Abstract
We have shown previously that perfluorocarbon-exposed sonicated dextrose albumin (PESDA) microbubbles bind to injured vascular tissue and can be detected with ultrasound imaging techniques. Prior studies have shown that scavenger receptors (SRs) are regulators of innate and adaptive immune responses and are involved in the progression of vascular disease such as atherosclerosis. In this study, we sought to determine the molecular mechanism of PESDA binding to balloon-injured vasculature. RT-PCR analysis of angioplastied aortas demonstrated a significantly (p ≤ 0.01) increased expression of SRs. Binding to SRs was confirmed using SR-expressing CHO cells, and this binding was blocked by competitive inhibition with the SR-binding ligands oxidized LDL and malondialdehyde-acetaldehyde-modified LDL. Confocal imaging confirmed the co-localization of PESDA microbubbles to CD36, SRB-1, and Toll-like receptor 4, but not to monocytes/macrophages. This study demonstrates that PESDA binds to SRs and that this binding is in major part dependent upon the oxidized nature of PESDA microbubble shell proteins. The extent of SR mRNA expression was increased with injury and associated with microbubble retention as defined by scanning electron microscopy and immunohistochemistry. These findings clarify the mechanisms of how albumin-based microbubbles bind to injured and inflamed vasculature and further support the potential of this imaging technique to detect early vascular innate inflammatory pathophysiologic processes.
Keywords: Atherosclerosis, Cell-surface Receptor, Cellular Immune Response, Endothelium, Innate Immunity, Ligand-binding Protein, Receptors, Albumin Microbubbles, Oxidized Proteins, Vascular Inflammation
Introduction
The complexities of vascular inflammation and disease are multifaceted, and although our current clinical treatments are effective, there is a need to refine patient treatment regimens and risk stratification tools. Standard risk factors for the development of coronary heart disease include age, hypertension, dyslipidemia, diabetes, and tobacco use (1). Although these measures are linked to the development of atheromatous plaques and coronary heart disease, there is an underestimation of subclinical atherosclerosis (2, 3). For example, a substantial proportion of men and women with an intermediate Framingham risk score (10–19%, 10-year coronary heart disease risk) had a higher than predicted atherosclerotic burden (3). This limitation in the predictive power of the Framingham risk score is postulated to be secondary to the lack of representation of other risk modifiers such as inflammation (2, 3).
Currently, there is no gold standard for detecting inflammation within the vascular wall. Perfluorocarbon-exposed sonicated dextrose albumin (PESDA)2 microbubbles interact with and bind to sites of endothelial dysfunction using contrast pulse sequencing ultrasound (4, 5). However, the mechanism(s) by which albumin-based microbubbles bind and interact with the injured vascular wall has yet to be fully determined. This study details in part the nature of PESDA binding to injured vessels and how this binding may play a role in the non-invasive detection of vascular wall injury and innate inflammatory responses.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and maintained on a Purina rat chow diet. All animals were allowed free access to food and water up to 1 h prior to killing. All procedures were approved by the Animal Subcommittee of the University of Nebraska Medical Center and are in accordance with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Synthesis and Injection of PESDA Microbubbles
PESDA microbubbles were prepared according to the method described previously (5). Briefly, a mixture of three parts 5% dextrose and one part 5% human serum albumin (total of 16 ml) was added to 8 ml of decafluorobutane gas, hand-agitated, and then sonicated with an electromechanical sonicator (Heat Systems Inc., Farmingdale, NY) for 70 ± 5 s until reaching a temperature of 80 ± 5 °C. The resulting average microbubble size, confirmed by hemocytometer, was 4.6 μm, and the mean concentration, measured by a Coulter counter, was 1.4 × 108 bubbles/ml.
