Abstract
Exposure of cells to hyperthermia is known to induce apoptosis, although the underlying mechanisms are only partially understood. Here, we examine the molecular requirements necessary for heat-induced apoptosis using genetically modified Jurkat T-lymphocytes. Cells stably overexpressing Bcl-2/Bcl-xL or stably depleted of Apaf-1 were completely resistant to heat-induced apoptosis, implicating the involvement of the mitochondria-mediated pathway. Pretreatment of wild-type cells with the cell-permeable biotinylated general caspase inhibitor b-VAD-fmk (biotin-Val-Ala-Asp(OMe)-CH2F) both inhibited heat-induced apoptosis and affinity-labeled activated initiator caspase-2, -8, and -9. Despite this finding, however, cells engineered to be deficient in caspase-8, caspase-2, or the caspase-2 adaptor protein RAIDD (receptor-interacting protein (RIP)-associated Ich-1/CED homologous protein with death domain) remained susceptible to heat-induced apoptosis. Additionally, b-VAD-fmk failed to label any activated initiator caspase in Apaf-1-deficient cells exposed to hyperthermia. Cells lacking Apaf-1 or the pro-apoptotic BH3-only protein Bid exhibited lower levels of heat-induced Bak activation, cytochrome c release, and loss of mitochondrial membrane potential, although cleavage of Bid to truncated Bid (tBid) occurred downstream of caspase-9 activation. Combined, the data suggest that caspase-9 is the critical initiator caspase activated during heat-induced apoptosis and that tBid may function to promote cytochrome c release during this process as part of a feed-forward amplification loop.
Keywords: Apoptosis, Caspase, Cell Death, Cytochrome c, Death Protease, Mitochondrial Apoptosis, shRNA, Jurkat, Heat Shock
Introduction
Sublethal heat exposure is known to induce an evolutionarily conserved adaptive response known as the heat shock response. A key feature of this response includes the transcriptional up-regulation of several heat shock proteins that are known to confer protection against a subsequent exposure to an otherwise lethal cellular stressor, including γ-radiation, hyperthermia, and chemotherapeutic agents (1, 2). By comparison, an initial exposure of cells to a more severe or prolonged bout of hyperthermia is known to overcome this protective heat shock response and induce apoptosis or necrosis (3). Significantly, because hyperthermia can induce apoptosis, it is currently being tested in combination with conventional anti-cancer therapy in clinical trials for advanced malignancies (4, 5). Additionally, there is a growing interest in developing ways to more selectively target tumor cells with hyperthermia for therapeutic use (6, 7).
There are two distinct apoptotic pathways: (i) the mitochondria-mediated (i.e. intrinsic) pathway and (ii) the receptor-mediated (i.e. extrinsic) pathway. The extrinsic pathway is activated upon binding of a death ligand to its cognate receptor (e.g. Fas binding to the Fas receptor), which causes the receptors to move within close proximity to one another and recruit the adaptor protein Fas-associated protein with death domain (FADD), followed by the recruitment of initiator procaspase-8 or -10 (8, 9). This protein complex is termed the death-inducing signaling complex (DISC) and serves as the activating platform for initiator caspase-8 and -10 during receptor-mediated apoptosis. In so-called type I cells, there is sufficient activation of caspase-8 at the DISC to directly cleave and activate effector caspase-3, resulting in execution of apoptosis. However, in so-called type II cells, activated caspase-8 does not directly cleave and activate a sufficient amount of effector caspase-3 to execute apoptosis. In this cell type, activated caspase-8 cleaves the pro-apoptotic Bcl-2 family member Bid to truncated Bid (tBid),2 which then engages the mitochondria-mediated pathway (10).
Mitochondria-mediated apoptosis is activated following exposure to cytotoxic stressors, including DNA damage, growth factor withdrawal, and γ-radiation. During intrinsic apoptosis, caspase activation is largely regulated by the Bcl-2 family of proteins. The Bcl-2 family of proteins contains both pro- and anti-apoptotic members that function to either promote or inhibit mitochondrial outer membrane permeabilization (MOMP). MOMP leads to the release of cytochrome c from the intermembrane space of mitochondria into the cytosol where it is required for caspase activation (11). Specifically, cytosolic cytochrome c interacts with the adaptor protein apoptotic protease activating factor-1 (Apaf-1) and dATP to form the apoptosome complex, which then recruits and activates initiator caspase-9 (11). Activated initiator caspase-9 cleaves and activates downstream effector caspases, which then cleave various target substrates, resulting in the biochemical and morphological characteristics associated with apoptotic cell death.
Although adaptive cellular responses to elevated temperatures have been studied for decades, heat-induced apoptosis has been studied to a much lesser extent, and conflicting results have emerged from these studies (3, 12–17). In this regard, the aim of the current study was to help determine the molecular requirements necessary for heat-induced apoptosis. Because several of the more recent studies have used Jurkat T-lymphocytes as a model system to investigate apoptosis induced by elevated temperatures, we also used a large panel of genetically modified Jurkat cells in which key steps in the intrinsic or extrinsic pathway were inhibited. In agreement with previous studies, our results indicated that heat-induced apoptosis relies heavily on the mitochondria-mediated apoptotic pathway. Significantly, although caspase-2, -8, and -9 were affinity-labeled as activated initiator caspases, subsequent experiments revealed that only the activation of caspase-9 was strictly required for heat-induced apoptosis in Jurkat cells. In addition, Bid was observed to play a role in this form of apoptosis as a regulator of MOMP, although its cleavage to tBid occurred downstream of Apaf-1, suggesting that tBid likely plays an amplification role in this process.
EXPERIMENTAL PROCEDURES
Cell Culture
Wild-type (clones E6.1 and A3) and caspase-8-deficient (clone I 9.2) Jurkat T-lymphocytes were cultured in RPMI 1640 complete medium (Invitrogen) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS) (HyClone, Logan, UT), 2% (w/v) glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a humidified 5% CO2 incubator. For transfected cells, 1 mg/ml Geneticin (Invitrogen) was substituted for penicillin and streptomycin. Cells were maintained in an exponential growth phase for all experiments. All cells were replated in fresh complete nonselective medium prior to apoptosis induction. Apoptosis was induced either by exposure to hyperthermia (44 °C) for 1 h followed by a 6-h incubation at 37 °C or by treatment with agonistic anti-Fas antibody (100 ng/ml) (clone CH-11, MBL International, Woburn, MA) for 6 h. The caspase inhibitor quinoline-Val-Asp-difluorophenoxymethylketone (Q-VD-OPh) (MP Biomedicals, Solon, OH) was used at a final concentration of 20 μm.
