Abstract
The triterpenoid 2-Cyano-3,12-dioxooleana-1,9-dien-28-oic-acid (CDDO) and its methyl ester (CDDO-Me) are undergoing clinical trials in cancer and leukemia therapy. Here we report that CDDO-Me ameliorates diabetes in high fat diet-fed type 2 diabetic mice and in Leprdb/db mice. CDDO-Me reduces proinflammatory cytokine expression in these animals. Oral CDDO-Me administration reduces total body fat, plasma triglyceride, and free fatty acid levels. It also improves glucose tolerance and insulin tolerance tests. Its potent glucose-lowering activity results from enhanced insulin action. Hyperinsulinemic-euglycemic clamp reveals an increased glucose infusion rate required to maintain euglycemia and showed a significant increase in muscle-specific insulin-stimulated glucose uptake (71% soleus, 58% gastrocnemius) and peripheral glucose clearance as documented by a 48% increase in glucose disposal rate. CDDO-Me activates AMP-activated protein kinase (AMPK) and via LKB1 activation in muscle and liver in vivo. Treatment of isolated hepatocytes with CDDO-Me directly stimulates AMPK activity and LKB1 phosphorylation and decreases acetyl-coA carboxylase activity; it also down-regulates lipogenic gene expression, suppresses gluconeogenesis, and increases glucose uptake. Inhibition of AMPK phosphorylation using compound C and lentiviral-mediated knockdown of AMPK completely blocks the CDDO-Me-induced effect on hepatocytes as well as C2C12 cells. We conclude that the triterpenoid CDDO-Me has potent anti-diabetic action in diabetic mouse models that is mediated at least in part through AMPK activation. The in vivo anti-diabetogenic effects occur at a dose substantially lower than that used for anti-leukemia therapy. We suggest that CDDO-Me holds promise as a potential anti-diabetic agent.
Keywords: AMP-activated Kinase (AMPK), Diabetes, Drug Action, Gluconeogenesis, Insulin Resistance, Anti-cancer Anti-diabetic Drug
Introduction
Triterpenoids, together with their close relatives, the steroids, are members of the cyclosqualenoid family (1). The semisynthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO)2 is structurally related to the pentacyclic triterpenoids oleanolic and ursolic acids. Studies with CDDO have revealed potent cellular differentiating, antiproliferative, proapoptotic (2), anti-inflammatory, and anticarcinogenic activities (3–5). This triterpenoid has been shown to induce monocytic differentiation of human myeloid leukemia cells, adipogenic differentiation of mouse 3T3-L1 fibroblasts, and nerve growth factor-induced neuronal differentiation of rat PC12 cells (6). CDDO enhanced the differentiation of acute promyelocytic leukemia cells in vitro and induced differentiation of all -trans retinoic acid-resistant acute promyelocytic leukemia cells (7). The mechanism responsible for the differentiating action of CDDO was in part associated with activation of CEBP-β (8). Micromolar concentrations of CDDO have been observed to induce apoptosis in different cancer cell lines (9–11). CDDO inhibited the growth of several ovarian cancer cell lines that express peroxisome proliferator-activated receptor γ, but co-treatment with the peroxisome proliferator-activated receptor γ antagonist T007 did not block the apoptogenic effects of CDDO, suggesting a peroxisome proliferator-activated receptor γ-independent action (12, 13).
The C-28 methyl ester of CDDO, CDDO-Me, has been shown to decrease the viability of leukemic cell lines, including multidrug resistance 1-overexpressing cells (14). It has been suggested that the combination of antitumorigenic, antiangiogenic, and proapoptotic effects and the ability of CDDO-Me to suppress cyclooxygenase 2 (COX-2), inducible nitric-oxide synthase, multidrug resistance gene 1, and FLIP is mediated by NF-κB activation through suppression of IκBα kinase (15). CDDO and CDDO-Me have shown differentiating effects in a clinical phase I study in acute myeloblastic leukemic patients and anti-tumor effects in solid tumors, alone and in combination with chemotherapy (8, 16). The experimental drugs appear to have little toxic side effects at the doses used. We hypothesized that CDDO-Me may have beneficial action in diabetes and investigated its potential anti-diabetic effects and possible mode of action in mouse models of type 2 diabetes.
EXPERIMENTAL PROCEDURES
Drug
CDDO-Me (supplemental Fig. S1A) was kindly provided by Dr. Edward Sausville (National Cancer Institute) under the Rapid Access to Interventional Development program and by Dr. Michael Sporn (Dartmouth Medical College). CDDO-Me was formulated in liposomes at a concentration of 2 mg/ml as follows. First, CDDO-Me was solubilized in t-butanol at 37 °C at a concentration of 2 mg/ml. Phospholipid distearoyl phosphatidyl choline was solubilized in t-butanol at 55 °C at a concentration of 10 mg/ml. Phospholipid distearoyl phosphatidyl choline and CDDO-Me were then mixed together and frozen by being placed at an angle in an acetone and dry ice bath and then quickly turned until the samples were frozen. The samples were then freeze-dried overnight. The lipid:drug ratio was 20:1. Empty liposomal controls were made using the same lipids without CDDO-Me. Liposomal CDDO was reconstituted in normal saline at 55 °C to form liposomes and then centrifuged at 13,000 rpm for 1 h. Pellets were resuspended at room temperature in normal saline at a concentration of 2 mg/ml (100 μm) for the in vivo studies.
Animals
Male C57BL/6J mice weighing 25–32 gm were used unless otherwise mentioned. Oral gavage was used to administer 0.25 ml of CDDO-Me dissolved in sesame oil (3 mg/kg of body weight) or sesame oil alone.
Plasma Profile and Lipids
Following manufacturer protocol, different enzymatic assay kits were used for the determination of plasma FFA (Wako), glycerol, glucose, total TG (Sigma), and insulin (Mercodia). EchoMRI was used for body fat quantification in live mice.
Glucose Tolerance Test (GTT) and Insulin Tolerance Test (ITT)
For intraperitoneal GTT, we injected 2.0 g of glucose/kg of body weight after a 6-h fast as described (17). For ITT, an intraperitoneal injection of regular insulin (Humulin R; 1.5–5 units/kg of body weight) was administered after a 4-h fast. Blood glucose levels were measured using a glucometer (Life Scan).
Euglycemic Hyperinsulinemic Clamp
The studies were performed in unrestrained mice using the insulin clamp technique (10 milliunits/kg of body weight) in combination with high pressure liquid chromatography purified [3-3H]glucose and [14C]2-deoxyglucose as described previously (18, 19). Overnight fasted conscious mice received a priming dose of HPLC-purified [3-3H]glucose (10 μCi) and then a constant infusion (0.1 μCi/min) of label glucose for ∼3.5 h. Blood samples were collected from the tail vein at 0, 50, 55, and 60 min to measure the basal glucose production rate. After 1 h of infusion, the mice were primed with regular insulin (bolus 40 milliunits/kg of body weight) followed by a 2-h constant insulin infusion (10 milliunits/kg/min). Using a separate pump, 25% glucose was used to maintain the blood glucose level at 100–140 mg/dl, as determined every 10 min using a glucometer (LifeScan). Hepatic glucose production under clamp condition, peripheral glucose disposal rates, and glucose infusion rate were then measured from collected plasma. At the end of the clamp, we sacrificed the mice and snap froze the soleus muscle, gastrocnemius, adipose tissue, and other tissue as required in liquid nitrogen. Glucose uptake in different tissue was calculated from plasma 2-[14C]deoxyglucose profile fitted with double exponential curve and tissue content of 2-[14C]deoxyglucose-6-phosphate.
