Abstract
CD200 is a cell-surface glycoprotein that functions through interaction with the CD200 receptor (CD200R) on myeloid lineage cells to regulate myeloid cell functions. Expression of CD200 has been implicated in multiple types of human cancer, however the impact of tumor expression of CD200 on tumor immunity remains poorly understood. To evaluate this issue, we generated CD200-positive mouse plasmacytoma J558 and mastocytoma P815 cells. We found that established CD200-positive tumors were often completely rejected by adoptively transferred CTL without tumor recurrence; in contrast, CD200-negative tumors were initially rejected by adoptively transferred CTL but the majority of tumors recurred. Tumor expression of CD200 significantly inhibited suppressive activity and IL-10 production by tumor-associated myeloid cells (TAMC), and as a result, more CTL accumulated in the tumor and exhibited a greater capacity to produce IFN-γ in CD200-positive tumors than in CD200-negative tumors. Neutralization of IL-10 significantly inhibited the suppressor activity of TAMC, and IL-10-deficiency allowed TAMC to kill cancer cells and their antigenic variants, which prevented tumor recurrence during CTL therapy. Thus, tumor expression of CD200 prevents tumor recurrence via inhibiting IL-10 production by TAMC.
Keywords: CD200, Tumor associated myeloid cells, Cytotoxic T Lymphocytes, Immune evasion
Introduction
CD200 (also known as OX-2) is a type 1 transmembrane glycoprotein expressed in diverse cell types and tissues [1]. Recent studies have revealed that CD200 is broadly expressed in a variety of cancer cells including those of B cell origin [2–4], human melanoma, ovarian cancer and renal cell carcinoma [5–6]. Mouse and human CD200 bind to their respective receptors (CD200R). The distribution of mouse and human CD200R is similar, with strong expression in macrophages, neutrophils and other leukocytes, including monocytes and mast cells [7]. Triggering CD200R suppresses myeloid cell activity in vitro and engagement of CD200R by CD200 inhibits activation of myeloid cells [8]. CD200 appears to limit autoimmune inflammation in animal models of multiple sclerosis and arthritis [9] and lung injury caused by viral infection [10], as CD200-deficient mice were found to have a significantly increased disease severity due to hyper activation of macrophages. These findings indicate that CD200-CD200R interactions are involved in limiting the cellular functions of myeloid lineages of cells.
The role of CD200-CD200R interaction in tumor immunity is poorly understood. An earlier study [11] has shown that CD200 expression on tumor cells inhibits activation of tumor-specific T cells. In a recent study [6], Petermann et al. reported that CD200 expressed on melanoma cells suppressed the ability of dendritic cells to activate allogenic T cells. Kretz-Rommel et al [12–13] have demonstrated that anti-CD200 antibody treatment can enhance tumor rejection by peripheral blood mononuclear cells in a hu-SCID adoptive transfer model. While the published works mainly studied the impact of the CD200-CD200R interaction in the induction phase of anti-tumor immune responses and the tumor rejection process, no studies have addressed the roles of tumor associated myeloid cells (TAMC), including myeloid-derived suppressor cells (MDSC) and tumor associated macrophages (TAM), the major lineages of cells expressing CD200R. Further, the significance of the CD200-CD200R interaction in tumor immunity has not been evaluated in definitive, syngenic T cell-tumor interaction models.
The tumor rejection antigen P1A was the first unmutated CTL antigen identified in the mouse. While it was initially identified in the mastocytoma P815 tumor cell line by Boon et al. [14], further studies have shown that the antigen is also expressed in several other lineages of tumor cells including plasmacytoma J558 [15]. The CTL recognized epitope of mouse P1A was identified as P1A 35–43 and restricted by H-2Ld [16]. Transgenic mice expressing a CTL TCR recognizing H-2Ld: P1A 35–43 (named P1CTL) have been produced [17]. The T cell activation and co-stimulation requirements have been well characterized [18–20]. By performing adoptive transfer therapy of mice with large established J558 tumors using P1CTL [21–22], we have revealed that, P1CTL are very efficient in reducing tumor burden. However, some cancer cells managed to evade P1CTL destruction in vivo via antigenic drift or antigen loss mutations [21–22]. In this study we have used the P1A/P1CTL model to evaluate the role of tumor expression of CD200 on tumor immunity. We found that tumor expression of CD200 altered tumor microenvironment via inhibition of suppressive activity and IL-10 production by TAMC, which resulted in the prevention of tumor recurrence, a phenomenon frequently observed during CTL adoptive transfer therapy of mice with established tumors.
Materials and Methods
Experimental animals
Transgenic mice expressing a TCR specific for the tumor rejection antigen H-2Ld:P1A35-43 complex (P1CTL) have been described [17]. P1CTL TCR transgenic mice were backcrossed with BALB/c mice for at least 12 generations before they were used for this study. BALB/c mice with a targeted mutation of the RAG-2 gene were purchased from Taconic Farms (Germantown, New York, USA). Through breeding P1CTL TCR transgenic mice with RAG-2−/−BALB/c mice we have generated RAG-2-deficient P1CTL TCR transgenic mice (RAG-2−/−P1CTL). BALB/c mice and IL-10-deficient BALB/c mice (IL-10−/−BALB/c) were originally purchased from The Jackson Laboratories. Through breeding IL-10−/−BALB/c mice with RAG-2−/−BALB/c mice we have generated RAG-2 and IL-10 double deficient mice (IL-10−/−RAG-2−/−). PCR was used for genotyping of IL10-deficiency and the primers used were: mIL10.G: 5’-ATA GAC TTG CTC TTG CAC TAC CAA AG-3’ (forward) and 5’-CTC ATG GCT TTC CCT AGG ACT CTC TA-3’ (reverse). All mice were maintained in OSU laboratory animal facilities which are fully accredited by Institutional Animal Care and Use Committee.
Generation of CD200-positive cancer cells and controls
The mouse plasmacytoma J558 (BALB/c, H-2Ld) and mastocytoma P815 (DBA/2, H-2d) cells have been previously described [18, 22]. We have cloned the full-length cDNA of mouse CD200 from a CD200-positive J558 variant cell line (with low expression of CD200) into PCDNA3 (Invitrogen) expression vector and used it to transfect CD200-negative J558 and P815 cells. The resulting G418-resistant J558 and P815 cells were selected for CD200 expression using flow cytometry. The empty PCDNA3 expression vector was also used to transfect J558 and P815 cells to generate J558-ctrl and P815-ctrl cells. All cell lines were cultured in RPMI 1640 medium containing 5% FCS, 100 µg/ml of penicillin and streptomycin.
