Abstract
In this issue, a study by Hayer et al. (2010. J. Cell Biol. doi: 10.1083/jcb.201003086) provides insights into the trafficking of caveolins, the major membrane proteins of caveolae. As well as providing evidence for ubiquitin-mediated endosomal sorting and degradation of caveolin in multivesicular bodies (MVBs), the new findings question the existence of a unique organelle proposed nine years ago, the caveosome.
Caveolae and their trafficking have fascinated cell biologists for decades. These bulb-shaped pits in the cell surface can occupy up to 40% of the area of the plasma membrane in mammalian cell types such as adipocytes and muscle cells (for review see Parton and Simons, 2007). Their general morphological similarity to clathrin-coated pits immediately suggested a role as a parallel endocytic pathway for specific cargo proteins (Montesano et al., 1982). However, the study of caveola dynamics has proven difficult. Few totally specific caveolar cargo proteins are known, precluding simple marker uptake experiments, and most studies have suggested that, compared with their clathrin-coated cousins, caveola budding is infrequent (for review see Hommelgaard et al., 2005). A small fraction of putative caveolar cargo molecules missorted into clathrin-coated pits would hinder studies of internalization via caveolae and have a strong influence on the measured kinetics of uptake. Caveolin itself, which is an essential component of caveolae present in ∼140 copies per caveola (Pelkmans and Zerial, 2005), has no external epitopes available for uptake experiments, and both N and C termini face the cytoplasm (for review see Parton and Simons, 2007).
With the advent of genetically encoded fluorescent protein tags, the possibility of following caveolae in real time using caveolin fusion proteins became a reality. Caveola trafficking could now be compared with the transport of putative cargoes. Pelkmans et al. (2001) reported the use of caveolin-GFP fusion proteins and state of the art microscopy to follow trafficking of caveolin and the uncoated virus SV40 (Pelkmans et al., 2001). They provided evidence for internalization of SV40 and caveolin-GFP into a previously undescribed endocytic compartment, which they termed the caveosome, an essential intermediate in trafficking to the ER where viral translocation to the cytoplasm occurs. Further studies complemented by new EM methods suggested that caveolae can bud into the cell but are far less dynamic and individual caveolae more variable in their budding frequency (Thomsen et al., 2002; Kirkham et al., 2005; Pelkmans and Zerial, 2005) than clathrin-coated pits (which have a lifetime from formation to budding of <1 min).
The discovery of the caveosome, a previously unrecognized membrane-bound organelle, almost 50 years after the discovery of caveolae, created huge interest in the field. The caveosome was described as immobile and was not labeled by either fluid phase markers or ligands of the clathrin-coated pit pathway (Pelkmans et al., 2001). Importantly, the compartment had a neutral pH and did not accumulate a lysosomal dye (lysotracker). Therefore, the caveosome was clearly distinct from any existing endocytic compartment. However, several other studies had linked caveolae and caveolin to conventional endocytic trafficking pathways. Markers internalized through caveolae were suggested to meet ligands internalized via the clathrin-coated pit pathway in the early endosome (Tran et al., 1987; Parton et al., 1994; Pol et al., 2000), and endogenous caveolin was localized to recycling endosomes (Gagescu et al., 2000) and EEA1-positive early endosomes (Liebl et al., 2006). Caveolin trafficking to conventional endosomes could also be modulated. Cholesterol disruption of fibroblasts and muscle cells caused endogenous caveolin-1 and caveolin-3 to redistribute to conventional early and late endosomes (Carozzi et al., 2000). More recently, down-regulation of an essential caveolar coat protein, PTRF/cavin-1, which disassembles caveolae, caused noncaveolar endocytosis of caveolin, accelerated turnover, and increased association with endosomal compartments, including the appearance in the internal membranes of multivesicular late endosomes (Fig. 1; Hill et al., 2008). Ultrastructural studies have also added to the confusion, as vacuolar structures covered in caveolae have been observed in many different cell types and, in some cases, have been assumed to be caveosomes with caveolae fusing to their surface. However, in most cases, these remarkable structures are actually caveolae-covered structures connected to the cell surface (Novikoff et al., 1980; Parton et al., 2002).