Tyrosine Phosphorylation of Insulin Receptor (IR), IRS-1, and IRS-2
Muscle samples were snap frozen in liquid nitrogen and homogenized using polytron in ice-cold buffer containing 50 mm HEPES, pH 7.5, 150 mm NaCl, 10 mm sodium pyrophosphate, 2 mm Na3VO4, 1 mm MgCl2, 1 mm CaCl2, 10 mm NaF, 2 mm EDTA, 2 mm phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, 1% Nonidet P-40, and 10% glycerol. Solubilized proteins were then immunoprecipitated with IR, IRS-1, or IRS-2 antibody and resolved by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. The membranes were then blotted with phosphotyrosine antibody (4G10 clone) to check for tyrosine phosphorylation of IR, IRS-1, and IRS-2.
Measurement of AMPK Activity, ACC Activity, Gluconeogenesis, and Palmitate Oxidation in Primary Hepatocytes and Glucose Uptake in Differentiated C2C12 Cells
Mouse hepatocytes were isolated by collagenase digestion (20, 21). For the AMPK assay, the cells were seeded in six-well plates at 1.5 × 106 cells/well in DMEM containing 100 units/ml penicillin, 100 μg/ml streptomycin, 10% FBS, 100 nm insulin, 100 nm dexamethasone, and 5 μg/ml transferrin for 4 h. The cells were then cultured in serum-free DMEM for 16 h followed by treatment for 3 or 7 h with control medium, 5-amino-imidazole carboxamide riboside (AICAR), or CDDO-Me at the indicated concentrations. After treatment, the cells were directly lysed in digitonin and phosphatase inhibitor-containing buffer A (22) followed by precipitation with ammonium sulfate at 35% saturation. AMPK activity was determined using a synthetic peptide substrate specific for AMP-activated protein kinase (SAMS) as substrate following the method published by Davies et al. (23). ACC activity was determined using 35% ammonium sulfate precipitate from digitonin-lysed hepatocytes (4 μg each) and the 14CO2 fixation method in the presence of 20 mm citrate as previously described (22). Glucose production, palmitate oxidation, and glucose uptake were measured following the standard method as described previously (24, 25).
Quantification of mRNA
For hepatocytes, we isolate RNA using RNeasy mini-kit (Qiagen) and treated all of the samples (10 μg) with RNase-free DNase-I before using Omniscript RT kit (Qiagen). For tissues, we extracted total RNA using guanidine thiocyanate (TRIzol; Invitrogen) and then treated the RNA with DNase-1 before amplification using Omniscript RT kit (Qiagen). Two μl of the RT products were used to do quantitative PCR using iQ-SYBR Green Supermix (Bio-Rad) under MX3000P system (Stratagene).
shRNA-mediated Knockdown of AMPK
We knocked down the AMPKα2 catalytic subunit by RNA interference using a vector-based small hairpin approach. We bought three lentiviral constructs in pGIPZ that contain a specific AMPKa2 shRNA region (accession numbers V2LMM_65055, V2LMM_62911, and V2LMM_77170) from Open Biosystems. Then we packaged and produced lentivirus against these constructs and infected C2C12 cells to knock down AMPKα2. Internal GFPs were used for initial screening of positive transduction.
Immunoblot Analysis
For phospho-AMPK (Thr-172) and phospho-LKB1 detection, 35% ammonium sulfate precipitate from treated mouse hepatocytes or liver and muscle homogenate was used for Western blot analysis using polyclonal antibodies to phospho-AMPKα (Thr-172; Cell Signaling Technology, Beverly, MA) or phospho-LKB1 (S428; Cell Signaling Technology).
Immunoprecipitation
We incubated tissue homogenate or hepatocyte lysate (0.5–0.6 ml) with 1.0 μl of antibody against either AMPK or LKB1 in a rotator at 4 °C overnight. Protein G/A-Sepharose (25 μl; Amersham) was added, and rotational incubation was continued for an additional 4 h. We collected the immunoprecipitated complex by centrifugation in an Eppendorf Micro 5415C centrifuge at 10,000 rpm for 2 min and aspirated the supernatant. The Sepharose beads were washed several times with ice-cold immunoprecipitation buffer containing protease and phosphatase inhibitors. The samples were kept at 4 °C during the washing and centrifugation steps. The Sepharose beads were then boiled directly with SDS gel loading buffer.
Statistical Analysis
Student's t test was used for statistical analysis. Differences were considered significant when p < 0.05.
RESULTS
CDDO-Me Reduces Body Fat, without Affecting Total Body Weight or Food Intake, and Reverses Diabetes in High Fat Diet-induced Type 2 Diabetic Mice
C57BL/6 mice (6–7 weeks old) were fed a high fat diet (40% calories from fat; Test Diet, Richmond, IN) for 12–16 weeks to induce obesity and T2DM and then treated with CDDO-Me (in sesame oil) or vehicle (sesame oil alone) by gavage for 2 weeks.
The T2DM mice treated with CDDO-Me or vehicle had similar food intake but significantly reduce total body fat content and increased lean body mass (supplemental Fig. S1, B–E). Fasting plasma triglyceride and free fatty acid levels were lower in CDDO-Me-treated as compared with vehicle-treated mice (Fig. 1, A and B). CDDO-Me-treated mice also displayed lower fasting blood glucose and plasma insulin levels (Fig. 1, C and D). Similarly, in diet-induced diabetic mice, the total lipid contents were significantly reduced in muscle and liver; the TG concentration is lower in these organs, but the difference is significant only in muscle and not in liver. The tissue FFA level tends to be lower in liver and muscle (Fig. 1, E–G). Histological examination revealed substantial reduction in lipid accumulation in the liver, although the changes may be patchy, and markedly reduced adipocyte size in white adipose tissue (WAT, epididymal fat) (Fig. 1H). Except for visceral fat, which showed a 50% reduction in weight, we observed no major changes in the weight of other organs after treatment (liver, spleen, kidney, and brown adipose tissue; supplemental Fig. S1F). Treatment led to a ∼10% increase in oxygen consumption (VO2, by indirect calorimetry) in CDDO-Me-treated mice compared with oil (vehicle)-treated control mice (supplemental Fig. S1G).
FIGURE 1.
CDDO-Me treatment lowers plasma TG, FFA, blood glucose, and plasma insulin levels. A–D, after 2 weeks of CDDO-Me treatment, Western diet-fed mice showed significant reduction in plasma TG level (A), plasma FFA level (B), random-fed blood glucose (C), and insulin levels (D). E–G, after 2 weeks of CDDO-Me treatment, liver and muscle tissues were extracted for total lipid (E), TG (F), and FFA (G). H, histology of liver and WAT. The liver section was stained with Oil Red O, whereas WAT was stained with hematoxylin and eosin. Shown is the number of cells presented in WAT from three different slides. Solid bar, CDDO-Me; empty bar, vehicle. The results are expressed as the means ± S.D. (n = 5 mice/group). *, p < 0.05, control versus CDDO-Me.
CDDO-Me Improves Glucose Homeostasis and Insulin Sensitivity
Intraperitoneal GTT showed that CDDO-Me treatment led to a marked improvement in GTT. Plasma insulin and glucose concentrations during the intraperitoneal GTT were lower in CDDO-Me-treated mice (Fig. 2, A and B), indicating that the improvement in glucose tolerance in CDDO-Me-treated mice was likely the result of improved peripheral insulin sensitivity. As a first measure of insulin sensitivity, we performed an intraperitoneal ITT and found that CDDO-Me significantly enhanced the blood glucose-lowering effect of injected insulin (Fig. 2, C and D).
FIGURE 2.