Tumorigenesis and T cell adoptive transfer therapy of mice with established tumors
For tumor establishment in vivo, 5 × 106 of J558 cells or 1 × 106 of P815 cells or their variant cells were injected into each mouse subcutaneously. Tumor volumes were measured for length (a) and width (b) every three days and calculated as ab2/2 [23]. For CTL therapy of mice with established tumors, pools of spleen and lymph node cells from P1CTL-transgenic mice were incubated with a cocktail of mAbs (anti-CD4 mAb GK1.5, anti-FcR mAb 2.4G2 and anti-CD11c mAb N418). After removal of unbound mAbs, cells were incubated with anti-Ig coated magnetic beads (Dynal Biotech). The antibody-coated cells were removed by a magnet. The unbound cells consisted of more than 90% CD8+ T cells, with no detectable CD4+ T cells. The purified CD8+ T cells (5 × 106/mouse) were injected intravenously (i.v.) into mice bearing established tumors.
Antibodies and flow cytometry
For CD200 and CD200R staining, PE-labeled anti-CD200 (clone OX-90) and FITC-labeled anti-CD200R (OX-110) antibodies (Serotech) were used. FITC-, PE-, APC- or Percp- labeled antibodies to CD11b, CD11c, CD8α, Vα8.3, F4/80, Gr-1, Ly6G, Ly6C, IFN-γ and isotype control antibodies were purchased from BD Biosciences (San Diego, CA). For staining of cell surface markers, cells (cancer cells, splenocytes and single cell suspensions of tumors) were stained with various antibodies in staining buffer (PBS with 1% FCS) on ice for 30 min. After washing with staining buffer, cells were fixed in 1% Paraformaldehyde in PBS. For detection of intracellular cytokines, cells were stimulated in vitro with PMA (50 ng/ml) and ionomycin (50 ng/ml) for 5 h. GolgiStop (BD Pharmingen) were added (1/1500) during the last 2 h of incubation. The cells were first stained for the cell surface markers such as Vα8.3, followed by a standard intracellular cytokine staining procedure for IFN-γ. Cells were analyzed on a FACSCalibur flow cytometer. Data were analyzed using the flowjo software (Tree Star, Inc., OR).
Realtime RT-PCR
Quantitative real-time PCR was performed using an ABI 7900-HT sequence system (PE Applied Biosystems) with the QuantiTect SYBR Green PCR kit (Qiagen) in accordance with the manufacturer's instructions. PCR was done using previously determined conditions [21]. The following primers were used for amplifying specific genes: mArginase-1: 5’-ACAACCAGCTCTGGGAATCT-3’ (forward) and 5’-TGTACACGATGTCTTTGGCA-3’ (reverse). mCox2: 5’-ACCTGGTGAACTACGACTGCT-3’ (forward) and 5’-GACTGCTCATGAGTGGAGGA-3’ (reverse). mNOS2: 5’-ACCTTGTTCAGCTACGCCTT-3’ (forward) and 5’-CATTCCCAAATGTGCTTGTC-3’ (reverse). mVEGF: 5’-AGAGAGCAACATCACCATGC-3’ (forward) and 5’-GGTCTGCATTCACATCTGCT-3’ (reverse). mIL10: 5’-ACA GCC GGG AAG ACA ATA AC-3’(forward) and 5’-CAG CTG GTC CTT TGT TTG AA-3’ (reverse). mTNFα: 5’-ATG AGA AGT TCC CAA ATG GC-3’ (forward) and 5’-CTC CAC TTG GTG GTT TGC TA-3’ (reverse). mTGF-β1: 5’-ACAATTCCTGGCGTTACCTT-3’ (forward) and 5’-GAAAGCCCTGTATTCCGTCT-3’ (reverse). mIL-1β: 5’-CACTACAGGCTCCGAGATGA-3’ (forward) and 5’-TTTGTCGTTGCTTGGTTCTC-3’ (reverse). mIL-6: 5’-ACTTCACAAGTCGGAGGCTT-3’ (forward) and 5’-TCTGCAAGTGCATCATCGT-3’ (reverse). The HPRT gene was simultaneously amplified as endogenous control. The primers were 5'-AGCCTAAGATGAGCGCAAGT-3' (forward) and 5'-TTACTAGGCAGATGGCCACA-3' (reverse). Each sample was assayed in triplicate and the experiments were repeated twice. The relative amount of mRNA was calculated by plotting the Ct (cycle number) and the average relative expression for each group was determined using the comparative method (2−ΔΔCt).
Cytokine ELISA
ELISA kits for the detection of IL-10, TNF-α and IFN-γ were obtained from BD Biosciences. Standard procedures were followed to detect releases of cytokines in culture supernatants in a variety of settings.
TAMC suppression assay
Purification of TAMC and their subsets were performed by staining single cell suspensions of tumors and/or spleens using PE-anti–GR-1 (RB6-8C5; BD Biosciences) or PE-anti-CD11b (BD Biosciences), followed by magnetic antibody cell separation using anti-PE microbeads (Miltenyi Biotec). Typically the purity of the resulted CD11b+ or Gr-1+ cells was greater than 90%. To assess the suppressive activity of the purified TAMC, 1 × 106/ml of lymphocytes (spleen and lymph node cells) from RAG-2−/−P1CTL mice were cultured in the presence of P1A35-43 (0.1 µg/ml) with graded numbers of TAMC for 48–72 h. For detection of proliferation of P1CTL, 3H-Thymidine were added in the culture at the last 12 h and incorporation of 3H-Thymidine were quantitated using a scintillation counter.
Cytotoxicity assay
Splenocytes from P1CTL TCR transgenic mice were stimulated with P1A peptide (0.1µg/ml) for 5 days and used as effectors. 51Cr-labeled tumor cells were used as targets. The effector T cells and the targets were incubated together for 6 h, and the percentages of specific lysis were calculated based on the following formula: specific lysis % = 100 × (cpmsample−cpmmedium)/(cpmmax−cpmmedium).
Statistics
Student’s t test was used to compare tumor size and number differences between two groups. A chi square (χ2) statistic was used to determine differences for numbers of mice with tumor recurrence. For comparison of mice survival, Kaplan-Meier survival analysis and log-rank test were used (version 10.0, SPSS, Inc., Chicago, IL). A p value less than 0.05 was considered significant.