Vascular Injury Models
Male Sprague-Dawley rats were anesthetized with isoflurane. The left femoral artery was exposed by cutdown, and a 2.0 × 20-mm coronary balloon was inserted into the distal 2 cm of the infrarenal aorta. Using ultrasound without microbubbles, aortic diameter and aortic flow velocities were documented. Balloon position was verified and inflated to 4 or 6 atm for 30 s while imaging with ultrasound (130–150% of the original vessel diameter) and pulled back and forth to denude the endothelium. This process was repeated three times. The balloon was removed; femoral bleeding was controlled by pressure; the cutdown incision was closed; and the animals were allowed to recover and rest for 48 h. Control animals (i.e. non-balloon-injured animals) were not manipulated prior to imaging at 48 h. Following the 48-h recovery period, ballooned and control animals were anesthetized; the right femoral vein was exposed via cutdown; and a 22-gauge angiocatheter were inserted and secured. Microbubbles (synthesized as outlined above) were diluted 1:10 in normal saline and injected as a bolus of 0.2 ml (2.8 × 106 microbubbles/injection as determined by hemocytometer and/or Coulter counter), followed by a 0.5-ml saline flush into the femoral vein. Treatment and control rats were killed 5 min later to ensure that non-retained microbubbles had cleared from the blood pool. The infrarenal aorta and the remaining thoracic and infrarenal aortas were harvested for analysis by RT-PCR, light microscopy, scanning electron microscopy, and immunohistochemistry.
RNA Extraction and Scavenger Receptor and Biomarker RNA Expression
RNA was extracted from the thoracic and infrarenal aortas using an RNeasy mini kit (Qiagen, Valencia, CA) following the manufacturer's protocol. RNA was quantified, and its integrity was verified by electrophoresis of 1 μg of RNA on a 1% agarose gel stained with ethidium bromide. Gel bands were detected by exposure on a Fluor-S MultiImager and analyzed by Quantity One software (Bio-Rad).
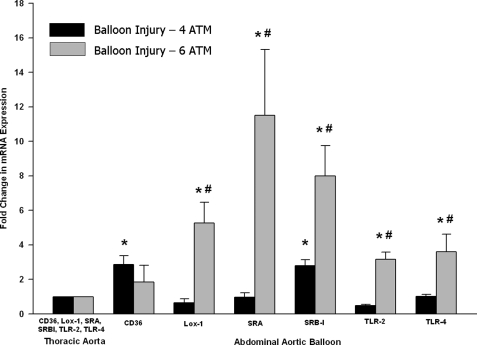
To assess the potential scavenger receptors involved in the binding of microbubbles to injured vessels, thoracic and infrarenal aortic tissue was obtained from control and balloon-injured animals using 4 and 6 atm of pressure. RNA was isolated, and RT-PCR was performed. RNA from thoracic or infrarenal aortas was converted into cDNA using a High Capacity cDNA Archive kit from Applied Biosystems (Foster City, CA). The cDNA sample was subjected to RT-PCR using TaqMan gene expression assay primers from Applied Biosystems. Using a Model 7500 RT-PCR system from Applied Biosystems, the primers for scavenger receptor A (SRA), scavenger receptor B-1 (SRB-1), the oxidized LDL receptor Lox-1, Toll-like receptor (TLR) 2, TLR-4, and CD36 were added, and mRNA was amplified according to the manufacturer's directions. Amplified infrarenal mRNA of each animal was normalized to its own thoracic aortic mRNA and is presented as the -fold change in mRNA expression.
Scanning Electron Microscopy
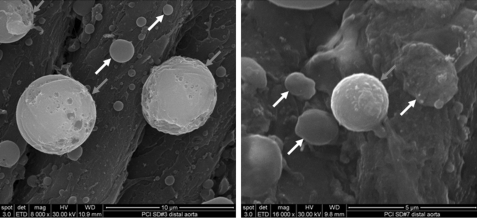
For scanning electron microscopy (SEM), samples were dehydrated through a series of ethanol concentrations increasing from 50 to 100%, immersed in Freon 113, critical point-dried, mounted on aluminum stubs, and sputter-coated with gold (Polaron E5100, Polaron Inc., Hertfordshire, United Kingdom). Specimens were viewed under a JEOL scanning electron microscope. Adherent microbubbles were characterized as a less electron-dense structure, spherically shaped (differentiating them from the biconcave-shaped erythrocytes), and not exhibiting any of the surface characteristics of hematopoietic cells such as microvilli or microridges. The entire extension of non-injured thoracic or injured abdominal aorta samples was analyzed.