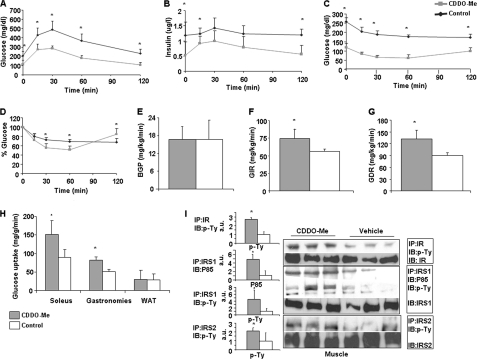
A and B, treatment improves glucose tolerance. Male, 22-week-old wild type mice fed with high fat for 16 weeks starting at 6 weeks of age showed significant improvement in intraperitoneal glucose (2 mg/kg) tolerance test (A) and glucose-stimulated insulin level during GTT (B). C and D, increased insulin sensitivity in the CDDO-Me-treated groups. C, insulin tolerance (1.5 units/kg, intraperitoneal) on Western diet-fed mice after 2 weeks of gavage treatment. Glucose level checked at 0, 15, 30, 60, and 120 min after insulin injection. D, represents the percentage blood glucose value. E–H, effect of CDDO-Me on glucose kinetics during hyperinsulinemic-euglycemic clamp. After 2 weeks of treatment, intravenous catheters were implanted surgically in the jugular vein of the mice, and the mice were allowed 4 days to recover. When the mice were able to access food and water freely, clamp studies were performed on these Western diet-fed mice using a continuous infusion of 10 milliunits/kg of body weight/min. E, basal glucose production rate (BGP) was not changed. F, glucose infusion rate (GIR) to maintain the euglycemic state was significantly higher in drug-treated mice. G, glucose disposal rate (GDR) was significantly increased. Solid bar, CDDO-Me; empty bar, control. H, glucose uptake was measured using 2-deoxy glucose under hyperinsulinemic-euglycemic clamp condition. Increased insulin-stimulated glucose transport activities were observed in isolated soleus and gastrocnemius muscles but not in epidymal fat tissue after CDDO-Me treatments in comparison with control mice. The data represent the means ± S.D. *, p < 0.05, control versus treated mice (n = 5 mice/group). I, Western blot of soleus muscle after clamp showed increased insulin signaling as detected by increased phosphotyrosine levels in CDDO-Me treated mice in comparison with vehicle-treated mice. Increased phosphorylation at tyrosine residue of IR, IRS1, IRS2, and p85 subunit of phosphatidylinositol 3-kinase, when immunoprecipitated with corresponding antibody in muscle sample after clamp, indicates insulin-stimulated glucose uptake by muscle. The corresponding bar graph shows quantification of the blot. The results are expressed as the means ± S.D. (n = 5 mice/group). *, p < 0.05, control versus CDDO-Me. IP, immunoprecipitation; IB, immunoblot.
We next performed hyperinsulinemic-euglycemic clamp to further assess the effect of CDDO-Me. The plasma insulin level after the clamp was similar between the two groups (CDDO-Me: 4.9 ± 0.95 and oil: 5.2 2 ± 0.7, μg/liter). Basal hepatic glucose production was similar in CDDO-Me and vehicle-treated mice (16.7 ± 4.2 mg/kg/min in CDDO-treated versus 16.8 ± 6.3 mg/kg/min in vehicle) (Fig. 2E). However, the CDDO-Me-treated group required a higher rate of exogenous glucose infusion (74.36 ± 14.5 mg/kg/min versus 56.48 ± 3.4 mg/kg/min; p = 0.04) to maintain euglycemia, indicating enhanced whole body sensitivity to the insulin infusion. This was accompanied by a 48% increase in the mean glucose disposal rate in this group (132.28 ± 20.92 mg/kg/min versus 89.4 ± 8.29 mg/kg/min; p = 0.006), reflecting an increased peripheral insulin responsiveness induced by CDDO-Me (Fig. 2, F and G). We estimated the rates of insulin-stimulated glucose transport activity in individual tissues by measuring 2-deoxy-d-[1-14C]glucose uptake during the clamp and determined the tissue content of 2-deoxyglucose-6-phosphate, because 2-deoxyglucose is phosphorylated but not further metabolized by the cell. We found that CDDO-Me treatment increased insulin-stimulated glucose transport activity in skeletal muscles, by 71% in the soleus (88 ± 22 versus 151 ± 35 mg/g/min; p < 0.015) and by 58% in the gastrocnemius (51.8 ± 5.21 versus 82.2 ± 8.7 mg/g/min; p < 0.002). In contrast, the insulin-stimulated glucose transport activity in WAT was not changed by CDDO-Me (Fig. 2H). To obtain corroborative evidence of increased insulin sensitivity in muscle at the biochemical level, we studied insulin signaling by analyzing the tyrosine phosphorylation of IR, IRS1, and IRS2 and the abundance of active subunit of phosphatidylinositol 3-kinase, p85. Western blot analysis revealed increased phosphorylation of all of these molecules in the muscles of treated mice (Fig. 2I), further supporting the capacity of CDDO-Me to up-regulate insulin signaling in skeletal muscle.
CDDO-Me Decreases Proinflammatory Cytokine Gene Expression
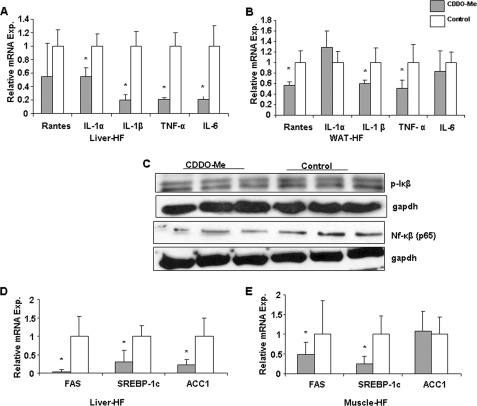
The expression of proinflammatory cytokines has been reported to be increased in mice with obesity and T2DM (26–28). By quantitative RT-PCR, we found that CDDO-Me treatment led to significant down-regulation of the hepatic mRNAs for the following cytokines: IL-1a (by 45%), IL-1b (by 80%), TNF-α (by 78%), and IL-6 (by 78%). Treatment also tended to reduce the RANTES mRNA level (Fig. 3A). In WAT, CDDO-Me treatment significantly lowered the mRNA levels for RANTES (by 32%), IL-1b (by 35%), and TNF-α (by 46%), whereas those for IL-1a and IL-6 remained unchanged (Fig. 3B). These CDDO-Me-induced improvements in cytokine expression in liver and WAT may have contributed to the observed improvement in insulin sensitivity (29, 30). Moreover, Western blot analysis of Iκβ (CDDO-Me: 1 ± 0.08 and control: 1.26 ± 0.12) and NFκβ (CDDO-Me: 1 ± 0.3 and control: 1.44 ± 0.2) in liver homogenate also suggests that CDDO-Me treatment decreased inflammation (Fig. 3C). On the other hand, complete blood counts were not altered by CDDO-Me treatment (supplemental Fig. S2).
FIGURE 3.
Effect of CDDO-Me on pro-inflammatory cytokine gene expression in vivo. mRNAs were extracted from WAT and liver tissue samples of mice treated with CDOO-Me and vehicle for 2 weeks. After DNase-1 digestion, expression of different cytokine genes was determined and compared as the relative mRNA level using real time RT-PCR. Solid bars, CDDO-Me; empty bars, vehicle. A and B, cytokine expression in liver tissue of Western diet-fed mice (A) and from WAT tissue (epidymal fat pad) (B). C, nuclear fraction of liver homogenate showed lower accumulation of NFκB (P65) after CDDO-Me treatment in comparison with oil (control) and reduced phosphorylation in IκB was also observed after drug treatment. D and E, CDDO-Me treatment reduced lipogenic gene expression in liver and muscle tissue of Western diet fed mice. Relative mRNA expression levels were quantified using real time RT-PCR and showed down-regulation for transcripts FAS, SREBP-1c, and ACC1 in liver tissue of treated mice (D) and FAS, SREBP, but not in ACC1 in muscle tissue (E). In each case, the data were normalized to the expression level of actin and are expressed as 2 −ΔCT, and then the relative mRNA expression level was calculated. The error bars represent the S.D. (n = 9, which is 3 mice in triplicate). Statistical analysis revealed that CDDO-Me significantly down-regulates most of the pro-inflammatory cytokine and lipogenic gene expression in comparison with vehicle control (p < 0.05).