Flow Cytometry for Cell Death and Mitochondrial Membrane Potential (ΔΨ) Measurements
Phosphatidylserine exposure on the outer leaflet of the plasma membrane was detected using the annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit II (BD Pharmingen) according to the manufacturer's instructions. In brief, 106 cells were pelleted following heat or anti-Fas treatment and washed in phosphate-buffered saline (PBS). Next, cells were resuspended in 500 μl of binding buffer, and 100 μl of the suspension (2 × 105 cells) were mixed with annexin V-FITC and propidium iodide and incubated at room temperature (22 °C) for 5–10 min in the dark. Prior to flow cytometric analysis, 400 μl of binding buffer were added to the cells. For ΔΨ determination, the MitoProbe DiIC1(5) kit (Invitrogen) was used. Briefly, cells (106) were pelleted following drug treatment, washed once in PBS, and resuspended in 1 ml of warm PBS. Next, 5 μl of 10 μm DiIC1(5) were added to the cells and incubated in a humidified 5% CO2 incubator at 37 °C for 15 min. Cells were pelleted, resuspended in 500 μl of PBS, and analyzed by flow cytometry.
Western Blotting
Pelleted cells (5 × 106) were resuspended and lysed in 200 μl of ice-cold lysis buffer (10 mm Tris/HCl, pH 7.4, 10 mm NaCl, 3 mm MgCl2, 1 mm EDTA, 0.1% Nonidet P-40) supplemented with a mixture of protease inhibitors (Complete mini EDTA-free, Roche Applied Science). Protein concentrations were determined using the bicinchoninic acid assay (Pierce), and equal amounts were mixed with Laemmli buffer, boiled for 5 min, and subjected to 12–15% SDS-PAGE at 195 V followed by electroblotting to nitrocellulose for 1 h at 115 V. Membranes were blocked for 1 h with 5% nonfat milk in PBS at room temperature (22 °C) and subsequently probed overnight at 4 °C with primary antibody suspended in PBS containing 1% bovine serum albumin. Following overnight incubation, membranes were rinsed and incubated with a horseradish peroxidase-conjugated secondary antibody (Pierce). Following the secondary antibody incubation, membranes were rinsed, and bound antibodies were detected using enhanced chemiluminescence according to the manufacturer's instructions (GE Healthcare). The primary antibodies used were anti-β-actin (clone AC-15, Sigma), anti-caspase-2 (clone 35, BD Transduction Laboratories), anti-caspase-3 (clone 8G10, Cell Signaling), anti-caspase-8 (clone 1C12, Cell Signaling), anti-caspase-9 (Cell Signaling), anti-cytochrome c (clone 7H8.2C12, BD Pharmingen), and anti-RAIDD (StressGen).
Measurement of Caspase Activity
Cells (5 × 105) were pelleted and washed once with ice-cold PBS. Cells were resuspended in 25 μl of PBS, added to a microtiter plate, and combined with DEVD-aminomethylcoumarin (Peptide Institute, Osaka, Japan) dissolved in a standard reaction buffer (100 mm Hepes, pH 7.25, 10% sucrose, 10 mm dithiothreitol, 0.1% CHAPS). Cleavage of DEVD-aminomethylcoumarin was monitored by aminomethylcoumarin production in a FLx800 multi-detection microplate reader (BioTek Instruments, Winooski, Vermont) using 355 nm excitation and 460 nm emission wavelengths.
b-VAD-fmk Affinity Labeling of Initiator Caspases
Cells (2.5 × 106/ml) were incubated with 120 μm cell-permeable biotinylated form of the general caspase inhibitor VAD-fmk (b-VAD-fmk; Kamiya Biomedical Co., Seattle, Washington) for 3 h at 37 °C prior to heat (44 °C) exposure or anti-Fas treatment. Following b-VAD-fmk pretreatment, cells were diluted with RPMI 1640 complete growth medium to a density of 106/ml and either incubated with anti-Fas (100 ng/ml) for 6 h or exposed to 44 °C for 1 h followed by a 6-h incubation at 37 °C. Afterward, cells were washed with PBS and lysed with 500 μl of lysis buffer (50 mm Tris/HCl, pH 7.4, 150 mm NaCl, 1% Nonidet P-40, 1 mm DTT) supplemented with a mixture of protease inhibitors (Complete mini EDTA-free, Roche Applied Science). Lysates were incubated on ice for 30 min and subsequently centrifuged at 12,000 × g for 10 min at 4 °C. Resulting supernatants were collected and incubated with 130 μl of prewashed streptavidin-Sepharose beads (GE Healthcare) followed by gentle rocking overnight at 4 °C. Following overnight incubation, beads were washed four times with wash buffer (same as lysis buffer except that the concentration of Nonidet P-40 was 0.5%), and bound proteins were eluted with Laemmli buffer and subjected to Western blot analysis.
RNA Interference
The vector-based pSUPER RNAi system (OligoEngine, Seattle, WA) was used to suppress gene expression. The gene-specific targeting insert for RAIDD (receptor-interacting protein (RIP)-associated Ich-1/CED homologous protein with death domain; RefSeq accession number NM_003805) specifies a 19-nucleotide sequence corresponding to nucleotides 957–975 (5′-GGCAGGTGTCTCATATGTA-3′) downstream of the transcription start site, which is separated by a 9-nucleotide noncomplementary spacer (TTCAAGAGA) from the reverse complement of the same 19-nucleotide sequence. The targeting sequences used to suppress APAF-1, BID, and CASP2 gene expression have been reported previously (18–20). In each case, the sequence was ligated into the BglII and XhoI sites of the pSUPER.neo vector, which was subsequently transformed into TOP10-competent cells (Invitrogen) according to the manufacturer's instructions. Several clones were obtained, and the correct insert was verified by sequence analysis.
Transfections
Wild-type Jurkat T-lymphocytes (107) were transfected with 20 μg of plasmid DNA (pSUPER-neo, pSUPER-Apaf-1, pSUPER-Bid, pSUPER-Caspase-2, pSUPER-RAIDD, pSFFV-neo, pSFFV-Bcl-2, and pSFFV-Bcl-xL,) by electroporation using a Bio-Rad Gene Pulser Xcell system (0.4-cm cuvette, 300 V, and 950 microfarads). Cells were allowed to recover in RPMI 1640 complete growth medium minus antibiotics for 48 h at 37 °C in a humidified 5% CO2 incubator. Selection of transfected cells was performed in the presence of 1 mg/ml Geneticin for several weeks, at which time serial dilutions were performed to obtain single-cell clones of Apaf-1-silenced cells, Bid-silenced cells, caspase-2-silenced cells, RAIDD-silenced cells, or cells overexpressing full-length human Bcl-2 or Bcl-xL.
