Abstract
Graft-vs-host disease (GVHD) is a negative effect of stem cell transplant, but the graft-vs-leukemia (GVL) effect has been shown to positively affect outcomes. GVHD and GVL have similar but not identical alloreactivity, and GVL may be affected by the source of grafts, the preparative conditioning regimen, and donor genetic elements. Human leukocyte antigen (HLA) mismatch does not appear to augment the GVL effect, and increased GVHD does not necessarily lead to decreased relapse.
Keywords: graft-vs-leukemia effect, GVL, graft-vs-host disease, GVHD, acute leukemia, human leukocyte antigen, HLA, mismatch, unrelated donor, cord blood, conditioning regimen
INTRODUCTION
The effectiveness of allogeneic stem cell transplantation for leukemia results from more than the high-dose therapy that precedes transplant. While graft-vs-host disease (GVHD) is a negative consequence of transplant, the graft-vs-leukemia (GVL) effect significantly benefits outcome for transplant. However, GVL might just be a manifestation of GVHD.
Graft-vs-host reactivity involves a cytolytic attack on target tissues, which could also result in immunodeficiency or GVL, depending on the efficacy of the attack in various tissues. In general GVHD reactions, CD8+ T cells lyse human leukocyte antigen (HLA) class I-expressing targets, usually in tissues of the gut, skin, and liver. In immunodeficiency, the CD8+ cells attack HLA class I-expressing thymic tissue. Also in GVHD, natural killer (NK) cells lyse targets lacking class I inhibitory killer-immunoglobulin receptor-ligand (KIR-L). Other mediators of GVHD include B cells, cytokines, and antibody-dependent cell-mediated cytotoxicity (ADCC), among others. For the GVL effect, CD8+ T cells seek HLA class I-expressing targets, such as myeloid or lymphoid tissue. NK cells, antibodies, and cytokines attack targets expressing minor histocompatibility, microbial pathogens, or tumor-associated antigens.
HLA mismatch and minor histocompatibility antigen mismatch, which is assumed to be present in fully matched unrelated and fully matched related donors, augments donor/recipient alloreactivity. This leads to more GVHD and more immunodeficiency, but it is unclear whether it also augments the GVL effect. It is unclear whether a mismatched or unrelated donor transplant will yield augmented GVL and less relapse. To address this question in a clinical way, two large studies were conducted with data from the Center for International Blood and Marrow Transplant Research (CIBMTR) and donor/recipient specimens from the National Marrow Donor Program repository.
UNRELATED VS HLA-IDENTICAL SIBLING DONOR TRANSPLANTS
The first study used a homogeneous cohort of chronic myeloid leukemia (CML) patients in first chronic phase (CP1).1 All patients received myeloablative conditioning and were transplanted between 1988 and 2003. Donors were either HLA-identical siblings (n = 3537) or unrelated but with allele-level HLA typing (n = 1076). Data were reported to CIBMTR. Age was similar between recipients of both donor sources (median, 36 years). For unrelated donor recipients, 51% received a transplant with an 8 of 8 HLA match, while 24% received a transplant with mismatch at 1 allele, 15% received transplant with mismatch at 2 alleles, and the remaining 10% received a transplant with mismatch at 3 or more alleles. However, most of these transplants were considered to be a complete match at the time they were performed. HLA-identical sibling transplants were conducted closer to time of diagnosis, while unrelated donor transplants occurred later. Median follow-up for HLA-identical sibling transplant recipients was 97 months (range, 2–209 months), and the median follow-up for patients who received transplants from unrelated donors was 106 months (range, 8–219 months).