Results
Expression of CD200 on cancer cells prevents tumor evasion of CTL adoptive transfer therapy
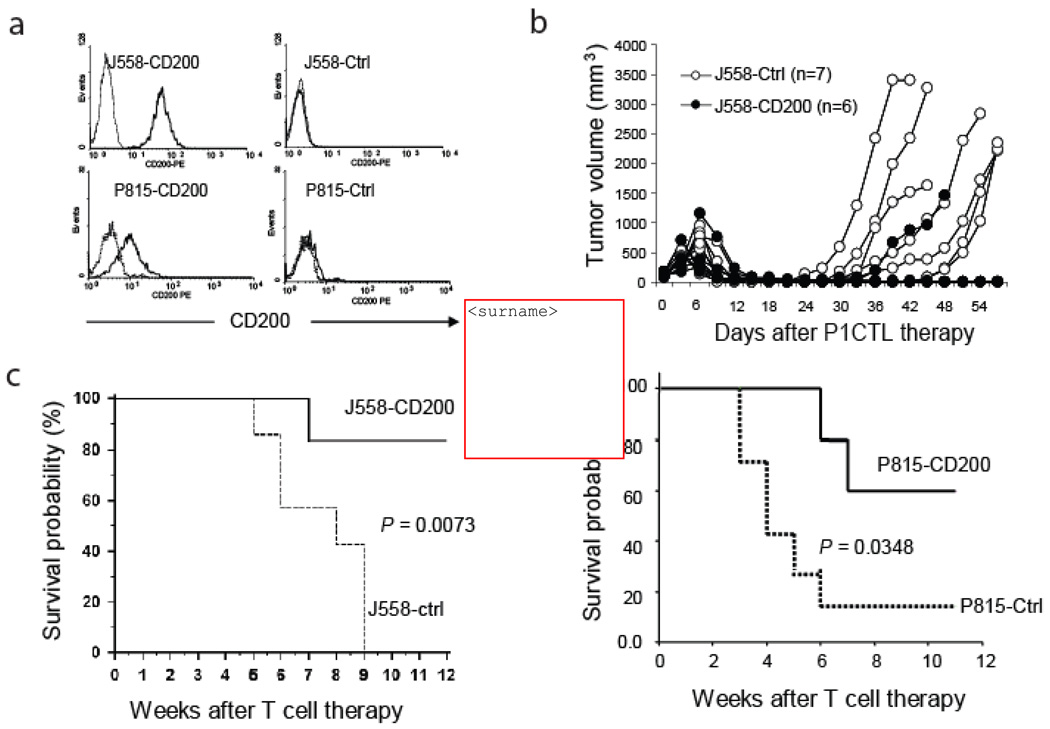
Recent studies have revealed that CD200 is broadly expressed in a variety of malignancies including malignant B cells [2–4] and a number of human solid cancers such as melanoma, ovarian cancer and renal cell carcinoma [5–6]. To test the role of tumor expression of CD200 on tumor immunity, we transfected CD200 negative plasmacytoma J558 cells and mastocytoma P815 cells with either a control expression vector or a vector containing CD200 cDNA and generated CD200-positive J558 and P815 cells (Figure 1a). We then injected the CD200-positive and negative J558 cells into RAG-2−/−BALB/c mice subcutaneously. When tumors were fully established (0.6–0.8 cm in diameter), we evaluated the tumor responses to CTL therapy. As shown in Figure 1b and c, upon CTL adoptive transfer therapy, 5 out 6 CD200-positive tumors were completely rejected without tumor recurrence; in contrast, 0 out of 7 CD200-negative tumors were completely rejected by P1CTL. We obtained similar results when we injected tumor cells into wild type BALB/c mice followed by CTL therapy (not shown). Thus, CD200 expression on J558 cells facilitates better rejection of large established tumors by P1CTL. We also tested if expression of CD200 on metastatic tumor P815 had the same effect. As shown in Figure 1d, P1CTL protection for mice bearing CD200-positive P815 tumors was more pronounced than mice bearing CD200-negative tumors.
Figure 1.
Expression of CD200 on tumor cells prevents tumor recurrence after P1CTL therapy. a. Full-length CD200 cDNA was cloned from a variant of J558 cells and an expression vector was generated to transfect CD200-negative J558 cells and mastocytoma P815 cells. We also generated cells transfected with the empty expression vector. The solid lines represent CD200 staining while the dotted lines represent staining with an isotype control antibody. b. Tumor growth following P1CTL therapy. Tumor cells were injected into RAG-2−/−BALB/c mice. When tumors were established, mice were treated with purified P1CTL. Numbers of mice with recurrent tumors were significantly different between the two groups (P<0.05, X2 test). Data shown are representative of three independent experiments. c. Tumor free survival of J558 tumor-bearing mice that received P1CTL therapy. Data from the same group of mice shown in b is presented. d. Tumor cells were injected into RAG-2−/−BALB/c mice at a dose of 1 × 106/mouse s.c., 7 days after tumor cell injection, mice were treated with purified P1CTL (5 × 106/mouse). Data are representative of two independent experiments.
Tumor expression of CD200 enhances CTL accumulation and effector function in the tumor
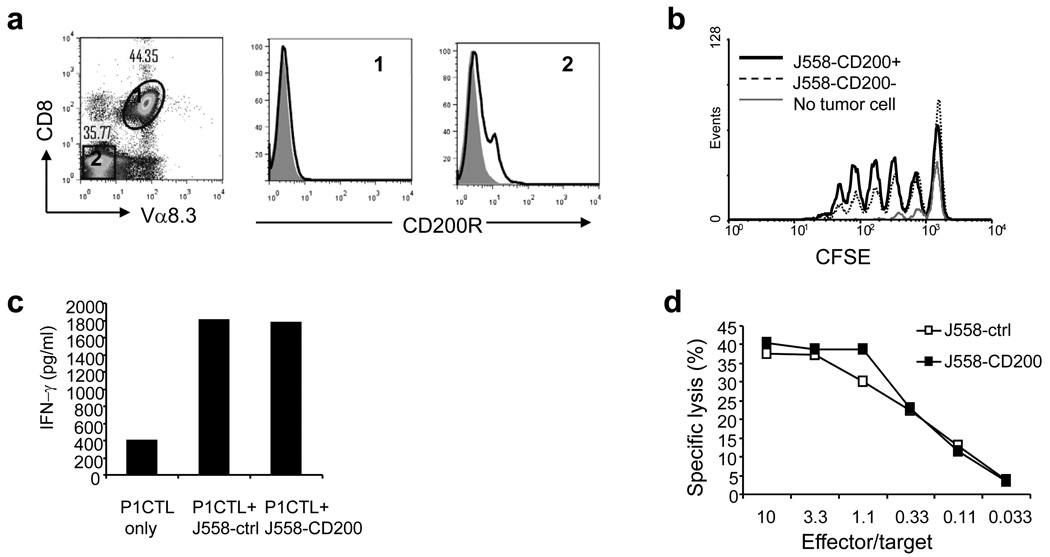
To understand the mechanisms of enhanced rejection of CD200-positive tumors by adoptively transferred CTL, we injected purified CD8+ T cells from P1CTL transgenic mice into tumor-bearing RAG-2−/−BALB/c mice. At different time points after T cell injection, we quantitated tumor antigen specific T cells in spleens and tumors. As demonstrated in Figure 2a, T cells were undetectable in tumors at 48 h after injection, while they started to accumulate in both CD200-positive and CD200-negative tumors by 72 hours. However, we observed that T cells infiltrated into CD200-positive tumors more efficiently than they did in CD200-negative tumors. By day 3 and 5, significantly higher numbers of CTL were detected in CD200-positive tumors (Figure 2b). By day 5, as high as 70–80% of P1CTL in CD200-positive tumors were capable of producing IFN-γ (Figure 2c and 2d), which was much higher than that of CD200-negative tumors (20–50%, Figure 2c and 2d). Thus, tumor expression of CD200 resulted in enhanced CTL accumulation and differentiation in the tumor.