Determination of Bak Activation
For detection of activated Bak by flow cytometry, cells (106) were washed in PBS and fixed in 400 μl of 0.25% paraformaldehyde for 5 min, subsequently washed two times with 1% fetal bovine serum in PBS, and incubated in 50 μl of staining buffer (1% fetal bovine serum and 100 μg/ml digitonin in PBS) with a conformation-specific mouse monoclonal antibody against Bak (1:30, AM03, Calbiochem) for 30 min at room temperature (22 °C). Then, cells were washed and resuspended in 50 μl of staining buffer containing 0.25 μg of Alexa Fluor 488-labeled chicken anti-mouse for 30 min in the dark. Cells were washed again and analyzed by flow cytometry. Analysis and histogram overlays were performed using FlowJo software (Tree Star, Ashland, OR).
Subcellular Fractionation
Following heat shock, cells (106) were washed in PBS, resuspended in 50 μl of buffer (140 mm mannitol, 46 mm sucrose, 50 mm KCl, 1 mm KH2PO4, 5 mm MgCl2, 1 mm EGTA, 5 mm Tris, pH 7.4) supplemented with a mixture of protease inhibitors (Complete mini EDTA-free), and permeabilized with 3 μg of digitonin (Sigma) on ice for 10 min. Plasma membrane permeabilization was monitored by trypan blue staining, and cell suspensions were centrifuged at 12,000 × g for 10 min at 4 °C. Supernatant and pellet fractions were subjected to Western blot analysis.
RESULTS AND DISCUSSION
Hyperthermia Induces Jurkat Cells to Undergo Apoptosis in a Bcl-2- and Bcl-xL-inhibitable Manner
Previous studies have reported different mechanisms for the initiation of heat-induced apoptosis that, in some instances, are difficult to reconcile. For instance, some evidence in the literature suggests that caspase-2 is the most apical caspase activated during heat-induced apoptosis (17, 21) and functions by cleaving Bid to tBid, which, in turn, triggers Bax/Bak-dependent MOMP (12). However, a separate line of investigation found that caspase-2 is dispensable for heat-induced apoptosis and instead suggested that an unknown Z-VAD-inhibitable protease is the earliest protease activated in response to heat (13). According to that model, the unknown protease functions to initiate apoptosis, at least in part, by directly cleaving and activating caspase-3, suggesting that caspase-9 may not always be necessary for this form of apoptosis (13). Other evidence suggests that Bax and Bak are activated directly by heat (13, 14), an effect that can be enhanced by the ubiquitination and degradation of Mcl-1 (16).
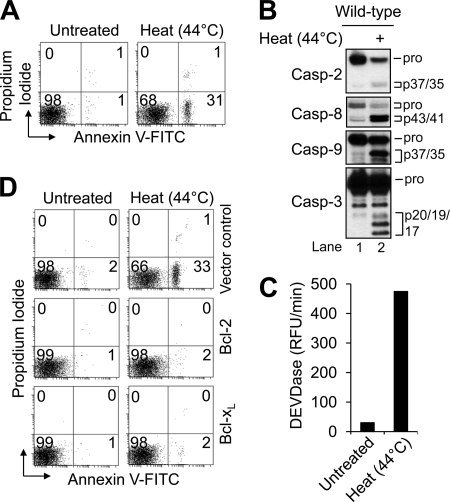
In light of the conflicting and, in some cases, contradictory nature of the reported data, we set out to better understand the molecular requirements necessary for heat-induced apoptosis. As illustrated in Fig. 1A, exposure of wild-type Jurkat cells to 1 h of hyperthermia at 44 °C followed by a 6-h incubation at 37 °C resulted in ∼32% of the cells undergoing apoptosis as determined by annexin V-FITC and propidium iodide co-staining. Heat-induced apoptosis was accompanied by the proteolytic cleavage of caspase-9, -8, -3, and -2, as well as an increase in caspase-3-like (DEVDase) activity (Fig. 1, B and C). In addition, incubation of cells with the pan-caspase inhibitor Q-VD-OPh (20 μm) for 1 h prior to heat exposure prevented cell death and caspase processing (data not shown), indicating that heat-induced apoptosis is a caspase-mediated event. Next, to determine whether the mitochondrial apoptotic pathway was involved in this form of apoptosis, we evaluated the heat sensitivity of clones of Jurkat cells that do not undergo MOMP due to the overexpression of Bcl-2 or Bcl-xL (22). As illustrated in Fig. 1D, Jurkat cells overexpressing either Bcl-2 or Bcl-xL were completely resistant to heat-induced apoptosis. Taken together, these data suggest that heat-induced cell death is a caspase-mediated apoptotic event that requires MOMP.
FIGURE 1.
Bcl-2 or Bcl-xL overexpression inhibits heat-induced apoptosis. A, wild-type Jurkat cells (106/ml) were either left untreated or cultured for 1 h at 44 °C followed by a 6-h incubation at 37 °C and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. B and C, duplicate aliquots of cells in A were harvested and processed for Western blotting (B) or caspase (DEVDase) activity determination (C). pro, pro-form; Casp, caspase; pro, pro-apoptotic; RFU, relative fluorescence units. D, control-transfected, Bcl-2-overexpressing, and Bcl-xL-overexpressing cells (106/ml) were left untreated or heated for 1 h at 44 °C followed by a 6-h incubation at 37 °C processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. In A and D, quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant.
Initiator Caspase-2, -8, and -9 Are Affinity-labeled by b-VAD-fmk in Response to Hyperthermia
Execution of apoptosis requires the activation of the caspase cascade, where active initiator caspases cleave and thereby activate downstream effector caspases that, in turn, dismantle the cell. Although proteolytic cleavage of an effector caspase is indicative of its activation, the same is not necessarily true with initiator caspases. There are currently two prevailing models for initiator caspase activation. The induced proximity model suggests that the adaptor molecules required for initiator caspase activation function to bring caspases into close proximity, leading to caspase homodimerization and subsequent activation (8). By comparison, the induced conformation model suggests that initiator caspases undergo a conformational change upon binding to an adaptor protein that leads to their activation (23). Neither model suggests that cleavage is an activating event, although it was recently shown that dimerization and cleavage are necessary for caspase-8 activation (24).
Because proteolytic cleavage of initiator caspases may not always reflect their activation and because synthetic peptide substrates are not specific for a particular caspase (25), it has been technically challenging to identify apical caspases activated in response to a given insult. A recent study, however, used an innovative approach to affinity-label or ”trap“ initiator caspases as they become activated inside cells (17). The method relies on b-VAD-fmk and immobilized streptavidin to pull down the labeled caspase(s) (26).