CDDO-Me Down-regulates Hepatic Lipogenic Gene Expression in T2DM Mice
Treatment with CDDO-Me significantly reduced whole body fat content. Analysis of hepatic lipogenic gene expression showed that CDDO-Me treatment significantly reduced the levels of transcripts for fatty acid synthase (FAS), ACC-1, and the transcription factor sterol response element-binding protein (SREBP)-1c in the livers of these animals (Fig. 3D). Interestingly, CDDO-Me lowered FAS and SREBP-1c transcript levels but not ACC1 expression in skeletal muscle (Fig. 3E).
CDDO-Me Down-regulates the Expression of Lipogenic Genes in Hepatocytes in Vitro
The effect of CDDO-Me on glucose and lipid homeostasis in vivo could be a direct or indirect consequence of the compound because of possible downstream compensatory effects and/or interorgan cross-talk in response to the triterpenoid under in vivo conditions. To determine whether CDDO-Me has a direct action on the liver, we isolated primary hepatocytes from wild type mice and treated them with CDDO-Me (Fig. 4). Under these conditions, CDDO-Me again suppressed the transcripts for FAS (by ∼30%), ACC1 (∼50%), and SREBP-1c (∼50%) but did not affect the expression of HMGCoA-reductase (Fig. 4, A and B). Therefore, the effects of CDDO-Me on hepatic lipogenic gene expression are reproduced in isolated hepatocytes, indicating that they represent a direct effect of the triterpenoid on these cells.
FIGURE 4.
CDDO-Me down-regulates ACC activity in primary murine hepatocytes. Treatment of hepatocytes with CDDO-Me suppresses lipogenic genes in primary murine hepatocytes. A and B, Cyber green-based real time RT-PCR was used to measure relative mRNAs level of FAS, SREBP-1c, and HMGR (A) and ACC1 (B). In each case, the data were normalized to the expression (Exp.) level of actin and are expressed as 2−ΔCT, and then the relative mRNA expression level was calculated. Deactivation of ACC (dephosphorylation) and ACC protein level were checked by Western blot using anti-phospho-ACC and anti-ACC antibody in hepatocytes (C) and liver samples (D). E, CDDO-Me (solid bar, 100 nm) and AICAR (hatched bar, 250 μm) significantly inactivate ACC activity in mouse primary hepatocytes in comparison with controls (empty bars) when treated for 7 h. F, palmitate oxidation in hepatocytes plated in 60-mm tissue culture plates. 14CO2 were trapped using hyamine hydroxide soaked blotting paper in each cover. The amount of CO2 produced was then normalized using total protein and expressed as a percentage of production of 14CO2 (fold over basal). The corresponding bar graph shows the quantification of the blot. All of the values are shown as the means ± S.D. *, p < 0.05, versus control.
CDDO-Me Lowers ACC Protein Level and Inhibits ACC Activity in Primary Mouse Hepatocytes
At the protein level, we proved by Western blotting that CDDO-Me treatment led to a reduction in total immunoreactive ACC in both isolated hepatocytes and total liver extracts (Fig. 4, C and D, right panels). Furthermore, the proportion of phosphorylated ACC (p-ACC; Fig. 4, C and D, left panels) was also increased.
To determine whether the CDDO-Me effects on ACC transcript and protein levels are also reflected by changes in enzyme activity, we measured ACC enzyme activity in isolated hepatocytes and found that CDDO-Me suppressed ACC activity by ∼70% compared with vehicle-treated hepatocytes (Fig. 4E). As a positive control, AICAR, a known AMPK activator (31, 32), was found to potently inactivate ACC activity in liver cells.
Effect of CDDO-Me on Palmitate Oxidation
To confirm our initial findings that CDDO-Me treatment significantly reduced liver fat in T2D model, we measured the rate of palmitate oxidation in hepatocytes with and without CDDO-Me. Compared with vehicle, 1 h of treatment with CDDO-Me increased CO2 production by ∼50%. Moreover, the addition of Comp C completely blocked the effect of CDDO-Me (Fig. 4F).
CDDO-Me Stimulates AMPK Activity in Isolated Hepatocytes
To determine whether AMPK mediates CDDO-Me effects in the liver, we measured AMPK activity in isolated hepatocytes in the presence and absence of CDDO-Me using AICAR as a positive control and found that the addition of CDDO-Me led to significant dose-dependent activation of AMPK. At 250 nm CDDO-Me, there was a ∼7-fold activation compared with control, whereas AICAR produced a ∼9-fold activation at a concentration of 500 μm (Fig. 5A).
FIGURE 5.
CDDO-Me mediates AMPK activation in primary hepatocytes. All of the these experiments were repeated a minimum of three times with similar results. A, control (empty bar), AICAR (hatched bars, 500 μm), and CDDO-Me (solid bar; 10, 100, and 250 nm) significantly activate AMPK in mouse primary hepatocytes when treated for 3 h. The means ± S.D. values are shown. *, p < 0.05 versus control medium. B and C, Western blot of stimulated AMPK Thr-172 phosphorylation in primary hepatocyte (B) and differentiated C2C12 cells (C) incubated for 1 h either with CDDO-Me (250 nm) or AICAR (1000 μm) in comparison with controls (vehicle-treated). D, time course of CDDO-Me activation. CDDO-Me treatment activated AMPK phosphorylation as early as 15 min in hepatocytes. E, preincubation with inhibitor (Comp C) for 1 h followed by 3 h of incubation with CDDO-Me in hepatocytes completely blocked stimulation of AMPK Thr-172 phosphorylation in AICAR and CDDO-Me. F, like primary hepatocytes, differentiated C2C12 cells showed higher phosphorylation with CDDO-Me (250 nm) treatment than control. AICAR (500 μm) was used as a positive control and led to even higher phosphorylation. Compound C blocked AMPK activation.
Activation of AMPK involves the phosphorylation of Thr-172 in the activation domain of the α subunit of AMPK (31, 33). We investigated whether CDDO-Me affected the phosphorylation of AMPK Thr-172 and found that, like AICAR, CDDO-Me stimulated AMPK phosphorylation in hepatocytes as well as in differentiated C2C12 cells (Fig. 5, B and C). To determine the time course of AMPK activation, we incubated hepatocytes with timed exposure to CDDO-Me and found that CDDO-Me activated AMPK as early as 15 min after incubation; the p-AMPK increased further at 30 and 60 min (Fig. 5D), indicating the time dependence of CDDO-Me-induced AMPK activation. As an additional control, we preincubated hepatocytes or C2C12 cells with Comp C, a low molecular weight inhibitor of AMPK phosphorylation (34), and showed that it blocked CDDO-Me-stimulated Thr-172 phosphorylation (Fig. 5, E and F), further supporting the conclusion that CDDO-Me activates AMPK in hepatocytes as well as in C2C12 cells.
CDDO-Me Reduces Gluconeogenesis in Hepatocytes
We next examined the effect of CDDO-Me on glucose production in hepatocytes and found that it suppressed glucose production by ∼40%, an effect that was blocked by prior incubation with Comp C (Fig. 6A). Consistent with this effect of CDDO-Me is the fact that the mRNA level of two key gluconeogenic enzymes, glucose-6-phosphatase and PEPCK, was significantly down-regulated by the treatment (Fig. 6B).
FIGURE 6.