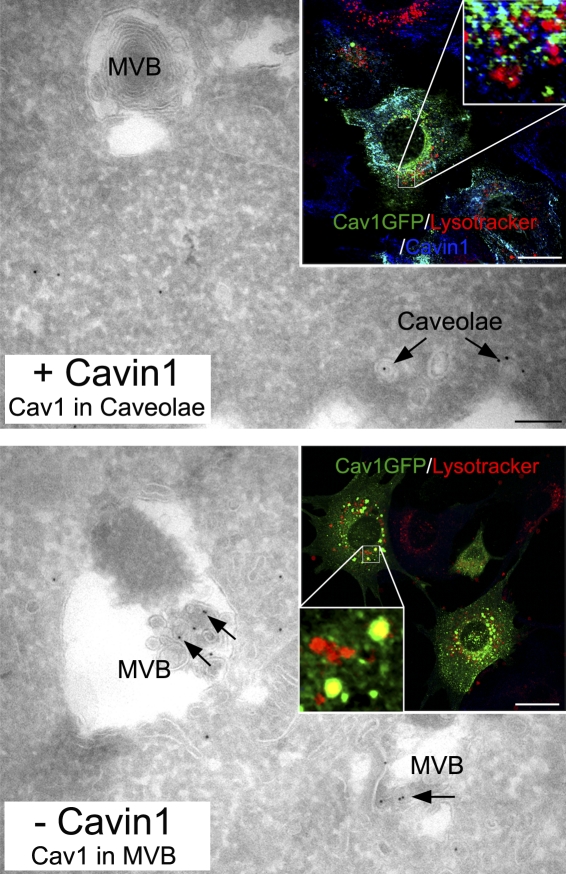
Figure 1.
Cavin-1 regulation of caveolin trafficking. In the presence of cavin-1 and available cholesterol, endogenous caveolin-1 is predominately found within caveolae at the cell surface and not within MVBs. (top) Under these conditions, expressed cavin-1 (Cav1)–GFP colocalizes with cavin-1 but not lysotracker (a marker for MVBs). When caveolae do not stably form, such as in the absence of cavin-1, caveolin-1 increased association with MVBs (bottom) and expressed cavin-1–GFP colocalized with lysotracker. Arrows show immunogold labeling for caveolin-1 on frozen sections. Bars: (main panels) 100 nm; (insets) 10 µm.
In this issue, Hayer et al. clarify many of these issues and, importantly, find no evidence for a distinct compartment with the properties expected of the caveosome. The authors propose that without convincing additional evidence, the term caveosome should not be used, a recommendation that is particularly admirable and should carry considerable weight given that it is made by the group that first suggested the existence of the caveosome. Using several elegant complementary approaches, the authors demonstrate that the level of caveolin expression is critical to the trafficking of the protein. The results suggest that caveolae bud from the plasma membrane carrying caveolin and cavins to the early endosome. However, a different pathway is followed by caveolin when highly expressed or after caveola disruption. Consistent with the aforementioned previous studies, cholesterol perturbation or cavin down-regulation causes disassembly of caveolae, endocytosis of caveolin as a cargo protein, caveolin ubiquitination, and accumulation of caveolin in the internal membranes of late endosomes. This results in dramatically accelerated degradation of caveolin.