Figure 2.
Tumor infiltrating CTL preferentially accumulate in CD200-positive tumors and are capable of producing more IFN-γ. Purified P1CTL were injected into RAG-2−/−BALB/c mice with established J558-CD200 or J558-Ctrl tumors. At various times after P1CTL injection, single cell suspensions were prepared from tumors and were stained for Vα8.3, CD8 and/or IFN-γ. For IFN-γ staining, cells were stimulated with 50 ng/ml of PMA/Ionomycin for 5 h followed by a standard intracellular staining procedure. a. Flow cytometry analysis of frequencies of tumor antigen-specific T cells in CD200-positive and CD200-negative tumors. b. Numbers of tumor-infiltrating P1CTL at different times after injection. Data show mean + SD (n= 3–7 mice). c. Flow cytometry analysis of IFN-γ production by tumor infiltrating P1CTL. d. Quantitation of tumor-infiltrating P1CTL that were IFN-γ positive. Data show mean + SD (n=3–7 mice). Data shown in panels c and d were at day 5 after P1CTL transfer. Paired student’s t test was used for the statistical analysis.
Tumor expression of CD200 does not directly affect CTL effector functions
Since CD200 functions through interaction with CD200R, we asked if CD200 expressed on tumor cells was directly interacting with CD200R on tumor infiltrating CTL and was affecting their functions. We first stained in vivo activated CTL (CTL from tumor-bearing mice) for the expression of CD200R. As shown in Figure 3a, activated P1CTL were completely devoid of CD200R expression. In contrast, consistent with previous reports that CD200R expression is expressed predominantly on cells of the myeloid lineage [7, 24], we found that CD200R was expressed on non-T population of cells in spleen (Figure 3a). Lack of CD200R expression on T cells suggests that a direct interaction between tumor expressed CD200 and T cells is unlikely. Indeed, we found that CD200-positive and CD200-negative J558 cells had similar capacity to stimulate proliferation of P1CTL in vivo (Figure 3b). Activated P1CTL produced the same amount of IFN-γ when co-cultured with CD200-positive or CD200-negative J558 cells in vitro (Figure 3c). Moreover, activated CTL can equally kill CD200-positive and negative target cells in vitro (Figure 3d). Thus, CD200-CD200R interaction does not seem to directly affect CTL-cancer cell interaction.
Figure 3.
Tumor expression of CD200 does not inhibit effector functions of CTL. a. Splenocytes from P1CTL-treated tumor-bearing mice were stained for CD8, Vα8.3 and CD200R. CD8+Vα8.3+ and CD8−Vα8.3− cells were analyzed for CD200R expression. Shaded histograms represent isotype controls, solid lines represent CD200R staining. b. In vivo proliferation of P1CTL. 20 × 106 irradiated (1000 rad) tumors cells were injected into RAG-2−/−BALB/c mice intravenously (i.v.), 24 h later, mice received 5 × 106 of CFSE-labeled P1CTL. 72 h after P1CTL injection, mice were sacrificed and splenocytes were stained for CD8 and Vα8.3. Data show CFSE dilution of CD8+Vα8.3+ cells and are representative of three independent experiments. c. IFN-γ production by activated P1CTL. Splenocytes from RAG-2−/−P1CTL mice were activated in vitro with P1A 35–43, 72 h later, activated P1CTL were co-cultured with J558-CD200 or J558-Ctrl cells. 72 h after co-culture, IFN-γ release in the culture supernatants were measured by ELISA. Data are representative of two independent experiments. d. Effect of cancer cell CD200 expression on CTL-mediated destruction of target cells. A standard 51Cr-release assay, using activated P1CTL as effectors, 51Cr-labeled J558-Ctrl and J558-CD200 cells as targets, was performed. Data are representative of three independent experiments.
Myeloid cells from CD200-positive tumors are less suppressive for tumor antigen specific T cells
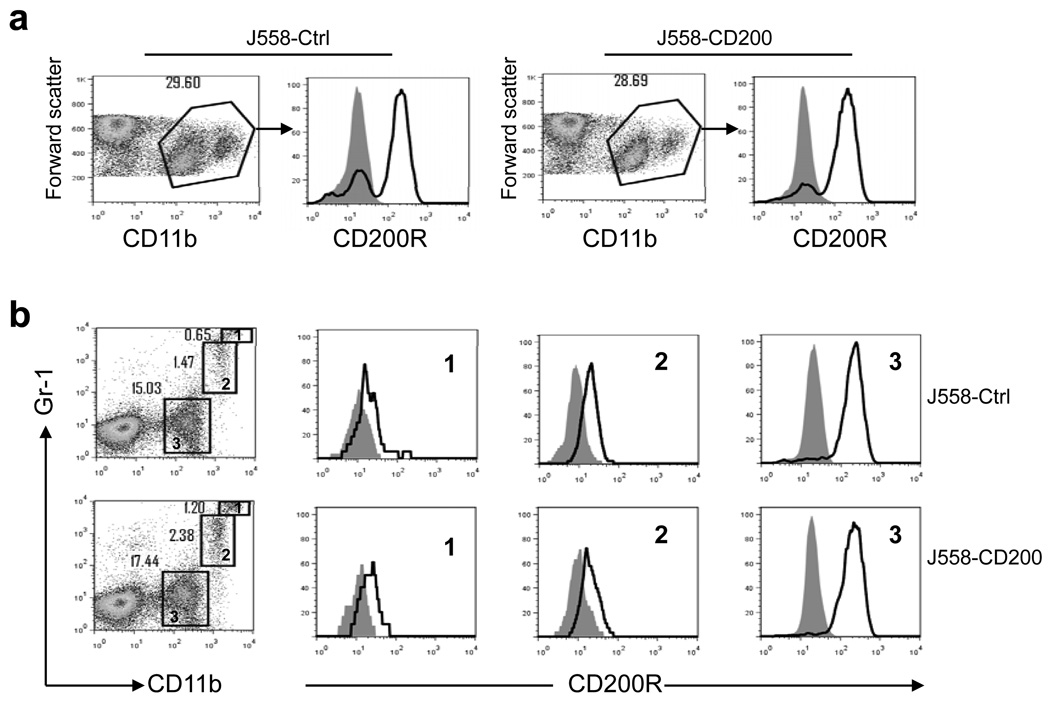
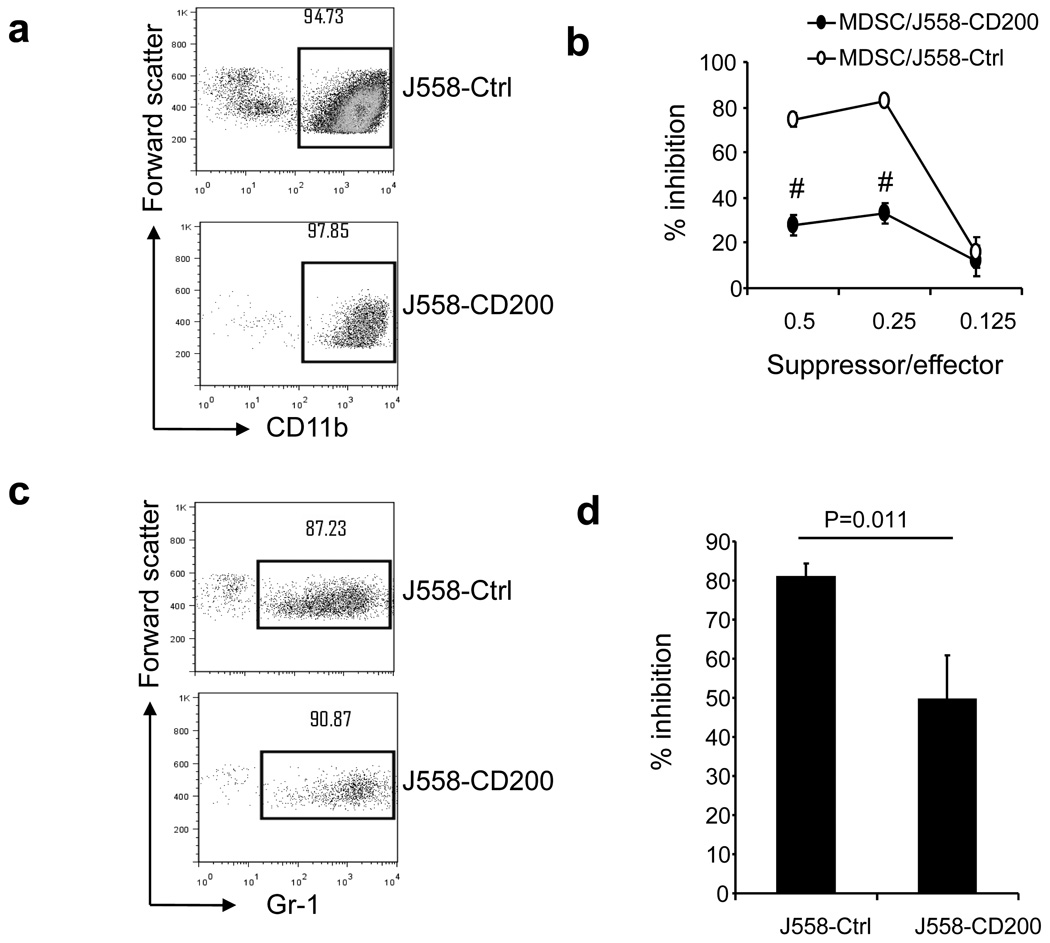