Immunohistochemical Staining for Albumin
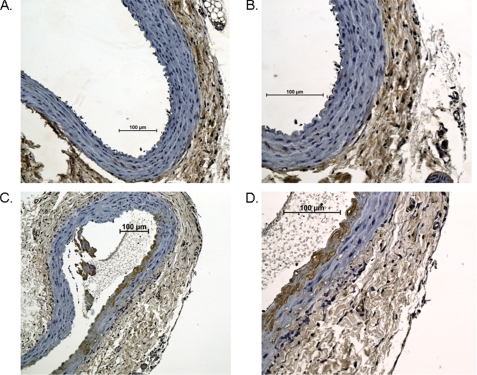
Paraffin-embedded sections were cut and subjected to immunohistochemical staining for the presence of human albumin. For light microscopy, sections were incubated with a human-specific, anti-human albumin polyclonal antibody (Sigma), blocked in peroxide, and incubated with HRP-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA). DAB (3,3′-diaminobenzidine) substrate was used as the detection reagent, and sections were counterstained with hematoxylin. Slides were analyzed using a Nikon Eclipse 80i at ×10 and ×20 power and NIS-Elements 3.0 software (Nikon, Melville, NY).
Co-localization Studies Using Confocal Microscopy
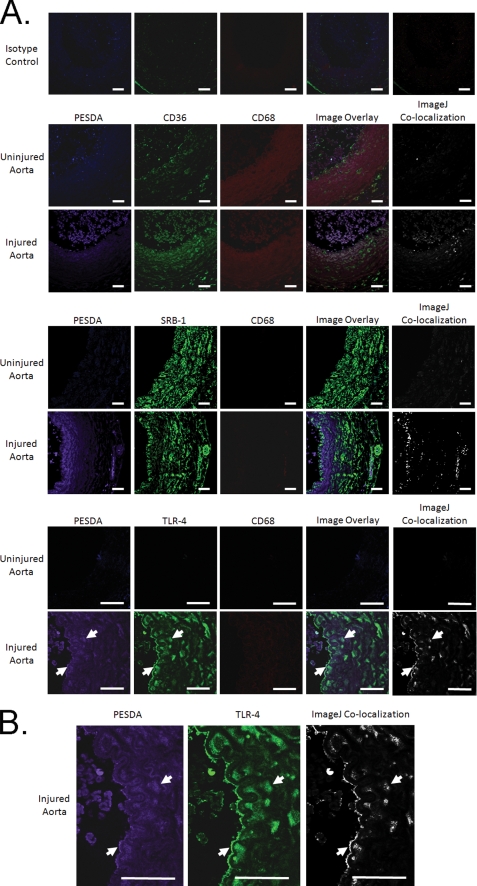
Paraffin-embedded sections were processed as described above. However, epitope retrieval was performed to increase antigen availability. Samples were blocked using 5% donkey serum, washed, and incubated with the following combinations of primary antibodies: rabbit anti-rat CD36 (Novus Biologicals, Littleton, CO), TLR-4 (Rockland, Gilbertsville, PA), mouse anti-rat CD68 (AbD Serotec, Raleigh, NC), and goat anti-human albumin (Bethyl Laboratories, Montgomery, TX). Following a 1-h incubation, slides were washed, and the following three different detection antibodies were incubated on the tissue: DyLight 405-conjugated donkey anti-mouse IgG, DyLight 549-conjugated donkey anti-rabbit IgG, and DyLight 649-conjugated donkey anti-goat IgG (Jackson ImmunoResearch Laboratories). Secondary antibodies were incubated for 30 min, washed, and tissue-mounted using Fluoromount-G (SouthernBiotech, Birmingham, AL). Slides were analyzed using a Zeiss 510 Meta confocal microscope at ×20 and ×40 power. Images were then analyzed for co-localization using ImageJ software (6).