CDDO-ME effects on hepatocytes in vitro. Hepatocytes were incubated with either CDDO-Me, insulin, or pretreatment with Comp C and then incubated with CDDO-Me for 1 h. A, glucose productions were then assayed in the presence of gluconeogenic substances. After subtracting the glucose production (normalized with total protein) in the absence of gluconeogenic substances, the percentages of glucose production normalized against control were plotted. Insulin (100 nm) was used as a negative control. CDDO-Me suppressed gluconeogenesis and Comp C completely reverse the effect. B, relative mRNAs level of G6Pase and PEPCK measured by quantitative real time PCR from hepatocytes in the presence and absence of CDDO-Me. C, glucose uptake in differentiated C2C12 cells were measured using 2-deoxy glucose (14C) with and without CDDO-Me; insulin (100 nm) was used as a positive control. CDDO-Me increased glucose uptake in comparison with vehicle-treated cells, and Comp C effectively blocked the effect. C2C12 cells were transduced with lentiviral vector containing different shRNA against AMPKα2. The cells were grown in the presence of polybrene for a few days or until 70–80% of them are green. D, relative mRNA (normalized) levels of AMPKα2 were then determined by quantitative PCR. E and F, positively transduced cells were then differentiated and checked for AMPK (E) and glucose uptakes (F) in presence of CDDO-Me. Empty virus transduced cells and untransduced cells were used as a control. C1, C2, and C3 indicate different shRNA constructs. Ctr, control; N, normal; EV, empty virus; Ins, insulin.
CDDO-Me Stimulates Glucose Uptake by C2C12 Cells and Involvement of AMPK in This Action
To examine the action of CDDO-Me in differentiated C2C12 cells, we determined glucose uptake using 2-deoxy glucose (14C) (Fig. 6C). As a positive control, insulin was found to cause a 5-fold increase in uptake. CDDO-Me treatment caused a significant ∼3-fold increase in this parameter, an effect that was completely blocked by Comp C (Fig. 6C), suggesting the involvement of AMPK in this action.
To corroborate the observation that CDDO-Me action in C2C12 cells may be mediated by AMPK activation (Fig. 5), we examined the effect of shRNA-mediated knockdown of AMPK. We tested three different lentiviral-based vectors expressing shRNAs in C2C12 cells that targeted AMPKα2 mRNA, the level of which was monitored by real time quantitative PCR, and the protein level, which was determined by Western blotting (Fig. 6, D and E). Empty vector expressing enhanced GFP was used as a negative control. shRNA construct (C1) led to ∼65% knockdown of AMPK, whereas construct C3 had no effect. We examined the effect of CDDO-Me treatment on C2C12 cells transduced with C1 and C3, respectively, and found that transduction with C1-shRNA, but not C3-shRNA, blocked the CDDO-Me-mediated simulation of glucose uptake in these cells (Fig. 6F), supporting the involvement of AMPK in this action of CDDO-Me.
CDDO-Me Stimulates AMPK via LKB1 Activation
The tumor suppressor LKB1 is an AMPK kinase that activates AMPK by phosphorylating the latter at Thr-172. To determine whether LKB1 is involved in AMPK activation by CDDO-Me, we isolated mouse hepatocytes and treated them with CDDO-Me (250 nm) or vehicle. CDDO-Me was found to stimulate the phosphorylation of both AMPK-Thr-172 and LKB1 (Fig. 7, A and B). We next used an anti-LKB1 antibody to immunoprecipitate LKB1 in CDDO-Me-treated and vehicle-treated liver homogenates and examined for the presence of phosphorylated AMPK-Thr-172 in the immunoprecipitate by immunoblotting. The analysis showed that CDDO-Me up-regulated the amount of phospho-AMPK that is associated with immunoprecipitated LKB1 protein (Fig. 7C). The converse experiment of blotting of phospho-LKB1 in AMPK immunoprecipitated by an AMPK antibody also revealed an increased amount of phospho-LKB1 associated with the complex (Fig. 7D). Analogous results for the AMPK and LKB1 association were also obtained with AICAR, a known AMPK activator that works via LKB1 (data not shown). Taken together, these data suggest that AMPK activation may be mediated by LKB1 activation in hepatocytes.
FIGURE 7.
CDDO-Me activates AMPK in a LKB1-dependent manner in isolated primary murine hepatocytes. A and B, cell extract of hepatocytes treated with CDDO-Me (250 nm) and solvent (control) for 3 h and then immunoblotted with p-AMPK antibody (A) and p-LKB1 antibody (B) showed increased phosphorylation with CDDO-Me in comparison with control cells. AMPK and LKB1 antibodies were used as loading controls of the respective blots. C and D, immunoprecipitation (IP) of CDDO-Me and solvent-treated cell lysates with LKB1 antibody (C) and AMPK antibody (D) and immunoblot (IB) with p-AMPK and p-LKB1, respectively, showed increased association of LKB1 with AMPK and vice versa after drug treatment for 3 h. In both cases immunoblots with pulldown antibodies were used as loading controls. The corresponding bar graphs show quantification of the blots.
To further explore the relationship of LKB1 and AMPK activation under in vivo conditions, we examined the association of AMPK and LKB1 in liver and skeletal muscle of CDDO-Me-treated mice. Western blot analysis of liver and skeletal muscle homogenates showed higher activation of AMPK in CDDO-Me treated mice as compared with vehicle-treated animals (Fig. 8, A and B). In support of a key role of LKB1 in the observed AMPK activation, we also detected up-regulated phospho-LKB1 in CDDO-Me-treated animals as compared with vehicle controls (Fig. 8, C and D). To determine whether there is direct interaction between AMPK and LKB1, we immunoprecipitated AMPK from liver and skeletal muscle homogenates and performed Western blotting on the immunoprecipitate using anti-phospho-LKB1 antibody. This experiment showed that CDDO-Me treatment stimulated the amount of phospho-LKB1 associated with AMPK in both tissues (Fig. 8, E and F). The converse experiment of immunoprecipitating with anti-LKB1 antibody first and blotting with anti-phospho-AMPK antibody showed that CDDO-Me treatment also increased the amount of phospho-AMPK associated with LKB1 in both liver and skeletal muscle (Fig. 8, G and H). Taken together, these complementary in vitro and in vivo experiments indicate that CDDO-Me activates AMPK via LKB1 activation through a direct interaction between these two proteins.
FIGURE 8.
CDDO-Me treatment increases AMPK Thr-172 and LKB1 phosphorylation in vivo in a murine model. Activation of AMPK is LKB1-dependent. A–D, the tissue extracts were prepared from Western diet-fed mice treated with CDDO-Me and vehicle through gavage for 2 weeks. Western blot analysis showed that CDDO-Me increases AMPK Thr-172 and LKB1 phosphorylation in liver (A and C) and in muscle (B and D) homogenate. Blot with AMPK and LKB1 served as loading controls for muscle as well as liver tissue. E–H, immunoprecipitation of liver homogenate (E and G) and muscle homogenate (F and H) was further characterized to show the association between AMPK and LKB1. The precipitate was subjected to Western analysis with antibodies to p-AMPK and p-LKB1. The levels of precipitated AMPK (E and F) were assessed with p-LKB1-specific antibody and precipitated LKB1 (G and H) with p-AMPK-specific antibody and showed higher phosphorylation of LKB1 and AMPK in CDDO-Me over vehicle-treated mice. Western blot with same antibody used for immunoprecipitation served as a loading control. The corresponding bar graphs show the quantification of the blot. I and J, ERK1/2, an upstream target of the LKB1-AMPK pathway, was activated by CDDO-Me treatment and displayed increased phosphorylation with CDDO-Me treatment in C2C12 cells (I) and isolated hepatocytes (J). K, MAPK inhibitor U0126 blocked ERK1/2-mediated AMPK activation by CDDO-Me, suggesting ERK1/2 as a possible upstream target. IP, immunoprecipitation; IB, immunoblot.
To explore potential CDDO-Me target upstream of LKB1, we treated C2C12 and primary hepatocytes with CDDO-Me and found increased phosphorylation of ERK1/2 in MAPK cascade, which is upstream of LKB1. In support of this interpretation is the fact that U0126, a MAPK inhibitor, completely blocked the phosphorylation of ERK1/2 as well as the downstream phosphorylation of AMPK (Fig. 8, I–K). These findings suggest that ERK1/2 may play a role in CDDO-Me action.