Detailed analysis of caveolin trafficking using pH-sensitive fluorophores did not support the existence of a neutral pH compartment as an essential intermediate in the trafficking of caveolin (Hayer et al., 2010). The authors argue that despite the low level of caveolin-GFP overexpression in the original paper (50% of endogenous levels; Pelkmans et al., 2001), the overexpressed protein could saturate the system and lead to noncaveolar caveolin being directed to late endosomes, misidentified in the original study as caveosomes, for degradation. Thus, overexpression of caveolin would mimic the disruption of caveolae on cavin down-regulation or cholesterol perturbation. Despite many questions being still unresolved, the study raises many important issues. First, these results suggest that the relative levels of caveolin and cavin must be tightly balanced. Changes in the ratio of caveolin to cavin could potentially trigger increased caveolin degradation. Caveolin is generally long lived but, in some situations, may be turned over much more rapidly, e.g., in dividing fibroblasts (Fielding et al., 1999). Thus, cavins can act to regulate caveolin levels and, therefore, caveolae. Second, the experiments highlight the efficiency of caveolin sorting under steady-state conditions. Even a budding rate of 1% per minute, as estimated in fibroblasts (Kirkham et al., 2005), would lead to rapid caveolae turnover if recycling from the early endosome back to the plasma membrane was not highly efficient. This efficient sorting may be facilitated by highly stable caveolar domains, most probably containing caveolin and cavins, on the early endosome (Pelkmans et al., 2004; Hayer et al., 2010). Third, the experiments highlight the very different properties of caveolin within caveolae to noncaveolar caveolin. On release from caveolae, caveolin becomes highly mobile, is rapidly endocytosed by noncaveolar pathways (Hill et al., 2008), and as shown in Hayer et al. (2010), can be ubiquitinated and degraded. This contrasts with mutant caveolins associated with muscle diseases, which accumulate in the Golgi complex and are proteasomally degraded after ubiquitination (Galbiati et al., 2000). Caveolar caveolin, stabilized by the cavin complex, is relatively immobile and follows a defined trafficking pathway via dynamin-dependent caveolar budding to the early endosome followed by efficient recycling (Pelkmans et al., 2004). Release of caveolin from caveolae by changes in cavin levels or cavin interactions regulated by other processes (such as phosphorylation) allowing diffusion in the membrane could facilitate other interactions and functions. Noncaveolar trafficking and functions of caveolins are apparent from work in many different systems. For example, Caenorhabditis elegans caveolin may not generate caveolae (Kirkham et al., 2008) but is internalized through clathrin-coated pits (Sato et al., 2006). In the zebrafish embryo, caveolins are expressed before cavins, and therefore, before caveolae are generated (Hill et al., 2008), suggesting distinct noncaveolar roles, one candidate being the regulation of β-catenin signaling (Mo et al., 2010).
Clearly, more work is required to understand the trafficking of caveolins, particularly the fate and functions of caveolar and noncaveolar caveolin. In the meantime, the accumulating evidence against the existence of a discrete compartment with the characteristics of the caveosome means that, for now, the scheme of caveolin trafficking can be simplified, and the focus can turn to the molecules, mechanisms, and functions associated with caveolin and caveolar trafficking.
References
- Carozzi A.J., Ikonen E., Lindsay M.R., Parton R.G. 2000. Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic. 1:326–341 10.1034/j.1600-0854.2000.010406.x [DOI] [PubMed] [Google Scholar]
- Fielding C.J., Bist A., Fielding P.E. 1999. Intracellular cholesterol transport in synchronized human skin fibroblasts. Biochemistry. 38:2506–2513 10.1021/bi981012o [DOI] [PubMed] [Google Scholar]
- Gagescu R., Demaurex N., Parton R.G., Hunziker W., Huber L.A., Gruenberg J. 2000. The recycling endosome of Madin-Darby canine kidney cells is a mildly acidic compartment rich in raft components. Mol. Biol. Cell. 11:2775–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F., Volonte D., Minetti C., Bregman D.B., Lisanti M.P. 2000. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutanta and rescues wild-type caveolin-3. J. Biol. Chem. 275:37702–37711 10.1074/jbc.M006657200 [DOI] [PubMed] [Google Scholar]