Mismatching for minor or major histocompatibility antigens offered no additional protection against relapse and increased the risk of GVHD and transplant-related mortality (TRM). The cumulative incidence of TRM was lowest in the HLA-identical sibling cohort, followed by matched unrelated donor recipients.1 TRM increased further with each HLA mismatch. This increase can be attributed to GVHD, as the severity of GVHD increased with unrelated donors and with HLA mismatch. However, the difference in relapse rates was not significant between a fully matched unrelated donor and an HLA-identical sibling donor or with greater degrees of HLA disparity. Relapse rates were low in all groups, with 5-year relapse incidence rates between 7% and 14%, regardless of donor type. Furthermore, there was no significant relapse benefit to a higher risk of GVHD. Leukemia-free survival was significantly better in patients receiving transplants from HLA-identical siblings, followed by matched unrelated donors and then mismatched unrelated donors.
In a second study that took place between 1995 and 2004,2 adults with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), or CML received myeloablative treatment before transplant from HLA-identical siblings or unrelated matched donors. In contrast with the first study, only fully matched unrelated donor transplants were allowed, enabling this study to compare differences in other histocompatability antigens, but not differences in HLA. While 3158 patients received a transplant from an HLA-identical sibling, 941 received a transplant from a matched unrelated donor. The median age of recipients was approximately 38 years. Distribution among diseases was similar for the different types of donor transplants’. Most patients had CML (45% had sibling donors and 44% had unrelated donors), followed by AML (40% sibling and 36% unrelated donors), and ALL (15% sibling and 20% unrelated donors). Patients with sibling donors were more likely to receive a transplant early (70% vs 50% of unrelated donor transplants).
The 5-year relapse incidence was not significantly different between the different donor type transplants within each disease.2 Furthermore, patients at each disease stage, first or second CR or relapse, had no significant differences in relapse due to donor type. However, a multivariate analysis showed that in AML patients there was significantly more relapse with unrelated donors and significantly less relapse in those with chronic GVHD. In ALL patients, multivariate analysis showed a smaller reduction in risk of relapse with chronic GVHD, and the difference did not quite reach statistical significance (P = 0.058). In CML, chronic GVHD significantly lowered the risk of relapse, according to multivariate analysis. The 5-year leukemia-free survival was only significantly different between donor types for AML patients in CR1 or relapse and for CML patients in blast phase. In these three disease states, a sibling donor predicted a higher probability of leukemia-free survival. There was no difference in leukemia-free survival for the patients in other groups.
This study2 showed that, despite higher rates of severe acute and chronic GVHD with unrelated donor grafts, there was a similar risk of relapse and rate of leukemia-free survival after unrelated donor and HLA-identical sibling transplant for ALL and AML patients. There was also a higher relapse risk and a lower rate of leukemia-free survival after unrelated donor transplant in AML. Chronic GVHD was associated with lower relapse risk in AML, ALL, and CML, but there was no evidence for increased GVL effect after unrelated donor vs HLA-identical sibling transplant for AML, ALL, or CML.
GRAFT-VS-LEUKEMIA EFFECT
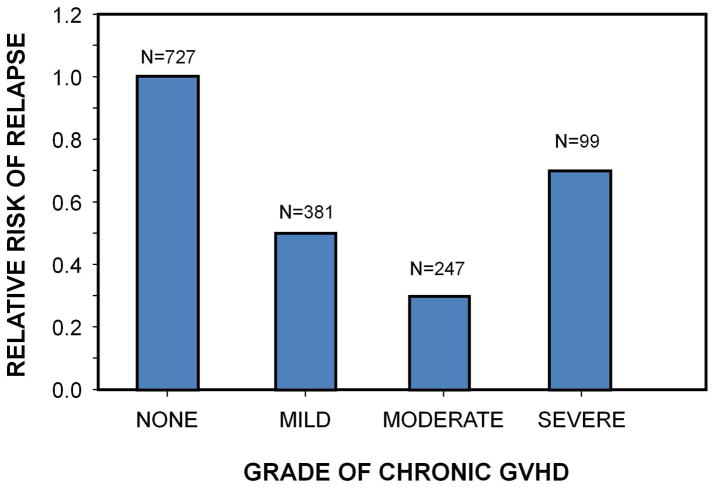
GVHD, particularly chronic GVHD in the myeloablative setting, is associated with a lower risk of relapse.3 In a large study of more than 2000 HLA-identical sibling transplants,4 there was a significantly lower risk of relapse, even with grade 1 acute GVHD. However, the relative risk of relapse did not significantly decrease with increasing grades of acute GVHD,4 and GVL effect did not increase proportionally with the grade of chronic GVHD severity in HLA-identical sibling transplant recipients in another study (Figure 1).5
Figure 1.