Anti-diabetic Action of CDDO-Me in Leprdb/db Mice, a Genetic T2DM Model
The experiments above showed that CDDO-Me reverses insulin resistance and diabetes in a diet-induced T2DM model. We examined a genetic model, Leprdb/db mice, which displayed severe obesity and T2DM because of leptin receptor deficiency. We used Leprdb/db mice at the age of 12–16 weeks (50–60 gm) when they had developed overt diabetes. To avoid the stress of daily gavage, we inserted an indwelling intravenous catheter in the jugular vein of conscious mice with the other end of the catheter threaded under the skin to provide a portal for drug delivery on the back of the animal. Three weeks later we started treating groups of unrestrained Leprdb/db mice in the same cage with twice daily injections of CDDO-Me-liposomes or empty liposomes. CDDO-Me treatment did not affect total body weight (Fig. 9A). However, random blood glucose levels dropped significantly by ∼50% within 2 days of drug treatment and stayed low thereafter as compared with the empty liposome group (Fig. 9B). Treatment also significantly reduced plasma FFA (p < 0.03) and a moderate reduction in plasma TG level (Fig. 9, C and D). Intravenous GTT at 2 weeks of treatment showed marked improvement in glucose tolerance; CDDO-Me reversed the severe hyperglycemia both at base line and at all time points during the 2-h GTT (Fig. 9E). After an overnight fast (16–19 h), using a steady state infusion technique with 3H glucose, we found that CDDO-Me significantly decreased hepatic glucose production in Leprdb/db mice (p < 0.05; Fig. 9F). The improvement in whole body insulin resistance in CDDO-Me-treated Leprdb/db mice was confirmed by the significantly enhanced blood glucose lowering during an intravenous ITT in CDDO-Me-treated mice (Fig. 9G). Therefore, CDDO-Me improved insulin resistance and reversed diabetes in two different T2DM models: via intravenous liposome treatment in Leprdb/db mice and per oral treatment in high fat diet-induced diabetic mice.
FIGURE 9.
Effects of CDDO-Me treatment in genetically diabetic mice (Leprdb/db). The mice were treated with vehicle (control liposomes) or with CDDO-Me (2 mg/ml) packed in liposomes (250 μl/mice/twice daily). A–D, treatment had no effect on body weight (A, BW), but CDDO-Me treatment lowered random blood glucose levels (B), plasma FFA level (C), and plasma TG level (D). E, two weeks of CDDO-Me liposome treatment clearly showed significant improvement of glucose (2 mg/kg) tolerance test. F, whole body glucose production was measured by [3H]glucose infusion and showed significant reduction in the treated group. G, treatment also increased insulin sensitivity after 2 weeks of treatment as determined by ITT (5 units/kg). Solid bars, Leprdb/db male mice treated with CDDO-Me liposomes; empty bars, Leprdb/db male mice treated with control liposomes. The error bars represent the means ± S.D. (n = 3 mice/group). *, p < 0.05, treated versus control.
DISCUSSION
This study is the first detailed metabolic analysis of T2DM mice treated with CDDO-Me, an agent currently in phase I clinical trials in cancer patients. Our data showed that CDDO-Me treatment greatly attenuates the hyperglycemia of diet-induced T2DM mice. Euglycemic-hyperinsulinemic clamp experiments indicate that an increased rate of glucose infusion is required to maintain euglycemia in CDDO-Me-treated mice, indicating increased glucose disposal in peripheral tissues (mainly skeletal muscle). Therefore, the improvement of the diabetic state appears to be the result of improved insulin sensitivity in skeletal muscle, whereas hepatic glucose production remains relatively unchanged. Interestingly, in Leprdb/db mice, a genetic T2DM model, we found significant reduction in basal glucose production measured by a steady state infusion method in CDDO-Me-liposome-treated as compared with empty liposome-treated mice. We believe that in diet-induced diabetic mice in which the CDDO-Me was administered by gavage, we could have missed a mild to moderate decrease in hepatic glucose production, because for 4 days after jugular vein catheterization, we had to temporarily suspend the gavage for technical reasons. Even then, we observed in these diet-induced diabetic mice that 2 weeks of drug treatment restored euglycemia and reduced plasma insulin levels (random (Fig. 1D) as well as during GTT (Fig. 1F)). Increased phosphorylation of IRS-1 and IRS-2 (Fig. 2E) in the setting of a constant insulin infusion suggests enhanced signaling at a proximal step in the insulin signaling pathway.
In addition to reversal of hyperglycemia, CDDO-Me treatment of diet-induced T2DM mice has a beneficial effect on all of these other comorbid conditions, i.e. it reverses obesity and insulin resistance, lowers TG and FFA, whereas it down-regulates the expression of a number of proinflammatory cytokines (35) (Fig. 3, A and B). CDDO-Me has been reported to act on multiple targets (1), including inhibition of NF-κB-mediated gene expression after translocation of activated NF-κB to the nucleus. CDDO was found to abolish NF-κB-dependent resynthesis of IκBα, which suggests that CDDO-Me targets a step downstream of NF-κB translocation into the nucleus (36). Our data are consistent with such an interpretation. In this study we also showed that this drug reduces lipogenesis in T2D mice, which corroborates a recent report by Shin et al. (37).
We showed that CDDO-Me action is mediated, at least in part, by AMPK activation. Although AMPK is ubiquitously expressed (38), the in vivo effects of CDDO-Me have been primarily associated with increased glucose uptake in skeletal muscle, which involves AMPK activation (32, 39, 40). The increased phosphorylation and activation of AMPK by CDDO-Me would provide a mechanism for the observed improvements in glucose and lipid metabolism. Phosphorylation and inactivation of ACC by AMPK activation would inhibit the proximal and rate-limiting step of lipogenesis. These effects are likely to contribute to the capacity of CDDO-Me to lower triglycerides and FFA in vivo.
AMPK activation is implicated as a mechanism for the induction of skeletal muscle glucose uptake; this effect is additive with insulin (41). Therefore, the observed association of increased glucose uptake and AMPK activation in isolated skeletal muscle suggests that the effect of CDDO-Me in augmenting muscle insulin action in vivo may be attributed to AMPK as well. AMPK mediates a decrease in SREBP-1 mRNA. FAS, a known lipogenic target gene for SREBP-1, is also down-regulated in CDDO-Me-treated hepatocytes, further contributing to the effect of CDDO-Me on modulating circulating lipids and reducing hepatic lipogenesis. It should be noted that increased SREBP-1 is postulated as a central mediator of insulin resistance in T2DM and related metabolic disorders (42, 43) and that increased liver lipid content is implicated in hepatic insulin resistance (44).
The α subunit of AMPK contains the catalytic site, and phosphorylation of Thr-172 in its activation loop by one or more upstream kinases (AMPK kinase) is required for activation (28, 46, 47). Recent work from several laboratories has demonstrated that LKB1 is a major upstream kinase for AMPK (48–50). LKB1 is a tumor suppressor kinase and can phosphorylate the T-loop of all 12 known members of the human AMPK family (51). It is not clear whether LKB1 phosphorylation is required for AMPK activation and, if so, which site of phosphorylation is involved in AMPK activation. LKB1 is phosphorylated at Ser-325, Thr-366, and Ser-431 by upstream kinases. In addition, LKB1 autophosphorylates at Ser-31, Thr-185, Thr-189, Thr-336, and Ser-404 (52). Mutation of any of these phosphorylation sites to Ala (to abolish phosphorylation) or Glu (to mimic phosphorylation) does not significantly affect the in vitro catalytic activity of LKB1 or its intracellular localization (53–55). Recently, Zou and co-workers (56) demonstrated that phosphorylation of LKB1 Ser-428 is required for metformin-enhanced AMPK activation. However, the precise mechanism(s) underlying LKB1 activation, the relevant phosphorylation sites, and the upstream activating kinase(s) are still not well understood. Neither the activity of LKB1 itself nor that of AMPK-related kinases is influenced directly by agents known to activate AMPK, e.g. AICAR and metformin (57–60). Thus, how these agents lead to LKB1-dependent AMPK activation remains unclear to date. Our results suggest, however, that phosphorylation of LKB1 is necessary for CDDO-Me-mediated AMPK phosphorylation.