In Vitro Microbubble Binding Assays
CHO cells transfected with the scavenger receptor CD36 (human), SRA (bovine), SRB-1 (human), Lox-1 (bovine), TLR-2 (human), or TLR-4 (human) were grown to confluence (1–3 days) in DMEM with 10% fetal bovine serum (7–10). CHO cells devoid of cellular receptors (CHO-K1) were used as control cells. To verify receptor integrity, cells were checked for receptor mRNA expression using RT-PCR as described above. Scavenger receptor protein expression was also evaluated by flow cytometry to ensure cell line expression and purity.
For these studies, PESDA microbubbles or PESDA microbubbles exposed to heat-inactivated serum (56 °C for 30 min) were incubated with CHO cells expressing CD36, Lox-1, SRA, SRB-1, TLR-2, or TLR-4 and with CHO-K1 control cells. CHO cells were subcultured and seeded with fresh medium into new flasks 24 h prior to testing. Flasks were seeded with sufficient CHO cell numbers to allow confluence at the time of testing. Tissue culture reagents were tested as detailed below to verify the absence of oxidized agents that would confound binding assay results.
Confluent flasks were injected with 0.5 ml of PESDA or PESDA exposed to heat-inactivated serum for 5 min. Flasks were mixed and incubated at room temperature for 2 min in an inverted fashion to allow layering and direct contact of microbubbles with the cellular monolayer. When performing inhibition assays, monolayers were preincubated for 10 min with oxidized LDL (ox-LDL) or malondialdehyde-acetaldehyde-modified LDL (MAA-LDL), washed three times with Dulbecco's PBS, and then injected and incubated with PESDA as noted above. Upright flasks were spun at 200 × g, and the number of microbubbles was counted across five random quadrants under a Nikon TS100 microscope at ×40 power. Individual experimental results are reported as the average of five ×40 image fields. The oxidative state of our tissue culture media reagents, PESDA, and scavenger receptor-binding agents ox-LDL and MAA-LDL was evaluated using an advanced oxidation protein products assay kit (Cell Biolabs, Inc., San Diego, CA).
RESULTS
In an effort to demonstrate microbubble binding to these injured vessels, aortic tissue was evaluated for microbubble retention by SEM (Fig. 1) and by immunohistochemistry (IHC) for anti-human albumin, the core component in the microbubble shell (Fig. 2). SEM of injured aortic tissue illustrated binding of PESDA microbubbles to the vessel border after balloon injury. This binding is consistent with our previously published data (4, 5). IHC for microbubble shell protein control aortas (Fig. 2, A and B) and balloon-injured aortas (Fig. 2, C and D) illustrated the absence of albumin staining in the control animal aortic tissue (Fig. 2, A and B). Staining was evident, however, in the intimal and medial layers of the balloon-injured aortas (Fig. 2, C and D), indicating binding and retention of microbubble proteins to the injured vessel wall. Confocal microscopy of aortic tissue for PESDA microbubbles, CD36, SRB-1, TLR-4, and CD68 is presented in Fig. 3. Isotype controls were negative for staining, whereas CD36 and SRB-1 staining was notable in non-injured aortic tissue. After balloon injury, staining for PESDA and CD36 increased with co-localization. Co-localization of PESDA also occurred when staining for TLR-4 and SRB-1. ImageJ processing (6) was performed to detail regions of co-localization. Importantly, staining for CD68 in control and injured tissue was negative.
FIGURE 1.
SEM imaging of balloon-injured rat aortic tissue for the presence of microbubbles. Note microbubble retention in the area of injured tissue (dark arrows). Protein aggregates consistent with red blood cells and platelet binding (white arrows) are noted in these same areas of injury at 48 h post-balloon. Scale bars = 10 (left panel) and 5 (right panel) μm.