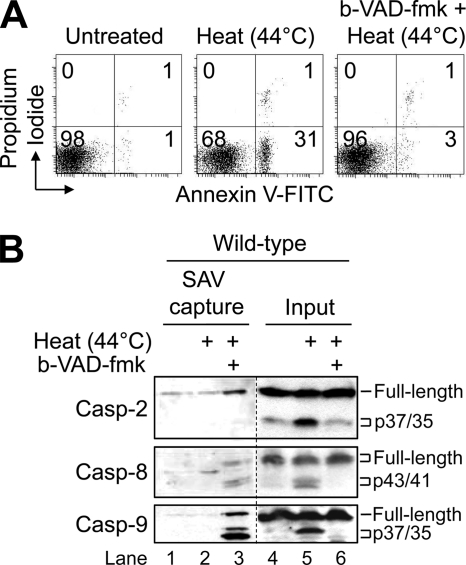
In using this approach, we initially sought to determine the extent to which b-VAD-fmk pretreatment could inhibit heat-induced apoptosis. To that end, wild-type Jurkat cells were cultured in the presence of b-VAD-fmk (120 μm) for 3 h at 37 °C prior to being exposed to hyperthermia at 44 °C for 1 h followed by a 6-h incubation at 37 °C. As illustrated in Fig. 2A, b-VAD-fmk strongly inhibited heat-induced apoptosis as determined by annexin V-FITC and propidium iodide co-staining. Subsequently, duplicate aliquots of cells were lysed and incubated in the presence of streptavidin-Sepharose beads to pull down any activated caspases. To our surprise, b-VAD-fmk affinity-labeled caspase-2, -8, and -9 as initiator caspases that are activated early in response to hyperthermia (Fig. 2B, lane 3). However, the fact that significantly more caspase-9 was pulled down as compared with caspase-2 and caspase-8 suggested to us that caspase-9 might perform a more central role as an initiator caspase in this setting.
FIGURE 2.
b-VAD-fmk inhibits heat-induced apoptosis, and at the same time, affinity-labels activated initiator caspase-2, -8, and -9. A, wild-type Jurkat cells (2.5 × 106/ml) were incubated in the presence or absence of 120 μm b-VAD-fmk for 3 h and subsequently heated at a density of 106/ml for 1 h at 44 °C followed by a 6-h incubation at 37 °C. Cells were harvested and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. Quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. B, duplicate aliquots of cells in A were harvested, lysed, and incubated with streptavidin-Sepharose beads, after which bound proteins were eluted and subjected to Western blotting. SAV, streptavidin; Casp, caspase.
Activation of Caspase-9, but Not Caspase-8, Is Required for Heat-induced Apoptosis
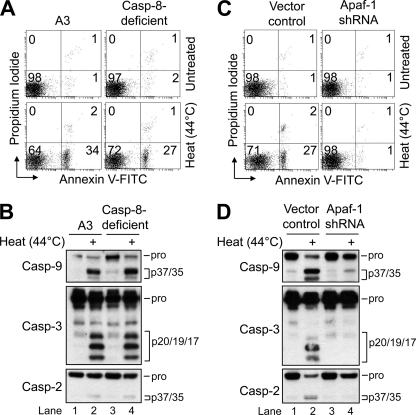
Interestingly, previous studies have reported that caspase-8 can play a role in heat-induced apoptosis through a Fas-dependent mechanism (15, 17). For this reason and because caspase-8 was affinity-labeled as an activated initiator caspase in response to heat (Fig. 2B, lane 3), we first sought to determine the extent to which caspase-8 was important for heat-induced apoptosis. To do so, caspase-8-deficient Jurkat cells that have been described previously (27), and are completely resistant to Fas-induced apoptosis (20, 27), were evaluated for their sensitivity to heat-induced apoptosis. As illustrated in Fig. 3, A and B, the results indicated that cells lacking caspase-8 underwent heat-induced apoptosis and processed caspase-9, -3, and -2 to the same extent as A3 control cells (Fig. 3, A and B), indicating that caspase-8 is not necessary in this setting.
FIGURE 3.
Caspase-9, but not caspase-8, activation is essential for heat-induced apoptosis. A, wild-type (A3) and caspse-8-deficient (Casp-8-deficient) (I9.2) cells (106/ml) were untreated or heated for 1 h at 44 °C followed by a 6-h incubation at 37 °C and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. B, duplicate aliquots of cells in A were harvested and lysed for Western blotting. C, control-transfected and Apaf-1-deficient cells (106/ml) were untreated or heated for 1 h at 44 °C followed by a 6-h incubation at 37 °C and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. D, duplicate aliquots of cells in C were harvested and lysed for Western blotting. In A and C, quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. In B and D, pro refers to pro-form.
Having ruled out a significant role for caspase-8, we next investigated whether caspase-9 activation, which strictly depends on Apaf-1 (28–30), was necessary for heat-induced apoptosis. To do so, we used well characterized Apaf-1-deficient Jurkat cells that were shown previously to be (i) incapable of activating caspase-9 in vitro (31), (ii) highly resistant to mitochondria-mediated apoptosis induced by etoposide (18, 22), and (iii) partially resistant to anti-Fas-induced apoptosis due to their type II origin (31). In stark contrast to caspase-8-deficient cells, the results showed that Apaf-1-deficient Jurkat cells were completely resistant to heat-induced apoptosis (Fig. 3C). In agreement with these findings, Western blot analysis of cell lysates showed that proteolytic processing of caspase-2, -3, and -9 was inhibited in Apaf-1-deficient cells following heat exposure (Fig. 3D). Taken together, these data demonstrate that caspase-8 is dispensable during heat-induced apoptosis, whereas caspase-9 activation is essential for this form of cell killing.
Caspase-2 and RAIDD Are Dispensable for Heat-induced Apoptosis
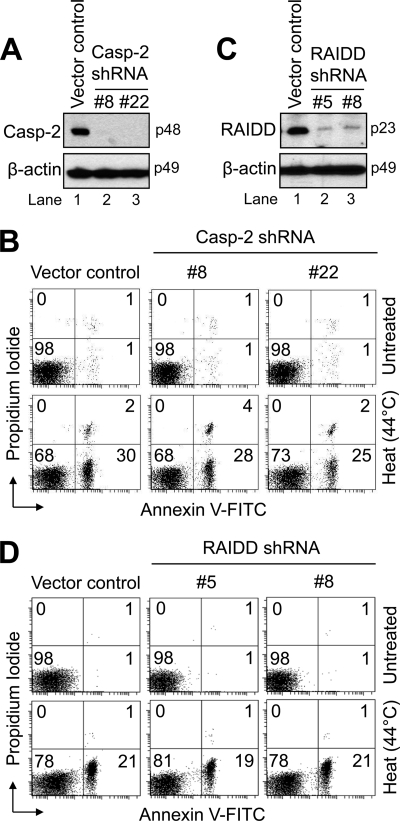
Because we had also observed, in agreement with a recent study (17), that caspase-2 was affinity-labeled as an activated initiator caspase in response to heat, albeit to a lesser degree than caspase-9 (Fig. 2B, lane 3), we next sought to determine the significance of this finding. To do so, we used the pSUPER vector-based system and a recently reported siRNA target to silence caspase-2 (19) in Jurkat cells. Several single-cell caspase-2-deficient clones were screened, and two (clones 8 and 22) with the highest level of knockdown (Fig. 4A, lanes 2 and 3) were used for these studies. To test whether caspase-2 activation was necessary for heat-induced apoptosis, we incubated the two clones for 1 h at 44 °C and evaluated them for differences in annexin V-FITC fluorescence after a 6-h recovery at 37 °C. As shown in Fig. 4B, clones 8 and 22 underwent heat-induced apoptosis to a similar extent as control-transfected cells. Although we also found another clone with a comparable level of caspase-2 knockdown as clones 8 and 22 that was partially resistant to heat-induced apoptosis (data not shown), the level of resistance observed was far lower than that conferred by Apaf-1 deficiency (Fig. 3C). Taken together, the data obtained with the caspase-2-deficient Jurkat cells suggested that this initiator caspase is not essential for heat-induced apoptosis.