Consistent with previous reports that CD200R expression is restricted predominantly to cells of the myeloid lineage [7, 24], we found that CD200R was abundantly expressed on CD11b+ tumor associated myeloid cells (TAMC). As shown in Figure 4a, CD11b+ cells from both CD200-positive and CD200-negative tumors expressed high levels of CD200R. Further analysis of myeloid cells from CD200-positive and CD200 negative tumors revealed that among CD11b+ cells there exists three populations of cells: CD11b+Gr-1hi cells, CD11b+Gr-1lo cells and CD11b+Gr-1− cells (Figure 4b). We found that CD11b+Gr-1+ cells (MDSC), expressed medium to low levels of CD200R. The highest expression of CD200R was observed in CD11b+Gr-1− cells (Figure 4b). No significant differences were observed regarding CD200R expression in subpopulations of CD11b+ cells between CD200-positive and CD200-negative tumors. To understand the impact of tumor expression of CD200 on the function of tumor associated myeloid cells, we purified CD11b-positive cells from both CD200-positive and CD200-negative tumors using anti-CD11b or anti-Gr-1 MACS beads. As demonstrated in Figure 5a and Figure 5c, CD11b-positive and Gr-1 positive cells were successfully isolated from tumors, with purities of about 90%. As demonstrated in Figure 5b, CD11b-positive cells from CD200-negative J558 tumors profoundly suppressed proliferation of P1CTL, while CD11b+ cells from CD200-positive J558 tumors were less suppressive. MDSC (Gr-1+) from CD200-positive tumors were also less suppressive compared with that of CD200-negative tumors. Thus, tumor expression of CD200 inhibited the suppressive activity of tumor associated myeloid cells (TAM and MDSC).
Figure 4.
CD200R expression on tumor-associated myeloid cells (TAMC). J558-Ctrl or J558-CD200 cells were injected into RAG-2−/−BALB/c mice subcutaneously. When tumors were fully established, single cell suspensions were prepared from tumors and were stained for various cell surface markers followed by flow cytometry analysis. a. CD200R expression on CD11b+ myeloid cells. b. CD200R expression on three subsets of TAMC, i.e. CD11b+Gr-1hi, CD11b+Gr-1lo and CD11b+Gr-1− cells. Shaded histograms represent isotype controls. Data are representative of at least 10 independent experiments.
Figure 5.
TAMC suppressive activity. Single-cell suspensions were prepared from tumors and were stained for CD11b or Gr-1 markers followed by anti-FITC or anti-PE MACS beads. The labeled cells went through MACS columns and CD11b+ or Gr-1+ cells (about 90% purity) were prepared. a. Flow cytometry analysis of CD11b+ cells after purification with MACS beads. b. Suppressive activity of CD11b+ cells. Spleen and lymph node cells from RAG-2−/−P1CTL mice were co-cultured with CD11b+ cells from J558-CD200 or J558-Ctrl tumors at different ratios in the presence of P1A35-43 in U-bottomed 96-well plates. 3H-thymidine incorporation was performed 48 h later. % inhibition of P1CTL proliferation relative to controls was calculated. c. Flow cytometry analysis of Gr-1+ cells after purification with MACS beads. d. Suppressive activity of Gr-1+ cells. Spleen and lymph node cells from RAG-2−/−P1CTL mice were co-cultured with Gr-1+ cells from J558-CD200 or J558-Ctrl tumors in the presence of P1A35-43. Data are representative of five independent experiments.
Expression of CD200 on cancer cells inhibits IL-10 production by tumor associated myeloid cells
High expression of CD200R on tumor associated myeloid cells suggests a direct interaction between CD200-positive cancer cells and myeloid cells. Murine TAM can produce an array of soluble factors and enzymes such as VEGF, TNF-α, IL-1β, IL-6, TGF-β, IL-10, inducible nitric-oxide synthases 2 (NOS2), arginase-1 (ARG-1) and cyclooxygenase-2 (COX-2) which have been implicated in their suppressive functions [25–28]. To understand the role of CD200-CD200R interaction between cancer cells and tumor associated myeloid cells, we injected J558-CD200 and J558-Ctrl cells into RAG-2−/−BALB/c mice. When tumors were fully established, we harvested tumors for the analysis of the above-mentioned TAM-associated factors. As shown in Figure 6a, real time PCR analysis of total tumor RNA revealed dramatically decreased (about 10-fold) expression of IL-10 gene in CD200-positive tumors compared with CD200-negative tumors. We observed increased (about three-fold) expression of TNF-α gene in CD200-positive tumors compared with CD200-negative tumors. Expression of other factors such as VEGF, IL-1β, IL-6, TGF-β, NOS2, ARG-1 and COX-2 did not differ between the two groups of tumors (not shown). Since the production of IL-10 by J558 cells was undetectable by ELISA (not shown), we concluded tumor stromal cells were the major source of IL-10 in established J558 tumors. We purified CD11b+ cells from CD200-positive or CD200-negative tumors, they were then stimulated with PMA and Ionomycin. We found that the culture supernatants of CD11b+ cells from CD200-negative tumors contained more IL-10 but less TNF-α than the supernatants of CD11b+ cells from CD200-positive tumors (Figure 6b).