Relapse by severity of chronic GVHD in 1827 HLA-identical bone marrow transplant recipients.5 Data includes only 100-day survivors.
There may be a few reasons why increased GVHD does not necessarily lead to decreased relapse. First, there may be an intrinsic lack of dose response. GVL may kill a certain population of leukemic cells no matter the level of GVHD. Secondly, the increased immune suppressive therapy some patients receive to prevent or treat GVHD may affect GVL. In patients who received unrelated donor transplants, the risk of GVHD is higher, and they are treated with agents, such as ATG, that patients who receive transplants from related donors do not receive. Furthermore, early forms of GVHD are treated faster and more aggressively when they are the result of an unrelated or mismatched donor. This immunosuppressive treatment, while meant to be therapeutic, may be obscuring the ability to see true GVL effect. Finally, there are differences in patient selection for unrelated or mismatched donor transplants. Patient selection is adjusted for disease, risk, and patient morbidity, and it is likely that diseases treated with unrelated donor transplants have a higher risk of recurrence.
Is it possible to select or manipulate grafts to enhance GVL? In a study of 177 cord blood transplants conducted at the University of Minnesota,6 the risk of relapse was significantly lower using double vs single unit cord blood transplants. This difference was not attributed to donor/recipient HLA mismatch. A follow-up study from the University of Minnesota and the Fred Hutchinson Cancer Research Center showed similar results and a lower risk of relapse with double cord transplants.7 Double unit cord blood transplants were also compared to matched sibling transplants and matched and mismatched unrelated donor transplants, and the double cord blood transplants had the highest probability of leukemia-free survival, due to decreased relapse in those recipients. The recipients of matched unrelated donor transplants also had superior survival to those receiving matched sibling transplants.
A study of 92 patients with acute leukemia who received T-cell-depleted haploidentical transplant showed that NK-cell alloreactivity, rather than the typical T-cell alloreactivity, results in reduced GVHD and relapse.8 Another study showed improved relapse-free survival after unrelated donor transplant for AML when using a donor with KIR B haplotype.9
Much of what is known about GVL effects and acute vs chronic GVHD comes from studies using myeloablative conditioning. A new study is comparing myeloablative conditioning to reduced-intensity conditioning before transplant for AML. The most dramatic GVHD-associated GVL effect occurs in patients who get chronic GVHD, either alone or following acute GVHD. With reduced-intensity conditioning, the relapse incidence is lowest for those with chronic GVHD following acute GVHD, while those with chronic GVHD who did not experience acute GVHD do not show the same benefit.
CONCLUSIONS
The alloreactivity of GVHD is similar but not identical to GVL; more GVHD is not necessarily associated with increased GVL and less relapse. Instead, GVL may involve multicellular responses that differ based upon graft source (whether cord blood or hematopoietic stem cells), conditioning intensity, and new genetic elements from the donor. Finally, HLA mismatch does not augment GVL, so HLA-identical sibling transplant can be considered for patients with high-risk leukemia.
Footnotes
Conflict of interest statement
Daniel J. Weisdorf, MD
Consulting Fees: Genzyme
Contracted Research: Genzyme
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arora M, Weisdorf DJ, Spellman SR, Haagenson MD, Klein JP, Hurley CK, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringden O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 4.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 6.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunstein C, Gutman J, Weisdorf D, Woolfrey A, DeFor T, Gooley T, et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: Relative risks and benefits of double umbilical cord blood. Blood. 2010 doi: 10.1182/blood-2010-05-285304. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]