It remains unclear how CDDO-Me activates LKB1. Our preliminary data suggest that it could work through activation of ERK1/2, a rate-limiting enzyme in MAPK pathway. A MAPK inhibitor that blocks ERK1/2 phosphorylation also inhibits AMPK phosphorylation, further supporting ERK1/2 as a possible upstream target for CDDO-Me. Much additional work will be needed to clearly define the underlying mechanisms involved.
CDDO-Me is undergoing phase I trial for different cancers (61). It is noteworthy that the dose of CDDO-Me used in the in vivo studies reported herein (2–3 mg/kg) is about 1 log lower than the doses required to achieve its anti-tumor effects (20–100 mg/kg) (45, 62). Therefore, it is likely that, clinically, the use of CDDO-Me as an anti-diabetic agent may require a substantially lower dose than that used in cancer therapy. To date, data from the ongoing phase I trials of CDDO-Me (RTA-402) indicate that toxicity of this agent even at the much higher anti-cancer dose is minimal. Therefore, it is possible that use of CDDO-Me for clinical management of T2DM could be achieved without serious side effects.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL51586 (to L. C.) and P30-DK079638. This work was also supported by the Betty Rutherford Chair in Diabetes Research, St. Luke's Episcopal Hospital, and the T. T. & W. F. Chao Global Foundation. M. A. and M. K. have stocks and consulting agreements with Reata Pharmaceuticals, Inc.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- CDDO
- 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid
- CDDO-Me
- CDDO methyl ester
- AMPK
- AMP-activated protein kinase
- ACC
- acetyl-coA carboxylase
- FFA
- free fatty acid
- GTT
- glucose tolerance test
- ITT
- insulin tolerance test
- IR
- insulin receptor
- AICAR
- 5-amino-imidazole carboxamide riboside
- WAT
- white adipose tissue
- FAS
- fatty acid synthase
- SREBP
- sterol response element-binding protein
- Comp C
- Compound C
- PEPCK
- phosphoenolpyruvate carboxykinase
- TG
- triglyceride
- T2DM
- type 2 diabetes mellitus.
REFERENCES
- 1.Sporn M. B., Suh N. (2000) Carcinogenesis 21, 525–530 [DOI] [PubMed] [Google Scholar]
- 2.Wang Y., Porter W. W., Suh N., Honda T., Gribble G. W., Leesnitzer L. M., Plunket K. D., Mangelsdorf D. J., Blanchard S. G., Willson T. M., Sporn M. B. (2000) Mol. Endocrinol. 14, 1550–1556 [DOI] [PubMed] [Google Scholar]
- 3.Huang M. T., Ho C. T., Wang Z. Y., Ferraro T., Lou Y. R., Stauber K., Ma W., Georgiadis C., Laskin J. D., Conney A. H. (1994) Cancer Res. 54, 701–708 [PubMed] [Google Scholar]
- 4.Nishino H., Nishino A., Takayasu J., Hasegawa T., Iwashima A., Hirabayashi K., Iwata S., Shibata S. (1988) Cancer Res. 48, 5210–5215 [PubMed] [Google Scholar]
- 5.Suh N., Honda T., Finlay H. J., Barchowsky A., Williams C., Benoit N. E., Xie Q. W., Nathan C., Gribble G. W., Sporn M. B. (1998) Cancer Res. 58, 717–723 [PubMed] [Google Scholar]
- 6.Suh N., Wang Y., Honda T., Gribble G. W., Dmitrovsky E., Hickey W. F., Maue R. A., Place A. E., Porter D. M., Spinella M. J., Williams C. R., Wu G., Dannenberg A. J., Flanders K. C., Letterio J. J., Mangelsdorf D. J., Nathan C. F., Nguyen L., Porter W. W., Ren R. F., Roberts A. B., Roche N. S., Subbaramaiah K., Sporn M. B. (1999) Cancer Res. 59, 336–341 [PubMed] [Google Scholar]
- 7.Tabe Y., Konopleva M., Kondo Y., Contractor R., Tsao T., Konoplev S., Shi Y., Ling X., Watt J. C., Tsutsumi-Ishii Y., Ohsaka A., Nagaoka I., Issa J. P., Kogan S. C., Andreeff M. (2007) Cancer Biol. Ther. 6, 1967–1977 [DOI] [PubMed] [Google Scholar]
- 8.Koschmieder S., D'Alò F., Radomska H., Schöneich C., Chang J. S., Konopleva M., Kobayashi S., Levantini E., Suh N., Di Ruscio A., Voso M. T., Watt J. C., Santhanam R., Sargin B., Kantarjian H., Andreeff M., Sporn M. B., Perrotti D., Berdel W. E., Müller-Tidow C., Serve H., Tenen D. G. (2007) Blood 110, 3695–3705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao X., Deeb D., Jiang H., Liu Y., Dulchavsky S. A., Gautam S. C. (2007) J. Neurooncol. 84, 147–157 [DOI] [PubMed] [Google Scholar]
- 10.Hyer M. L., Shi R., Krajewska M., Meyer C., Lebedeva I. V., Fisher P. B., Reed J. C. (2008) Cancer Res. 68, 2927–2933 [DOI] [PubMed] [Google Scholar]
- 11.Ikeda T., Nakata Y., Kimura F., Sato K., Anderson K., Motoyoshi K., Sporn M., Kufe D. (2004) Mol. Cancer Ther. 3, 39–45 [PubMed] [Google Scholar]
- 12.Kodera Y., Takeyama K., Murayama A., Suzawa M., Masuhiro Y., Kato S. (2000) J. Biol. Chem. 275, 33201–33204 [DOI] [PubMed] [Google Scholar]
- 13.Melichar B., Konopleva M., Hu W., Melicharova K., Andreeff M., Freedman R. S. (2004) Gynecol. Oncol. 93, 149–154 [DOI] [PubMed] [Google Scholar]
- 14.Konopleva M., Tsao T., Ruvolo P., Stiouf I., Estrov Z., Leysath C. E., Zhao S., Harris D., Chang S., Jackson C. E., Munsell M., Suh N., Gribble G., Honda T., May W. S., Sporn M. B., Andreeff M. (2002) Blood 99, 326–335 [DOI] [PubMed] [Google Scholar]
- 15.Shishodia S., Sethi G., Konopleva M., Andreeff M., Aggarwal B. B. (2006) Clin. Cancer Res. 12, 1828–1838 [DOI] [PubMed] [Google Scholar]
- 16.Hong D. S., Kurzrock R., Supko J. G., Lawrence D. P., Wheler J. J., Meyer C. J., Mier J. W., Andreeff M., Shapiro G. I., Dezube B. J. (2008) American Society of Clinical Oncology (ASCO) Annual Meeting2008, May 30–June 3, Chicago, IL [Google Scholar]
- 17.Chang B. H., Liao W., Li L., Nakamuta M., Mack D., Chan L. (1999) J. Biol. Chem. 274, 6051–6055 [DOI] [PubMed] [Google Scholar]
- 18.Saha P. K., Kojima H., Martinez-Botas J., Sunehag A. L., Chan L. (2004) J. Biol. Chem. 279, 35150–35158 [DOI] [PubMed] [Google Scholar]
- 19.Kim J. K., Michael M. D., Previs S. F., Peroni O. D., Mauvais-Jarvis F., Neschen S., Kahn B. B., Kahn C. R., Shulman G. I. (2000) J. Clin. Invest. 105, 1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soriano H. E., Lewis D., Legner M., Brandt M., Baley P., Darlington G., Finegold M., Ledley F. D. (1992) Transplantation 54, 717–723 [DOI] [PubMed] [Google Scholar]
- 21.Yaswen P., Hayner N. T., Fausto N. (1984) Cancer Res. 44, 324–331 [PubMed] [Google Scholar]