FIGURE 4.
Caspase-2 and RAIDD are not essential for heat-induced apoptosis. A, control-transfected and two single-cell caspase-2 (Casp-2)-deficient Jurkat clones (8 and 22) were harvested and lysed for Western blotting. B, control-transfected and the caspase-2-deficient cells (106/ml) were either left untreated or exposed to 44 °C for 1 h followed by a 6-h incubation and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. C, control-transfected and two single-cell RAIDD-deficient Jurkat clones (5 and 8) were harvested and lysed for Western blotting. D, control-transfected and the RAIDD-deficient cells (106/ml) were either left untreated or exposed to 44 °C for 1 h followed by a 6-h incubation and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. In B and D, quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant.
However, because we had observed partial resistance in one caspase-2-deficient clone and because a previous study reported that two distinct siRNA sequences that silenced caspase-2 to a similar extent had very different effects on the sensitivity of a single cell type to apoptosis induced by genotoxic stress (32), we sought to extend our findings by using a complementary approach to test the importance of caspase-2 for heat-induced apoptosis. Specifically, we used short hairpin RNA (shRNA) to generate RAIDD-deficient Jurkat cells. We hypothesized that because RAIDD is the adaptor protein responsible for activation of caspase-2 (34–36), such cells would offer additional information on the extent to which caspase-2 is important for heat-induced apoptosis. In addition, it was reported previously that RAIDD is required for caspase-2 activation during heat-induced cell death in mouse splenocytes (17). To that end, two single-cell RAIDD-deficient Jurkat clones (5 and 8) were produced, and the extent to which RAIDD was suppressed was confirmed by Western blot analysis (Fig. 4C). As illustrated in Fig. 4D, both RAIDD-deficient cell lines were as susceptible to heat-induced apoptosis as control-transfected cells. Collectively, although b-VAD-fmk labeled some caspase-2 as an initiator caspase that is activated early in response to hyperthermia, our findings using caspase-2-deficient and RAIDD-deficient Jurkat cells do not support an essential role for caspase-2 activation during this form of cell death.
b-VAD-fmk Does Not Associate with Any Initiator Caspase in Apaf-1-deficient Cells Exposed to Heat
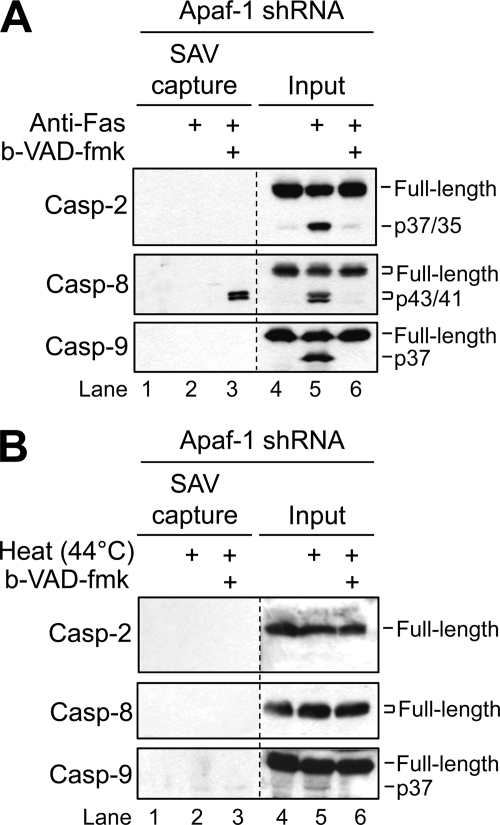
Because our collective data up to this point suggested that caspase-9 was the only initiator caspase whose activation was required for heat-induced apoptosis, we sought to extend these findings to test whether caspase-2 or -8 would be pulled down by b-VAD-fmk in the Apaf-1-deficient cells following heat exposure. We reasoned that if the activation of caspase-8 and/or caspase-2 occurred upstream of caspase-9 as reported previously (15, 17), then it should be possible to use b-VAD-fmk to label the activated caspase(s). As a proof of principle demonstration of the feasibility of using the b-VAD-fmk labeling approach in the Apaf-1-deficient cells, we pretreated the cells with b-VAD-fmk for 3 h and subsequently incubated them in the presence of agonistic anti-Fas antibody (100 ng/ml) for 6 h. Consistent with our previous results (31), Apaf-1-deficient Jurkat cells were partially sensitive to apoptosis induced by anti-Fas (data not shown). Further, as anticipated, caspase-8 was the only initiator caspase that was affinity-labeled by b-VAD-fmk (Fig. 5A, lane 3). The fact that only the cleaved form of caspase-8 was pulled down in this scenario is consistent with the findings of a recent study indicating that cleavage of the zymogen is necessary for activation (24). When Apaf-1-deficient cells were pretreated with b-VAD-fmk for 3 h at 37 °C prior to being exposed to hyperthermia at 44 °C for 1 h followed by a 6-h incubation at 37 °C, no single initiator caspase was affinity-labeled (Fig. 5B, lane 3), offering additional evidence that caspase-9 is the most critical initiator caspase activated during heat-induced apoptosis. It should be noted that a small amount of the p37 proteolytic fragment of caspase-9 was observed in heated Apaf-1-deficient cells (Fig. 3D, lane 4 versus lane 3 and Fig. 5B, lane 5 versus lane 4). However, evidence in the literature has shown that the p37 fragment of caspase-9 is catalytically inactive, whereas apoptosome-mediated activation of procaspase-9, which requires Apaf-1, produces a p35 proteolytic fragment (37, 38). Nevertheless, the fact that the appearance of the p37 fragment in Apaf-1-deficient cells was preventable by pretreatment of cells with b-VAD-fmk (Fig. 5B, lane 6 versus lane 5) suggests, in agreement with a previous study (13), that a Z-VAD-inhibitable protease, other than caspase-2 or -8, may be activated in response to hyperthermia and can cleave caspase-9; however, the extent to which this event is important for the execution of heat-induced apoptosis is not known. Overall, these findings, as well as those presented in Figs. 2 and 3, suggest that heat-induced activation and proteolytic cleavage of caspase-2 and caspase-8 in Jurkat cells depend upon the prior activation of caspase-9.