FIGURE 2.
Immunohistochemical staining of control and balloon-injured rat aortic tissue for the presence of microbubbles. Staining for PESDA microbubble proteins at sites of injury demonstrated microbubble retention (brown HRP staining of the intimal and media of the aorta). Paraffin-embedded aortic tissue from control animals at ×10 (A) and ×20 (B) were stained using an anti-human albumin antibody and compared with balloon-injured animals (C and D). Images are representative infrarenal aortas from three control animals and three balloon-injured animals. Scale bar = 100 μm.
FIGURE 3.
Immunohistochemical staining of balloon-injured and control rat aortic tissue for the presence of microbubbles (i.e. human albumin), CD36, TLR-4, and CD68. A, staining for PESDA microbubble proteins at sites of injury demonstrated microbubble retention in a focal pattern in the intimal and media of the aorta. Of note, PESDA shell proteins co-localized with CD36, SRB-1, and TLR-4 (white arrows), but not with CD68. ImageJ co-localization is also presented. B, images are enlarged to better illustrate co-localization of PESDA and TLR-4. However, note that PESDA and TLR-4 were not exclusively co-localized. ImageJ image processing was performed to determine areas of co-localization, which are presented in their respective panels as white pixels. Control tissues illustrate the basal expression of CD36 and were negative for PESDA and CD68. Isotype controls were negative for background staining. Pictures are representative of three infrarenal aortas from control animals and three balloon-injured animals. Scale bars = 50 μm.
RT-PCR of balloon-injured rat aortas showed an increase in mRNA expression of the scavenger receptors CD36 and SRB-1 after 4 atm of balloon injury (Fig. 4). However, a larger increase in receptor mRNA levels was observed when the balloon was increased to 6 atm. Lox-1, SRA, SRB-1, TLR-2, and TLR-4 were all increased following the increase in balloon stretch (i.e. 6 atm), with CD36 remaining unchanged with the increased aortic injury.
FIGURE 4.
Variable effects of scavenger receptor expression following balloon injury to the infrarenal aorta. Sprague-Dawley rats were subjected to balloon injury at 4 and 6 atm. Following a 48-h recovery period, the thoracic and infrarenal aortas were extracted; RNA was made; and RT-PCR was performed for scavenger receptor expression. The data are expressed as the mean ± S.E. of five separate experiments and are shown as the -fold increase over the thoracic aorta control. *, significantly increased compared with the thoracic control (p ≤ 0.04); #, significantly increased compared with balloon injury at 4 atm (p ≤ 0.05).
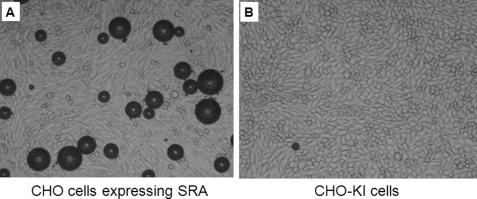
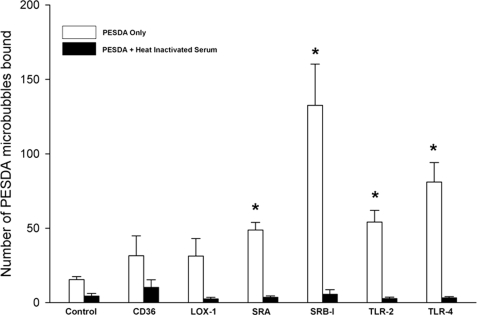
Because of the increased levels of mRNA of the various receptors tested above, CHO cells expressing these individual scavenger receptors were analyzed for their ability to directly bind PESDA microbubbles in vitro. Fig. 5 shows an example of SRA-expressing CHO cells binding PESDA microbubbles compared with the CHO-K1 control cells. When CHO cells expressing individual receptors were examined for PESDA retention without human serum, SRA, SRB-1, TLR-2, and TLR-4 were significantly different compared with the CHO-K1 control cells (Fig. 6). Of interest, when heat-inactivated serum was added, binding of microbubbles to the cells was absent (Fig. 6). In fact, previous in vivo studies have shown through complement depletion studies that serum complement is involved in PESDA retention and binding in injured tissue (4, 5).