- Hayer A., Stoeber M., Ritz D., Engel S., Meyer H.H., Helenius A. 2010. Caveolin-1 is ubiquitinated and targeted to intralumenal vesicles in endolysosomes for degradation. J. Cell Biol. 191:615–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M.M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S.J., Walser P., Abankwa D., Oorschot V.M., Martin S., et al. 2008. PTRF-cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 132:113–124 10.1016/j.cell.2007.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommelgaard A.M., Roepstorff K., Vilhardt F., Torgersen M.L., Sandvig K., van Deurs B. 2005. Caveolae: stable membrane domains with a potential for internalization. Traffic. 6:720–724 10.1111/j.1600-0854.2005.00314.x [DOI] [PubMed] [Google Scholar]
- Kirkham M., Fujita A., Chadda R., Nixon S.J., Kurzchalia T.V., Sharma D.K., Pagano R.E., Hancock J.F., Mayor S., Parton R.G. 2005. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J. Cell Biol. 168:465–476 10.1083/jcb.200407078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham M., Nixon S.J., Howes M.T., Abi-Rached L., Wakeham D.E., Hanzal-Bayer M., Ferguson C., Hill M.M., Fernandez-Rojo M., Brown D.A., et al. 2008. Evolutionary analysis and molecular dissection of caveola biogenesis. J. Cell Sci. 121:2075–2086 10.1242/jcs.024588 [DOI] [PubMed] [Google Scholar]
- Liebl D., Difato F., Horníková L., Mannová P., Stokrová J., Forstová J. 2006. Mouse polyomavirus enters early endosomes, requires their acidic pH for productive infection, and meets transferrin cargo in Rab11-positive endosomes. J. Virol. 80:4610–4622 10.1128/JVI.80.9.4610-4622.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo S., Wang L., Li Q., Li J., Li Y., Thannickal V.J., Cui Z. 2010. Caveolin-1 regulates dorsoventral patterning through direct interaction with beta-catenin in zebrafish. Dev. Biol. 344:210–223 10.1016/j.ydbio.2010.04.033 [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. 1982. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 296:651–653 10.1038/296651a0 [DOI] [PubMed] [Google Scholar]
- Novikoff A.B., Novikoff P.M., Rosen O.M., Rubin C.S. 1980. Organelle relationships in cultured 3T3-L1 preadipocytes. J. Cell Biol. 87:180–196 10.1083/jcb.87.1.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R.G., Simons K. 2007. The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8:185–194 10.1038/nrm2122 [DOI] [PubMed] [Google Scholar]
- Parton R.G., Joggerst B., Simons K. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199–1215 10.1083/jcb.127.5.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R.G., Molero J.C., Floetenmeyer M., Green K.M., James D.E. 2002. Characterization of a distinct plasma membrane macrodomain in differentiated adipocytes. J. Biol. Chem. 277:46769–46778 10.1074/jbc.M205683200 [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Zerial M. 2005. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 436:128–133 10.1038/nature03866 [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Kartenbeck J., Helenius A. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473–483 10.1038/35074539 [DOI] [PubMed] [Google Scholar]
- Pelkmans L., Bürli T., Zerial M., Helenius A. 2004. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 118:767–780 10.1016/j.cell.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Pol A., Lu A., Pons M., Peiró S., Enrich C. 2000. Epidermal growth factor-mediated caveolin recruitment to early endosomes and MAPK activation. Role of cholesterol and actin cytoskeleton. J. Biol. Chem. 275:30566–30572 10.1074/jbc.M001131200 [DOI] [PubMed] [Google Scholar]
- Sato K., Sato M., Audhya A., Oegema K., Schweinsberg P., Grant B.D. 2006. Dynamic regulation of caveolin-1 trafficking in the germ line and embryo of Caenorhabditis elegans. Mol. Biol. Cell. 17:3085–3094 10.1091/mbc.E06-03-0211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen P., Roepstorff K., Stahlhut M., van Deurs B. 2002. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol. Biol. Cell. 13:238–250 10.1091/mbc.01-06-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J.L., Sawano F., Gorden P., Orci L. 1987. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc. Natl. Acad. Sci. USA. 84:7957–7961 10.1073/pnas.84.22.7957 [DOI] [PMC free article] [PubMed] [Google Scholar]