Figure 6.
Role of IL-10 produced by TAMC. a. Analysis of TAMC-associated factors by real-time PCR. RNA samples were prepared from established J558-CD200 and J558-ctrl tumors (the hosts were RAG-2−/−BALB/c mice). Expression of genes associated with TAMC (IL-10 and TNF-α) was quantitated. b. CD11b+ cells from J558-CD200 or J558-Ctrl tumors were stimulated with100 ng/ml of PMA and ionomycin for 24 h, IL-10 and TNF-α releases in the culture supernatants were measured by ELISA. c. CD11b+ cells in spleens and tumors from mice bearing J558-Ctrl tumors were co-cultured with either J558-Ctrl or J558-CD200 cells at a 1:1 ratio for 24 h in the presence of 100 ng/ml of PMA and ionomycin. IL-10 concentrations in the culture supernatants were then detected by ELISA. Data are normalized to 1 × 106/ml of TAMC, show mean + SD (n=3) and are representative of three independent experiments. Student’s t test was used for the statistical analysis.
To test if expression of CD200 on cancer cells inhibits IL-10 production by TAMC in vitro, we purified CD11b+ cells from mice with J558-ctrl tumors, and then co-cultured CD200-positive or CD200-negative J558 cells with TAMC in the presence of PMA and Ionomycin. As shown in Figure 6c, TAMC co-cultured with CD200-positive J558 cells had dramatically reduced IL10 production. Since IL-10 production by J558-CD200 and J558-ctrl cells stimulated with PMA and Ionomycin was undetectable by ELISA (not shown), we conclude that tumor expression of CD200 inhibits IL10 production by TAMC.
IL10-deficient TAMCs are less efficient suppressors of T cells and have the ability to kill antigenic cancer cells and their variants
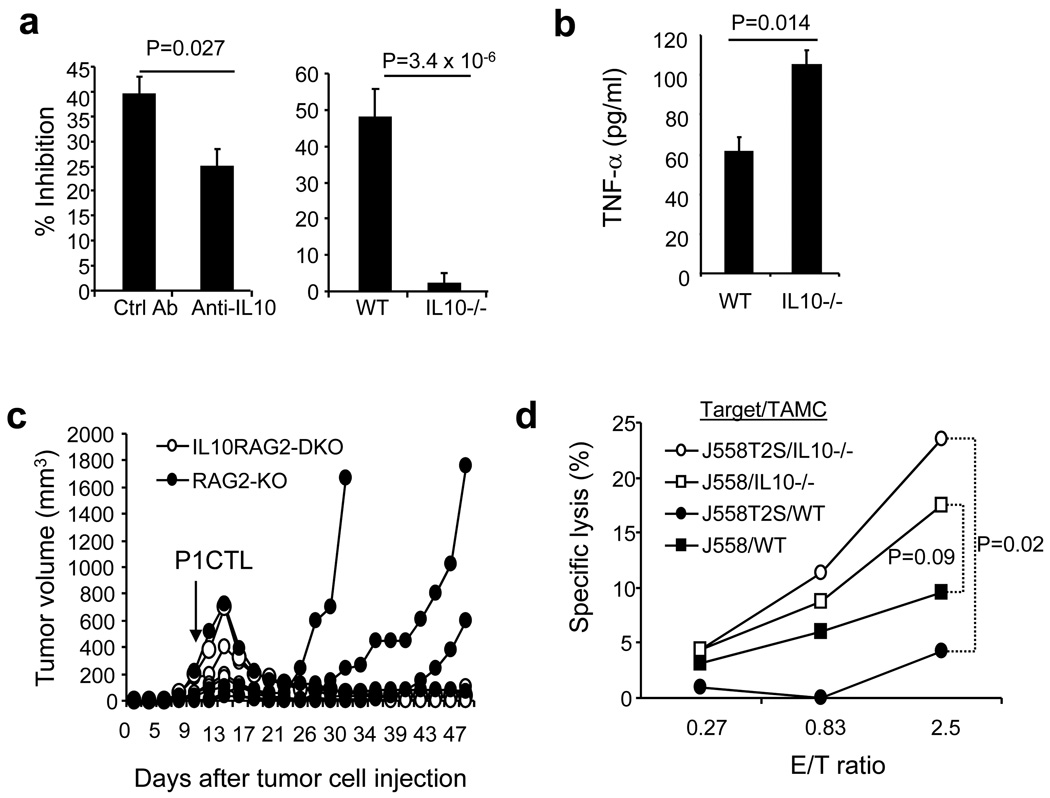
To determine if reduced IL-10 production by tumor associated myeloid cells from CD200-positive tumors was responsible for the reduced suppressive activity, we tested if IL-10 blockade or IL-10 gene knockout could affect suppressive activity of TAMC. As shown in Figure 7a (left panel), we found that antibody blocking of IL-10 significantly inhibited the suppressive activity of myeloid cells from J558 tumors, and IL-10-deficient myeloid cells lacked suppressive activity (Figure 7a, right panel).
Figure 7.
Role of TAMC-derived IL-10 in suppression of CTL activity. Spleen and lymph node cells from RAG2−/−P1CTL mice were co-cultured with CD11b+ cells from J558-Ctrl tumors in the presence of P1A35-43. 5 µg/ml of anti-IL-10 antibody or an isotype matched control antibody were added in the culture (left panel). CD11b+ cells from BALB/c or IL-10−/−BALB/c mice with J558-Ctrl tumors were compared for their suppressive activity using thymidine incorporation assay (right panel). Suppressor: effector =1:1. b. 0.3 × 106/ml CD11b+ cells from WT or IL-10−/− mice with J558-Ctrl tumors were stimulated with 100 ng/ml of PMA and 100 ng/ml of ionomycin for 24 h. TNF-α production in the culture supernatants were detected by ELISA. c. Effect of P1CTL treatment of mice with established J558-Ctrl tumors. 5 × 106 of CD8+ T cells from P1CTL transgenic mice were injected into each mouse with established J558-ctrl tumors. Numbers of mice with recurrent tumors were significantly different between the two groups (P<0.05, X2 test). d. CD11b+ cells from J558-Ctrl tumors grown in wild type and IL-10−/− mice were co-cultured with 51Cr-labeled J558 cells and J558T2S cells (with a P6R mutation in P1A epitope) for 12 h. 51Cr-releases into culture supernatants were measured in a scintillation counter. Specific lysis was calculated based on the following formula: specific lysis % = 100 × (cpmcoculture−cpmtarget minimal)/(cpmtarget max−cpmtarget minimal). Data are representative of 3–5 independent (a, b) and two (c, d). Paired student’s t test was used for statistical analysis.