- 22.Witters L. A., Kemp B. E. (1992) J. Biol. Chem. 267, 2864–2867 [PubMed] [Google Scholar]
- 23.Davies S. P., Carling D., Hardie D. G. (1989) Eur. J. Biochem. 186, 123–128 [DOI] [PubMed] [Google Scholar]
- 24.Cidad P., Almeida A., Bolaños J. P. (2004) Biochem. J. 384, 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H., Song X., Briggs M., Violand B., Salsgiver W., Gulve E. A., Luo Y. (2005) Biochem. Biophys. Res. Commun. 338, 793–799 [DOI] [PubMed] [Google Scholar]
- 26.Kahn B. B., Flier J. S. (2000) J. Clin. Invest. 106, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim F., Pham M., Luttrell I., Bannerman D. D., Tupper J., Thaler J., Hawn T. R., Raines E. W., Schwartz M. W. (2007) Circ. Res. 100, 1589–1596 [DOI] [PubMed] [Google Scholar]
- 28.Wellen K. E., Hotamisligil G. S. (2005) J. Clin. Invest. 115, 1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 30.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr. (2003) J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayashi T., Hirshman M. F., Fujii N., Habinowski S. A., Witters L. A., Goodyear L. J. (2000) Diabetes 49, 527–531 [DOI] [PubMed] [Google Scholar]
- 32.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurley R. L., Barré L. K., Wood S. D., Anderson K. A., Kemp B. E., Means A. R., Witters L. A. (2006) J. Biol. Chem. 281, 36662–36672 [DOI] [PubMed] [Google Scholar]
- 34.Lee M., Hwang J. T., Lee H. J., Jung S. N., Kang I., Chi S. G., Kim S. S., Ha J. (2003) J. Biol. Chem. 278, 39653–39661 [DOI] [PubMed] [Google Scholar]
- 35.Yang J., Park Y., Zhang H., Xu X., Laine G. A., Dellsperger K. C., Zhang C. (2009) Am. J. Physiol. Heart Circ. Physiol. 296, H1850–H1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stadheim T. A., Xiao H., Eastman A. (2001) Cancer Res. 61, 1533–1540 [PubMed] [Google Scholar]
- 37.Shin S., Wakabayashi J., Yates M. S., Wakabayashi N., Dolan P. M., Aja S., Liby K. T., Sporn M. B., Yamamoto M., Kensler T. W. (2009) Eur. J. Pharmacol. 620, 138–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardie D. G., Carling D., Carlson M. (1998) Annu. Rev. Biochem. 67, 821–855 [DOI] [PubMed] [Google Scholar]
- 39.Fujii N., Aschenbach W. G., Musi N., Hirshman M. F., Goodyear L. J. (2004) Proc. Nutr. Soc. 63, 205–210 [DOI] [PubMed] [Google Scholar]
- 40.Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi T., Hirshman M. F., Kurth E. J., Winder W. W., Goodyear L. J. (1998) Diabetes 47, 1369–1373 [DOI] [PubMed] [Google Scholar]
- 42.Kakuma T., Lee Y., Higa M., Wang Z., Pan W., Shimomura I., Unger R. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 8536–8541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimomura I., Matsuda M., Hammer R. E., Bashmakov Y., Brown M. S., Goldstein J. L. (2000) Mol. Cell 6, 77–86 [PubMed] [Google Scholar]
- 44.McGarry J. D. (1992) Science 258, 766–770 [DOI] [PubMed] [Google Scholar]
- 45.Ling X., Konopleva M., Zeng Z., Ruvolo V., Stephens L. C., Schober W., McQueen T., Dietrich M., Madden T. L., Andreeff M. (2007) Cancer Res. 67, 4210–4218 [DOI] [PubMed] [Google Scholar]
- 46.Kemp B. E., Stapleton D., Campbell D. J., Chen Z. P., Murthy S., Walter M., Gupta A., Adams J. J., Katsis F., van D. B., Jennings I. G., Iseli T., Michell B. J., Witters L. A. (2003) Biochem. Soc. Trans. 31, 162–168 [DOI] [PubMed] [Google Scholar]
- 47.Winder W. W., Hardie D. G. (1999) Am. J. Physiol. 277, E1–E10 [DOI] [PubMed] [Google Scholar]
- 48.Hawley S. A., Boudeau J., Reid J. L., Mustard K. J., Udd L., Mäkelä T. P., Alessi D. R., Hardie D. G. (2003) J. Biol. 2, 28.1–28.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 3329–3335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods A., Johnstone S. R., Dickerson K., Leiper F. C., Fryer L. G., Neumann D., Schlattner U., Wallimann T., Carlson M., Carling D. (2003) Curr. Biol. 13, 2004–2008 [DOI] [PubMed] [Google Scholar]
- 51.Lizcano J. M., Göransson O., Toth R., Deak M., Morrice N. A., Boudeau J., Hawley S. A., Udd L., Mäkelä T. P., Hardie D. G., Alessi D. R. (2004) EMBO J. 23, 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alessi D. R., Sakamoto K., Bayascas J. R. (2006) Annu. Rev. Biochem. 75, 137–163 [DOI] [PubMed] [Google Scholar]
- 53.Boudeau J., Baas A. F., Deak M., Morrice N. A., Kieloch A., Schutkowski M., Prescott A. R., Clevers H. C., Alessi D. R. (2003) EMBO J. 22, 5102–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sapkota G. P., Boudeau J., Deak M., Kieloch A., Morrice N., Alessi D. R. (2002) Biochem. J. 362, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sapkota G. P., Kieloch A., Lizcano J. M., Lain S., Arthur J. S., Williams M. R., Morrice N., Deak M., Alessi D. R. (2001) J. Biol. Chem. 276, 19469–19482 [DOI] [PubMed] [Google Scholar]
- 56.Xie Z., Dong Y., Scholz R., Neumann D., Zou M. H. (2008) Circulation 117, 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davis B. J., Xie Z., Viollet B., Zou M. H. (2006) Diabetes 55, 496–505 [DOI] [PubMed] [Google Scholar]
- 58.Sakamoto K., Göransson O., Hardie D. G., Alessi D. R. (2004) Am. J. Physiol. Endocrinol. Metab 287, E310–E317 [DOI] [PubMed] [Google Scholar]
- 59.Sakamoto K., Murata T., Chuma H., Hori M., Ozaki H. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 327–333 [DOI] [PubMed] [Google Scholar]
- 60.Zou M. H., Hou X. Y., Shi C. M., Kirkpatick S., Liu F., Goldman M. H., Cohen R. A. (2003) J. Biol. Chem. 278, 34003–34010 [DOI] [PubMed] [Google Scholar]
- 61.Dezube B. J., Kurzrock R., Eder J. P., Supko J. G., Meyer C. J., Camacho L. H., Andreeff M., Konopleva M., Lescale-Matys L., Hong D. (2007) Annual Meeting of the AACR-NCI-EORTC, International Conference on Molecular Targets and Cancer Therapeutics: Discovery, Biology, and Clinical ApplicationsOctober 22–26, San Francisco, CA [Google Scholar]
- 62.Liby K., Yore M. M., Roebuck B. D., Baumgartner K. J., Honda T., Sundararajan C., Yoshizawa H., Gribble G. W., Williams C. R., Risingsong R., Royce D. B., Dinkova-Kostova A. T., Stephenson K. K., Egner P. A., Yates M. S., Groopman J. D., Kensler T. W., Sporn M. B. (2008) Cancer Res. 68, 6727–6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.