FIGURE 5.
b-VAD-fmk fails to affinity-label any initiator caspase in Apaf-1-deficient cells exposed to hyperthermia. A and B, Apaf-1-deficient cells (2.5 × 106/ml) were left untreated or incubated in the presence or absence of 120 μm b-VAD-fmk for 3 h at 37 °C and subsequently incubated at a density of 106/ml in the presence of 100 ng/ml agonistic anti-Fas antibody for 6 h (A) or heated for 1 h at 44 °C followed by a 6-h incubation at 37 °C (B). Cells were then harvested, lysed, and incubated with streptavidin-Sepharose beads, after which bound proteins were eluted and subjected to Western blot analysis. Casp, caspase; SAV, streptavidin.
Heat-induced Mitochondrial Events Are Attenuated in Both Bid-deficient and Apaf-1-deficient Jurkat Cells
Several studies have concluded that MOMP is tightly regulated by pro- and anti-apoptotic members of the Bcl-2 family of proteins (39). It is widely accepted that Bax or Bak is required to promote MOMP (40), where activation coincides with their homo-oligomerization and pore formation spanning the outer mitochondrial membrane. It is through this pore that cytochrome c and other pro-apoptotic proteins are released from the intermembrane space of the mitochondria into the cytosol. Although it is widely accepted that the activation of Bak and Bax depends on the prior activation of a BH3-only Bcl-2 family member, the precise mechanism of Bax and Bak activation during mitochondria-mediated apoptosis remains poorly understood (41). It was, however, reported previously that caspase-2-mediated Bid cleavage to tBid is important for MOMP during heat-induced apoptosis (12).
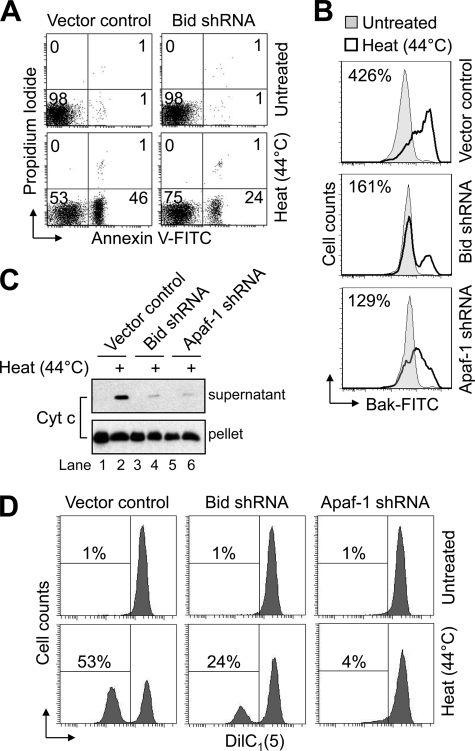
To investigate a potential role for Bid in our system, we evaluated the sensitivity of Bid-deficient Jurkat cells, which were described previously and shown to be highly resistant to receptor-mediated apoptosis due to their type II origin (20), to undergo heat-induced apoptosis. As illustrated in Fig. 6A, flow cytometric analysis showed that Bid-deficient cells were ∼50% resistant to heat-induced apoptosis as compared with control-transfected cells. Next, because Bid-deficient Jurkat cells were partially resistant and Apaf-1-deficient Jurkat cells were completely resistant to heat-induced apoptosis, mitochondrial events that characteristically define MOMP, such as Bak and/or Bax activation, release of intermembrane space proteins, and loss of ΔΨ, were evaluated. Previously, we have reported that Jurkat (E6.1) cells express Bak, but not Bax (20, 22). Bak activation was evaluated using an active conformation-specific monoclonal Bak antibody and flow cytometric analysis where activation causes a shift to the right of the resulting histogram. As illustrated in Fig. 6B, Bak activation was markedly decreased in both Apaf-1-deficient and Bid-deficient cells. Furthermore, consistent with the inhibition of Bak activation in cells lacking either Bid or Apaf-1, the release of cytochrome c in response to hyperthermia was also markedly decreased in both cell lines (Fig. 6C). Finally, as illustrated in Fig. 6D, the loss of ΔΨ was also decreased in the Apaf-1-deficient and Bid-deficient cells. Combined, these data suggest that Bid is important for Bak-mediated MOMP induced by hyperthermia. Our findings further suggest that events residing downstream of Apaf-1 play a vital role in amplifying initial heat-induced MOMP.
FIGURE 6.
Mitochondrial events induced by hyperthermia are attenuated in both Bid-deficient and Apaf-1-deficient Jurkat cells. A, control-transfected and Bid-deficient Jurkat cells (106/ml) were left untreated or heated for 1 h at 44 °C followed by a 6-h incubation time and processed for cell death determination by flow cytometric analysis of annexin V-FITC and propidium iodide staining. In A, quadrants are defined as live (lower left), early apoptotic (lower right), late apoptotic (upper right), and necrotic (upper left). Numbers refer to the percentage of cells in each quadrant. B, control-transfected, Bid-deficient, and Apaf-1-deficient cells (106/ml) were untreated or heated for 1 h at 44 °C followed by 6 h of incubation at 37 °C and processed for determination of Bak activation by flow cytometric analysis. Numbers in B refer to the percentage of increase in Bak-associated fluorescence between untreated and heated cells. C and D, duplicate aliquots of cells in B were harvested and processed for subcellular fractionation (C) or ΔΨ determination by flow cytometry (D). In C, supernatant and pellet fractions were analyzed by Western blotting. Cyt c, cytochrome c. In D, reduced DiIC1(5) fluorescence is indicative of a loss of ΔΨ, and numbers refer to the percentage of cells that underwent a dissipation of ΔΨ.