FIGURE 5.
Representative example of CHO cells expressing SRA and binding PESDA microbubbles. CHO cells expressing SRA (A) and CHO-K1 cells (B) were incubated with PESDA microbubbles in the presence of serum for 2 min and counted under a Nikon inverted microscope at ×40. Scale bars = 100 μm.
FIGURE 6.
Binding of PESDA microbubbles to CHO cells expressing scavenger receptors. CHO cells expressing either no receptors (CHO-K1) or scavenger receptors were exposed to PESDA microbubbles for 2 min in the presence or absence of heat-inactivated serum. Heat inactivation of serum resulted in the complete loss of PESDA binding to scavenger receptor-binding CHO cells. Microbubbles were counted using a Nikon inverted microscope. Data are expressed as the mean ± S.E. of five separate experiments scored by multiple individuals. *, significantly increased compared with CHO-K1 control cells without serum (p ≤ 0.002).
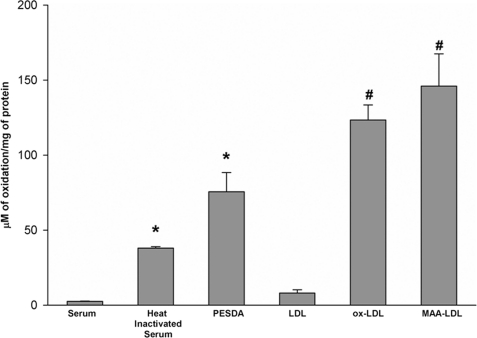
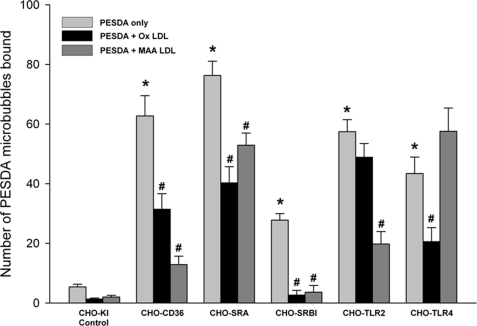
Because of the heat inactivation of serum and loss of PESDA binding to scavenger receptors, we evaluated the oxidative status of PESDA microbubble shell proteins and our culture reagents using an advanced oxidation protein products assay kit as described under “Experimental Procedures.” Comparison of fresh human serum, heat-inactivated serum, ox-LDL, and MAA-LDL illustrated the extent of protein oxidation of these different agents (Fig. 7). Importantly, PESDA had 75.6 μm oxidized protein/mg protein, and fresh human serum, heat-inactivated serum, LDL, ox-LDL, and MAA-LDL had 2.5, 37.9, 8.0, 123.4, and 145.9 μm oxidized protein/mg protein, respectively. Furthermore, competitive inhibition of scavenger receptor binding (Fig. 8) was performed with the scavenger receptor-binding agents ox-LDL and MAA-LDL. The inhibition of PESDA binding to CD36, SRA, SRB-1, TLR-2, and TLR-4 (Fig. 8) indicates that binding is due in major part to the oxidized nature of PESDA shell proteins.
FIGURE 7.
We used an advanced oxidation protein products assay kit and fresh human serum, heat-inactivated serum, PESDA, LDL, ox-LDL, and MAA-LDL to evaluate microbubbles for the extent of protein oxidation. Note the significant increase in oxidation when human serum was heat-inactivated and when human albumin was synthesized (i.e. sonicated and heated) into PESDA microbubbles. *, p ≤ 0.001 compared with serum; #, p ≤ 0.005 compared with LDL. Data are expressed as the mean ± S.E. of four separate experiments.
FIGURE 8.