To test if IL10-deficiency in TAMC affects CTL mediated tumor rejection in vivo, we tested whether P1CTL adoptive transfer therapy could eliminate established tumors in IL-10−/−RAG-2−/− mice. Our previous studies [21–22] have revealed that P1CTL therapy failed to control J558 tumors in IL-10+/+RAG-2−/− mice because tumor antigen mutation caused immune evasion. 5 × 106 of J558 cells were injected into each IL-10−/−RAG-2−/− and IL-10+/+RAG-2−/− mouse subcutaneously. When tumors were fully established, 5 × 106 purified P1CTL were injected into each mouse intravenously. As demonstrated in Figure 7c, P1CTL therapy prevented tumor recurrence in IL-10−/−RAG-2−/− mice (6/6) but rarely in IL-10+/+RAG2−/− mice (1/4).
Complete rejection of large established tumors requires destruction of both antigenic cancer cells and their variants. We therefore tested if IL-10-deficient TAMC could destroy J558T2S cells, whose tumor antigen P1A has a T>C mutation at N389 relative to P1A mRNA. This mutation resulted in a single amino acid replacement in P1CTL recognized epitope (P6W>R), leading to the loss of recognition by P1CTL [21–22]. As demonstrated in Figure 7d, while TAMC from tumors grown in wild type mice had limited tumor-killing activity, IL10-deficient TAMC killed both J558 and J558T2S cells efficiently.
Discussion
It is well-established that CD200, through interaction with CD200R1, regulates the functions of myeloid lineage of cells and other CD200R1 positive cells [29–30]. This interaction has been shown to limit inflammation through inhibition of tissue macrophage activation [9–10, 31]. Since CD200 is over-expressed in a number of human cancers, we evaluated the impact of tumor expression of CD200 on tumor rejection by adoptively transferred CTL. We found that expression of CD200 on cancer cells inhibited both suppressive activity and IL-10 production by tumor associated myeloid cells, which resulted in altered tumor microenvironments in CD200-positive tumors, permitting for better infiltration and better differentiation of adoptively transferred CTL. In addition, we showed that IL-10 deficiency allowed TAMC to kill cancer cells and their antigenic variants, which prevented tumor recurrence during CTL therapy.
In this study, we found that tumor associated myeloid cells from a solid tumor were composed of three populations of cells: CD11b+Gr-1hi, CD11b+Gr-1lo and CD11b+Gr-1− cells. The first two populations of cells should belong to the MDSC group [32–33], which represented about 1–5% of total tumor cells, while the latter population of cells, i.e. TAM, were about 15% of total tumor cells (Figure 4). Interestingly, we found that these three populations of cells expressed different levels of CD200R1, which was inversely correlated with Gr-1 expression (Figure 4). It is likely that CD200R1 expression reflects more matured stages of myeloid cell differentiation in an established tumor. Previous studies have demonstrated that both TAM and MDSC produce high amounts of IL-10 [34–35]. They are known to suppress CTL effector functions while promoting tumor invasion, growth and angiogenesis [25–27]. In addition, MDSC have been shown to convert M1 macrophages (TNF-α producing) into M2 macrophages (IL-10 producing) through the production of IL-10 [36]. We found here that in CD200-positive tumors, TAMC were converted to a M1 phenotype with lower production of IL-10 and higher production of TNF-α. Since IL-10-deficient TAMC also had increased production of TNF-α (Figure 7b), we consider that the increased TNF-α production by TAMC from CD200-positive tumors is a result of inhibition of IL-10 production.
Our previous studies [18, 21–22] have revealed that P1CTL therapy rarely leads to complete control of unmodified J558 and P815 tumors. Tumors almost always grew back after initial tumor rejection due to antigenic mutations. One notable observation in this study is that tumor expression of CD200 leads to near complete rejection of large established tumors by P1CTL without tumor recurrence (Figure 1). Complete rejection of large established tumors by CTL requires elimination of both antigen-positive cancer cells and their antigenic variants [37–38]. How did monoclonal P1CTL therapy eliminate both antigen positive cells and antigenic variants in mice with CD200-positive tumors? We propose the following two mechanisms. First, reduced IL-10 production and suppressor activity of TAMC enhances CTL effector functions. As previous studies [37, 39–40] have revealed, CTL can kill both antigenic tumor cells and tumor stromal cells that cross-present tumor antigen. This mechanism will result in the tumor bed destruction, leading to antigenic variant cancer cells failure to survive and grow out. Enhanced IFN-γ production by CTL can also inhibit angiogenesis in the tumor bed and results in tumor rejection [41–42]. Second, tumor expression of CD200 seems to interact with CD200R1 to switch M2 (IL-10 producing) TAMC into M1 TAMC (low or no IL-10 production). A recent study [43] has clearly shown that “re-educated” TAMC (devoid of IL-10 production) can efficiently kill cancer cells rather than promote tumor progression. Indeed, we have shown that IL-10-deficient TAMC but not wild type TAMC can efficiently kill both antigenic cancer cells and their variants (Figure 7d) and in IL-10−/−RAG-2−/− but not IL10+/+RAG2−/− mice, P1CTL completely eliminate established tumors without tumor recurrence (Figure 7c). CD200-educated TAMC may potentially kill cancer cells and their antigenic variants through the production of TNF-α, as TNFR signaling has been shown to be required for the complete eradication of tumors [40, 44].
The role of CD200-CD200R interaction in tumor immunity has been evaluated by a few other groups. An earlier study [11] has revealed that CD200 on tumor cells inhibits induction of tumor specific T cells. In a recent study [6], Petermann et al. reported that CD200 expressed on melanoma cells suppressed the function of dendritic cells to activate T cells. Kretz-Rommel et al [12–13] have demonstrated that anti-CD200 antibody treatment can enhance tumor rejection by T cells and macrophages in a hu-SCID adoptive transfer model. The discrepancy of the published results and ours can be clearly explained through the models and phases of anti-tumor immune responses evaluated. The published works mainly studied the impact of CD200-CD200R interaction in the induction phase of anti-tumor immune responses and the tumor rejection process. No study has evaluated its role in tumor recurrence. Our results do not rule out the possible inhibitory role of CD200 on the priming of CTL. In our study relatively high numbers of T cells were injected into tumor-bearing mice, and the recipient mice were devoid of CD4+ T cells, which may potentially bypass some priming needs. However, our data (Figure 3) and data from others [7] suggest that activated CTL do not express CD200R1. We have recently evaluated T cells with various specificities and found that activated T cells (both CD4 and CD8) do not normally express CD200R1. Thus, as we demonstrated in this study, it is unlikely that CTL effector functions are inhibited (Figure 3).