Cleavage of Bid Is a Caspase-mediated Event That Occurs Downstream of Apaf-1
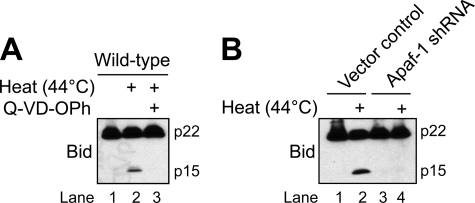
Previously, we reported that effector caspases cleaved Bid to tBid during etoposide-induced apoptosis and that tBid functioned as part of a feed-forward amplification loop to induce Bak oligomerization and cytochrome c release (20). In light of those observations, we next set out to determine whether heat-induced cleavage of Bid to tBid was a caspase-mediated event. To do so, wild-type Jurkat cells were incubated for 1 h with Q-VD-OPh (20 μm) prior to exposure to 44 °C for 1 h followed by a 6-h incubation at 37 °C. As illustrated in Fig. 7A (lane 3 versus lane 2), the inclusion of Q-VD-OPh completely inhibited the heat-induced generation of tBid. To extend these findings and to determine whether Bid was cleaved upstream or downstream of apoptosome formation, we evaluated the extent of heat-induced Bid cleavage in the Apaf-1-deficient cell line. As illustrated in Fig. 7B (lanes 4 and 3 versus lanes 2 and 1), cleavage of Bid to tBid did not occur in Apaf-1-deficient cells when exposed to 44 °C for 1 h followed by an incubation at 37 °C for 6 h. These data suggest that the cleavage of Bid to tBid is a caspase-mediated event that occurs downstream of apoptosome-dependent caspase activation during heat-induced apoptosis.
FIGURE 7.
Heat-induced cleavage of Bid to tBid is a caspase-mediated event that occurs downstream of Apaf-1. A, wild-type cells (106/ml) were incubated in the presence or absence of 20 μm Q-VD-OPh for 1 h at 37 °C and subsequently left untreated or heated at 44 °C for 1 h followed by a 6-h incubation at 37 °C. B, control-transfected and Apaf-1-deficient cells (106/ml) were left untreated or heated for 1 h at 44 °C followed by 6 h of incubation at 37 °C. Following the 6-h incubation at 37 °C (A and B), cells were harvested and lysed for Western blot analysis.
Concluding Remarks
Previous studies characterizing the molecular events necessary for heat-induced apoptosis have reported findings that, in some cases, are difficult to reconcile (12–17). In one study, caspase-2 was suggested to be the apical caspase activated in response to hyperthermia, in part because its activation was reported to occur even in Bcl-2- or Bcl-xL-overexpressing cells and because caspase-2-deficient mouse splenocytes were partially resistant to heat-induced apoptosis (17). In the same study, however, RAIDD-deficient mouse splenocytes, which did not activate caspase-2, were only marginally resistant to hyperthermia (17). A different study that was published around the same time reported that caspase-2-deficient mouse embryonic fibroblasts were sensitive to heat-induced apoptosis and, instead, suggested that an unknown Z-VAD-inhibitable protease was the apical protease activated in response to hyperthermia (13). The authors also suggested that the apoptosome was not necessary for heat-induced apoptosis, although the case was made that MOMP may nevertheless be necessary to antagonize inhibitor of apoptosis proteins via Smac release (13).
Although it is known that MOMP is required during intrinsic apoptosis and that this process is mediated by active Bak or Bax, the mechanisms by which Bak and Bax are activated during intrinsic apoptosis are only partially understood (41). By comparison, during extrinsic apoptosis in type II cells, caspase-8-mediated cleavage of the BH3-only protein Bid to its active form, tBid, is required for the activation of Bax and Bak (33, 42). A similar emergent role for any one of the BH3-only family members during true intrinsic apoptotic signaling has not been established. Nevertheless, the prevailing view is that BH3-only proteins are essential for Bax and Bak activation during mitochondria-mediated apoptosis. A possible exception to the rule is during heat-induced apoptosis, where it was reported that Bax and Bak can be activated by heat in vitro (14). However, because the authors reported that Bcl-xL could inhibit heat-induced Bax activation, the point was made that activation of Bax and Bak in intact cells may still require a BH3-only protein to antagonize the anti-apoptotic Bcl-2 family members (14). Along these lines, it has also been reported that the ability of caspase-2 to induce MOMP during heat-induced apoptosis requires the presence and cleavage of Bid (12), in part because Bid-deficient mouse embryonic fibroblasts were partially resistant to heat-induced apoptosis.
In agreement with previously published findings, our data support an essential role for caspases and the mitochondrial pathway during heat-induced apoptosis. Using an affinity labeling approach, we identified caspase-2, caspase-8, and caspase-9 as caspases that are activated early in response to hyperthermia. In addition, our data would seem to support the existence of a poorly characterized Z-VAD-inhibitable protease that can partially cleave caspase-9 to its catalytically inactive p37 fragment in response to hyperthermia as reported previously (13). Our findings also demonstrate that only the activation of initiator caspase-9 is essential for the execution of heat-induced apoptosis, whereas caspase-2 and caspase-8 more likely serve a supporting role during this form of cell death. We also found that the majority of MOMP and cytochrome c release occurs downstream of initial caspase-9 activation and is mediated to a large extent by tBid. Thus, although our data support a role for Bid during heat-induced apoptosis, most, if not all, cleavage of Bid occurs downstream of MOMP. In general, the data involving Bid support an emerging model of mitochondria-mediated apoptosis where the activation of caspases downstream of caspase-9 is necessary to feed forward and amplify the initial apoptotic signal to ensure cell death. In such a scenario, the earliest release of cytochrome c into the cytosol would remain sublethal unless or until it accumulated to an extent that is sufficient to promote sustained apoptosome-mediated activation of caspase-9.
Acknowledgments
We thank Dr. Joyce Slusser for invaluable assistance with flow cytometry, the late Dr. Stanley Korsmeyer for the pSFFV-neo, pSFFV-Bcl-2, and pSFFV-Bcl-xL mammalian expression vectors, and Drs. Shawn Bratton and Wen-Xing Ding for helpful discussion. The Flow Cytometry Core is supported in part by National Institutes of Health Grant P20 RR016443 from the National Center for Research Resources (NCRR).
This work was supported, in whole or in part, by National Institutes of Health Grants K22 ES011647 (to J. D. R.), P20 RR016475 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the NCRR, and T32 ES007079.
- tBid
- truncated Bid
- MOMP
- mitochondrial outer membrane permeabilization
- Apaf-1
- apoptotic protease activating factor-1
- Q-VD-OPh
- quinoline-Val-Asp-CH2-difluorophenoxy
- RAIDD
- receptor-interacting protein (RIP)-associated ICH-1/CED-3 homologous protein with a death domain
- BH3
- Bcl-2 homology 3
- ΔΨ
- mitochondrial membrane potential
- b-VAD-fmk
- biotin-Val-Ala-Asp(O-methyl)-CH2F
- Z-VAD
- benzyloxycarbonyl-Val-Ala-Asp.