CHO cells were incubated with PESDA only or with PESDA after pretreatment of the CHO cells with the oxidized ligands ox-LDL and MAA-LDL. Pretreatment with these oxidized proteins completely inhibited the binding of PESDA to SRB-1. Note that despite significant inhibition of binding to CD36, SRA, TLR-2, and TLR-4, inhibition was not complete and variable when comparing different receptors and inhibitors. *, p ≤ 0.01 compared with CHO-K1 cell binding; #, p ≤ 0.01 compared PESDA binding to each individual CHO cell expressing the scavenger receptor. Data are expressed as the mean ± S.E. of three separate experiments.
DISCUSSION
Vascular disease is a growing worldwide problem, and although there are excellent treatment options available, there is still a need for better diagnostic techniques for the detection of inflammation and/or plaque formation. Previous studies have demonstrated that the attachment of microbubbles to the vessel walls in areas of injury was partially due to a complement component (4, 11, 12). However, the mechanism by which this occurs early after injury (i.e. days) had not been completely determined.
Interestingly, innate immune responses are well recognized mechanisms involved in the induction (i.e. acute) and maintenance (i.e. chronic) of vascular injury and inflammation (i.e. ischemia-reperfusion injury and atherosclerosis) (13–16). Studies have implicated the potential pathogenic role of innate immune mediators such as TLR in the early and late progression of atherosclerotic disease and plaque rupture (17, 18). These TLRs, as well as other scavenger receptors, are expressed in atherosclerotic tissue on lymphocytes and on smooth muscle cells of the atherosclerotic plaques (19–23). Recently, TLRs and other scavenger receptors have become a target for the study of innate immune mechanisms associated with inflammatory vascular disease as well as targets for potential therapeutic approaches (24). Therefore, the purpose of this study was to determine the molecular mechanism(s) of PESDA microbubble binding to injured vascular tissue. Ultimately, if we understand these mechanisms, the use and imaging of albumin microbubbles, as published previously (4, 5), could be used to non-invasively evaluate vascular innate inflammation.
In these experiments, PESDA microbubbles were used as markers of inflammation, as they have been shown to adhere to injured vascular tissue (4, 5) and can be detected by SEM (Fig. 1) and IHC (Figs. 2 and 3). Importantly, inflammatory mRNA markers are up-regulated following aortic balloon injury (Fig. 4). As detailed in Figs. 1–4, balloon injury results in the retention of microbubbles in regions where there is a significant increase in local innate immune biomarker mRNA expression such as CD36, SRA, SRB-1, TLR-4, and Lox-1. The direct binding of PESDA to these biomarkers in vitro (Figs. 5 and 6) further corroborates the importance of PESDA interaction with scavenger receptors. Increases in the mRNA of these innate immune mediators have been shown to confer an increased risk of vascular disease such as atherosclerosis and support the importance of early detection from a risk management perspective. Innate immune mediators such as complement activation, IL-6, and C-reactive protein are central in the development and maintenance of vascular endothelial inflammation, injury, and dysfunction (14, 16, 18, 25, 26). IL-6 causes the release of acute phase proteins from the liver (i.e. C-reactive protein), promotes scavenger receptor expression, and is one of the major fundamental mediators of inflammation following injury and/or infection. IL-6 is also associated with loss of the cell membrane glycocalyx, which is important in the binding of albumin microbubbles to inflamed vessels (27). The pattern of co-localization of CD36, SRB-1, and TLR-4 to PESDA without co-localization to CD68-expressing monocytes/macrophages (Fig. 3) suggests that scavenger receptor expression occurs within the native cellular components of the artery. Additionally, PESDA, CD36, and TLR-4 tissue staining is also exclusive of each other (Fig. 3, A and B), and co-localization also occurs with cells of the blood pool. This further illustrates the complex interactions of PESDA shell proteins with their various receptors in vivo. It is likely that specific scavenger receptor-PESDA interactions will vary over the time course of injury and repair as well as with the specific tissue bed being observed.