Taken together, our study has revealed a novel function for tumor expression of CD200, i.e. alteration of tumor microenvironment via inhibition of M2 myeloid cell production of IL10 in an established tumor. Inhibition of IL-10 production by tumor associated myeloid cells not only makes tumors more permeable for activated CTL, but also allows the converted myeloid cells to kill cancer cells and their antigenic variants, and prevents tumor recurrence during CTL therapy. Given that many different lineages of human cancer express CD200, our data have important implications for immunotherapy of human cancer.
Acknowledgment
This study was supported by grants from the National Cancer Institute (R01CA138427) and American Cancer Society (RSG-09-188-01-LIB) to XFB.
Footnotes
Conflict of interest
The authors declare no financial or commercial conflict of interest.
References
- 1.Preston S, Wright GJ, Starr K, Barclay AN, Brown MH. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol. 1997;27:1911–1918. doi: 10.1002/eji.1830270814. [DOI] [PubMed] [Google Scholar]
- 2.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci U S A. 2006;103:1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De Vos J, Rossi JF, Goldschmidt H, Klein B. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–4197. doi: 10.1182/blood-2006-06-029355. [DOI] [PubMed] [Google Scholar]
- 4.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–568. doi: 10.1038/sj.leu.2404559. [DOI] [PubMed] [Google Scholar]
- 5.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2007 doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117:3922–3929. doi: 10.1172/JCI32163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171:3034–3046. doi: 10.4049/jimmunol.171.6.3034. [DOI] [PubMed] [Google Scholar]
- 8.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 9.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 10.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 11.Gorczynski RM, Chen Z, Hu J, Kai Y, Lei J. Evidence of a role for CD200 in regulation of immune rejection of leukaemic tumour cells in C57BL/6 mice. Clin Exp Immunol. 2001;126:220–229. doi: 10.1046/j.1365-2249.2001.01689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D, Frederickson S, Maruyama T, Wild MA, Nolan MJ, Wu D, Springhorn J, Bowdish KS. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol. 2007;178:5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 13.Kretz-Rommel A, Qin F, Dakappagari N, Cofiell R, Faas SJ, Bowdish KS. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol. 2008;180:699–705. doi: 10.4049/jimmunol.180.2.699. [DOI] [PubMed] [Google Scholar]
- 14.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. The gene coding for a major tumor rejection antigen of tumor P815 is identical to the normal gene of syngeneic DBA/2 mice. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramarathinam L, Sarma S, Maric M, Zhao M, Yang G, Chen L, Liu Y. Multiple lineages of tumors express a common tumor antigen, P1A, but they are not cross-protected. J Immunol. 1995;155:5323–5329. [PubMed] [Google Scholar]
- 16.Lethe B, van den Eynde B, van Pel A, Corradin G, Boon T. Mouse tumor rejection antigens P815A and P815B: two epitopes carried by a single peptide. Eur J Immunol. 1992;22:2283–2288. doi: 10.1002/eji.1830220916. [DOI] [PubMed] [Google Scholar]
- 17.Sarma S, Guo Y, Guilloux Y, Lee C, Bai XF, Liu Y. Cytotoxic T lymphocytes to an unmutated tumor rejection antigen P1A: normal development but restrained effector function in vivo. J Exp Med. 1999;189:811–820. doi: 10.1084/jem.189.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai XF, Bender J, Liu J, Zhang H, Wang Y, Li O, Du P, Zheng P, Liu Y. Local costimulation reinvigorates tumor-specific cytolytic T lymphocytes for experimental therapy in mice with large tumor burdens. J Immunol. 2001;167:3936–3943. doi: 10.4049/jimmunol.167.7.3936. [DOI] [PubMed] [Google Scholar]
- 19.Bai XF, Gao JX, Liu J, Wen J, Zheng P, Liu Y. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 2001;61:6860–6867. [PubMed] [Google Scholar]
- 20.Bai XF, Liu J, May KF, Jr, Guo Y, Zheng P, Liu Y. B7-CTLA4 interaction promotes cognate destruction of tumor cells by cytotoxic T lymphocytes in vivo. Blood. 2002;99:2880–2889. doi: 10.1182/blood.v99.8.2880. [DOI] [PubMed] [Google Scholar]
- 21.Bai XF, Liu J, Li O, Zheng P, Liu Y. Antigenic drift as a mechanism for tumor evasion of destruction by cytolytic T lymphocytes. J Clin Invest. 2003;111:1487–1496. doi: 10.1172/JCI17656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai XF, Liu JQ, Joshi PS, Wang L, Yin L, Labanowska J, Heerema N, Zheng P, Liu Y. Different Lineages of P1A-Expressing Cancer Cells Use Divergent Modes of Immune Evasion for T-Cell Adoptive Therapy. Cancer Res. 2006;66:8241–8249. doi: 10.1158/0008-5472.CAN-06-0279. [DOI] [PubMed] [Google Scholar]
- 23.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24:148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 24.Minas K, Liversidge J. Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal? Crit Rev Immunol. 2006;26:213–230. doi: 10.1615/critrevimmunol.v26.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 26.Nakao S, Kuwano T, Tsutsumi-Miyahara C, Ueda S, Kimura YN, Hamano S, Sonoda KH, Saijo Y, Nukiwa T, Strieter RM, Ishibashi T, Kuwano M, Ono M. Infiltration of COX-2-expressing macrophages is a prerequisite for IL-1 beta-induced neovascularization and tumor growth. J Clin Invest. 2005;115:2979–2991. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 28.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 29.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol. 2002;23:285–290. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- 31.Copland DA, Calder CJ, Raveney BJ, Nicholson LB, Phillips J, Cherwinski H, Jenmalm M, Sedgwick JD, Dick AD. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am J Pathol. 2007;171:580–588. doi: 10.2353/ajpath.2007.070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greifenberg V, Ribechini E, Rossner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 35.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 37.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10:294–298. doi: 10.1038/nm999. [DOI] [PubMed] [Google Scholar]
- 38.Spiotto MT, Schreiber H. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 2005;5:8. [PubMed] [Google Scholar]
- 39.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, Kranz DM, Schreiber H. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang B, Karrison T, Rowley DA, Schreiber H. IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J Clin Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qin Z, Schwartzkopff J, Pradera F, Kammertoens T, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 42.Lu Y, Yang W, Qin C, Zhang L, Deng J, Liu S, Qin Z. Responsiveness of Stromal Fibroblasts to Interferon-{gamma} Blocks Tumor Growth Via Angiostasis. J Immunol. 2009 doi: 10.4049/jimmunol.0901073. [DOI] [PubMed] [Google Scholar]
- 43.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. "Re-educating" tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Forster I, Huss R, Weber WA, Kneilling M, Rocken M. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–518. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]