REFERENCES
- 1.Parsell D. A., Taulien J., Lindquist S. (1993) Philos. Trans. R. Soc. Lond. B Biol. Sci. 339, 279–285; discussion 285–286 [DOI] [PubMed] [Google Scholar]
- 2.Mosser D. D., Morimoto R. I. (2004) Oncogene 23, 2907–2918 [DOI] [PubMed] [Google Scholar]
- 3.Milleron R. S., Bratton S. B. (2007) Cell Mol. Life Sci. 64, 2329–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kouloulias V., Plataniotis G., Kouvaris J., Dardoufas C., Gennatas C., Uzunoglu N., Papavasiliou C., Vlahos L. (2005) Am. J. Clin. Oncol. 28, 91–99 [DOI] [PubMed] [Google Scholar]
- 5.Wust P., Hildebrandt B., Sreenivasa G., Rau B., Gellermann J., Riess H., Felix R., Schlag P. M. (2002) Lancet Oncol. 3, 487–497 [DOI] [PubMed] [Google Scholar]
- 6.Ito A., Honda H., Kobayashi T. (2006) Cancer Immunol. Immunother. 55, 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pissuwan D., Valenzuela S. M., Cortie M. B. (2006) Trends Biotechnol. 24, 62–67 [DOI] [PubMed] [Google Scholar]
- 8.Boatright K. M., Salvesen G. S. (2003) Curr. Opin. Cell Biol. 15, 725–731 [DOI] [PubMed] [Google Scholar]
- 9.Kischkel F. C., Hellbardt S., Behrmann I., Germer M., Pawlita M., Krammer P. H., Peter M. E. (1995) EMBO J. 14, 5579–5588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnhart B. C., Alappat E. C., Peter M. E. (2003) Semin. Immunol. 15, 185–193 [DOI] [PubMed] [Google Scholar]
- 11.Jiang X., Wang X. (2004) Annu. Rev. Biochem. 73, 87–106 [DOI] [PubMed] [Google Scholar]
- 12.Bonzon C., Bouchier-Hayes L., Pagliari L. J., Green D. R., Newmeyer D. D. (2006) Mol. Biol. Cell 17, 2150–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milleron R. S., Bratton S. B. (2006) J. Biol. Chem. 281, 16991–17000 [DOI] [PubMed] [Google Scholar]
- 14.Pagliari L. J., Kuwana T., Bonzon C., Newmeyer D. D., Tu S., Beere H. M., Green D. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17975–17980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cippitelli M., Fionda C., Di Bona D., Piccoli M., Frati L., Santoni A. (2005) J. Immunol. 174, 223–232 [DOI] [PubMed] [Google Scholar]
- 16.Stankiewicz A. R., Livingstone A. M., Mohseni N., Mosser D. D. (2009) Cell Death Differ. 16, 638–647 [DOI] [PubMed] [Google Scholar]
- 17.Tu S., McStay G. P., Boucher L. M., Mak T., Beere H. M., Green D. R. (2006) Nat. Cell Biol. 8, 72–77 [DOI] [PubMed] [Google Scholar]
- 18.Franklin E. E., Robertson J. D. (2007) Biochem. J. 405, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi M., Vivian C. J., Lee K. J., Ge C., Morotomi-Yano K., Manzl C., Bock F., Sato S., Tomomori-Sato C., Zhu R., Haug J. S., Swanson S. K., Washburn M. P., Chen D. J., Chen B. P., Villunger A., Florens L., Du C. (2009) Cell 136, 508–520 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Shelton S. N., Shawgo M. E., Robertson J. D. (2009) J. Biol. Chem. 284, 11247–11255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bouchier-Hayes L., Oberst A., McStay G. P., Connell S., Tait S. W., Dillon C. P., Flanagan J. M., Beere H. M., Green D. R. (2009) Mol. Cell 35, 830–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shawgo M. E., Shelton S. N., Robertson J. D. (2008) J. Biol. Chem. 283, 35532–35538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bao Q., Shi Y. (2007) Cell Death Differ. 14, 56–65 [DOI] [PubMed] [Google Scholar]
- 24.Oberst A., Pop C., Tremblay A. G., Blais V., Denault J. B., Salvesen G. S., Green D. R. (2010) J. Biol. Chem. 285, 16632–16642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McStay G. P., Salvesen G. S., Green D. R. (2008) Cell Death Differ. 15, 322–331 [DOI] [PubMed] [Google Scholar]
- 26.Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I. M., Ricci J. E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. (2003) Mol. Cell 11, 529–541 [DOI] [PubMed] [Google Scholar]
- 27.Juo P., Kuo C. J., Yuan J., Blenis J. (1998) Curr. Biol. 8, 1001–1008 [DOI] [PubMed] [Google Scholar]
- 28.Malladi S., Challa-Malladi M., Fearnhead H. O., Bratton S. B. (2009) EMBO J. 28, 1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleh A., Srinivasula S. M., Acharya S., Fishel R., Alnemri E. S. (1999) J. Biol. Chem. 274, 17941–17945 [DOI] [PubMed] [Google Scholar]
- 30.Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. (1997) Cell 91, 479–489 [DOI] [PubMed] [Google Scholar]
- 31.Shawgo M. E., Shelton S. N., Robertson J. D. (2009) J. Biol. Chem. 284, 33447–33455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassus P., Opitz-Araya X., Lazebnik Y. (2002) Science 297, 1352–1354 [DOI] [PubMed] [Google Scholar]
- 33.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. (1998) Cell 94, 481–490 [DOI] [PubMed] [Google Scholar]
- 34.Duan H., Dixit V. M. (1997) Nature 385, 86–89 [DOI] [PubMed] [Google Scholar]
- 35.Park H. H., Logette E., Raunser S., Cuenin S., Walz T., Tschopp J., Wu H. (2007) Cell 128, 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinel A., Tschopp J. (2004) Science 304, 843–846 [DOI] [PubMed] [Google Scholar]
- 37.Srinivasula S. M., Ahmad M., Fernandes-Alnemri T., Alnemri E. S. (1998) Mol Cell 1, 949–957 [DOI] [PubMed] [Google Scholar]
- 38.Denault J. B., Eckelman B. P., Shin H., Pop C., Salvesen G. S. (2007) Biochem. J. 405, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 40.Lindsten T., Ross A. J., King A., Zong W. X., Rathmell J. C., Shiels H. A., Ulrich E., Waymire K. G., Mahar P., Frauwirth K., Chen Y., Wei M., Eng V. M., Adelman D. M., Simon M. C., Ma A., Golden J. A., Evan G., Korsmeyer S. J., MacGregor G. R., Thompson C. B. (2000) Mol. Cell 6, 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H., Zhu H., Xu C. J., Yuan J. (1998) Cell 94, 491–501 [DOI] [PubMed] [Google Scholar]