yeramian C, Deng C, Herbein G, Ferguson MR, Pappas TC, Decker JM, Singh A. et al. Potential role for CD63 in CCR5-mediated human immunodeficiency virus type 1 infection of macrophages. J Virol. 2003;77:3624–3633. doi: 10.1128/JVI.77.6.3624-3633.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dornadula G, Beumont M, Livornese L Jr, Van Uitert B, Henning K, Pomerantz RJ. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- Gartner S, Markovits P, Markovitz DM, Kaplan MH, Gallo RC, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Kuroda MJ. Macrophages: do they impact AIDS progression more than CD4 T cells? J Leukoc Biol. pp. 569–573. [DOI] [PubMed]
- Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995;69:2729–2736. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund G, Theodore TS, Freed EO, Engelman A, Martin MA. Integration is required for productive infection of monocyte-derived macrophages by human immunodeficiency virus type 1. J Virol. 1995;69:3216–3219. doi: 10.1128/jvi.69.5.3216-3219.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFemina RL, Schneider CL, Robbins HL, Callahan PL, LeGrow K, Roth E, Schleif WA, Emini EA. Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol. 1992;66:7414–7419. doi: 10.1128/jvi.66.12.7414-7419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Kawamura M, Sakuragi J, Sakuragi S, Shibata R, Ishimoto A, Ono N, Ueda S, Adachi A. Integration is essential for efficient gene expression of human immunodeficiency virus type 1. J Virol. 1993;67:1169–1174. doi: 10.1128/jvi.67.3.1169-1174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M, Stanwick TL, Dempsey MP, Lamonica CA. HIV-1 replication is controlled at the level of T cell activation and proviral integration. Embo J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky M, Sharova N, Stevenson M. Human immunodeficiency virus type 1 2-LTR circles reside in a nucleoprotein complex which is different from the preintegration complex. J Virol. 1993;67:6863–6865. doi: 10.1128/jvi.67.11.6863-6865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnet CM, Haseltine WA. Circularization of human immunodeficiency virus type 1 DNA in vitro. J Virol. 1991;65:6942–6952. doi: 10.1128/jvi.65.12.6942-6952.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- Konig R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, Irelan JT, Chiang CY, Tu BP, De Jesus PD, Lilley CE. et al. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell. 2008;135:49–60. doi: 10.1016/j.cell.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonza S, Maerz A, Deacon N, Meanger J, Mills J, Crowe S. Human immunodeficiency virus type 1 replication is blocked prior to reverse transcription and integration in freshly isolated peripheral blood monocytes. J Virol. 1996;70:3863–3869. doi: 10.1128/jvi.70.6.3863-3869.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandegraaff N, Kumar R, Burrell CJ, Li P. Kinetics of human immunodeficiency virus type 1 (HIV) DNA integration in acutely infected cells as determined using a novel assay for detection of integrated HIV DNA. J Virol. 2001;75:11253–11260. doi: 10.1128/JVI.75.22.11253-11260.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Tanaka C, Wu Y, Chang MO, Inagaki Y, Saito Y, Naito T, Ogasawara H, Sekigawa I, Hayashida Y. Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes. 2006;32:105–113. doi: 10.1007/s11262-005-5851-2. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Yu JJ, O'Doherty U. Detecting HIV-1 integration by repetitive-sampling Alu-gag PCR. Methods. 2009;47:254–260. doi: 10.1016/j.ymeth.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U, Swiggard WJ, Jeyakumar D, McGain D, Malim MH. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J Virol. 2002;76:10942–10950. doi: 10.1128/JVI.76.21.10942-10950.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Emery S, Kelleher AD, Law M, Chen J, Hazuda DJ, Nguyen BY, Teppler H, Cooper DA. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. Aids. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- Summa V, Petrocchi A, Bonelli F, Crescenzi B, Donghi M, Ferrara M, Fiore F, Gardelli C, Gonzalez Paz O, Hazuda DJ. et al. Discovery of raltegravir, a potent, selective orally bioavailable HIV-integrase inhibitor for the treatment of HIV-AIDS infection. J Med Chem. 2008;51:5843–5855. doi: 10.1021/jm800245z. [DOI] [PubMed] [Google Scholar]
- Yu JJ, Wu TL, Liszewski MK, Dai J, Swiggard WJ, Baytop C, Frank I, Levine BL, Yang W, Theodosopoulos T, O'Doherty U. A more precise HIV integration assay designed to detect small differences finds lower levels of integrated DNA in HAART treated patients. Virology. 2008;379:78–86. doi: 10.1016/j.virol.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington RD, Geballe AP. Cofactor requirement for human immunodeficiency virus type 1 entry into a CD4-expressing human cell line. J Virol. 1993;67:5939–5947. doi: 10.1128/jvi.67.10.5939-5947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodicka MA, Goh WC, Wu LI, Rogel ME, Bartz SR, Schweickart VL, Raport CJ, Emerman M. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology. 1997;233:193–198. doi: 10.1006/viro.1997.8606. [DOI] [PubMed] [Google Scholar]
- O'Brien WA, Koyanagi Y, Namazie A, Zhao JQ, Diagne A, Idler K, Zack JA, Chen IS. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1) J Clin Invest. 1992;89:176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SL, Hansen MS, Bushman FD. A quantitative assay for HIV DNA integration in vivo. Nat Med. 2001;7:631–634. doi: 10.1038/87979. [DOI] [PubMed] [Google Scholar]