It has been demonstrated previously that complement inactivation (heat inactivation of serum) results in a decrease in the binding of microbubbles not only to the endothelium (4) but also to activated leukocytes (11, 27). Thus, to extend our understanding of microbubble interactions with scavenger receptors, we hypothesized that binding to scavenger receptors was dependent upon the oxidized nature of PESDA shell proteins (i.e. sonicated human albumin). The extent of oxidation of fresh human serum, heat-inactivated serum, PESDA microbubbles, LDL, ox-LDL, and MAA-LDL is presented in Fig. 7. Fig. 7 illustrates the extensive oxidation that occurs during the sonication of albumin and synthesis of our microbubbles. This insight and understanding led us to perform competitive inhibition binding assays with PESDA and scavenger receptors. Fig. 8 illustrates the results of competitive inhibition binding assays of PESDA and scavenger receptors performed with the known scavenger ligands ox-LDL and MAA-LDL. There was significant inhibition of PESDA binding when CHO cells were pre-exposed to ox-LDL and MAA-LDL. There was complete inhibition of binding with SRB-1 and “heat-inactivated or oxidized” serum (Fig. 6). With CD36, SRA, TLR-2, and TLR-4, there was variable inhibition, which is consistent with the nature of our inhibitors (i.e. oxidized serum proteins and lipids versus ox-LDL versus MAA-LDL) and the interactions with individual receptors.
As reported previously (4, 12), complement is important in microbubble interactions with injured vessels. Historically, the complement cascade and scavenger pathways have been viewed as separate innate immune pathways. However, it is now recognized that there is a regulatory interplay between these two systems (28). Thus, the complex interplay of complement activation and subsequent modulation of scavenger receptor expression and function raises the possibility that prior published models of complement depletion simply altered the functional expression of albumin microbubble receptors (i.e. scavenger receptors) and thus decreased the observed microbubble binding to injured vasculature. These data illustrate the extremely complex nature of microbubble interactions with innate immune pathways and inflamed vessels.
The variable and focal immunohistochemical staining of the vessel wall for PESDA microbubbles illustrates that our model of balloon injury is not circumferentially uniform (Figs. 2 and 3). This focal histochemical staining of PESDA illustrates the complex interaction of PESDA with the injured aortic wall. Bubble interactions occur not only at the endothelium but possibly with inflammatory and/or native tissue cells at sites of injury. It is also reasonable to postulate that microbubbles are retained in or reflect the hyperemia of the vasa vasorum after balloon injury. The variable binding to and up-regulation of scavenger receptors are consistent with the focal and variable binding of microbubbles seen by SEM (Fig. 1) and IHC (Figs. 2 and 3).
In summary, this study demonstrated that 1) PESDA microbubbles bind to balloon-injured aortic endothelium as determined by contrast pulse sequencing ultrasound (4, 5), SEM, and IHC; 2) scavenger receptors are involved in the binding as demonstrated by increases in mRNA expression, in vivo co-localization, IHC, and CHO cell binding experiments; 3) scavenger receptor mRNA is dependent upon the extent of injury to the aorta; 4) PESDA microbubble binding is due to interactions of oxidized PESDA shell proteins; and 5) PESDA binding to balloon-injured aortic tissue may serve as a surrogate marker of innate inflammatory mediators. Thus, these findings detail the binding of PESDA microbubbles to sites of vascular injury and further suggest a role for innate immune mediators such as scavenger receptors.
The nature of in vitro and in vivo binding interactions of scavenger receptors (i.e. binding and/or receptor activation), as well as changes associated with the timeline (acute versus chronic) of injured vascular tissues, needs to be better understood. We expect that binding to various scavenger receptors in vivo will differ over the time course of injury repair and will be dependent on the production of mRNA as well as the differential expression and presentation of active and functional scavenger receptors. The ability to further define the relationship of innate inflammatory mediators to inflammatory diseases such as arrhythmias and coronary artery disease is expected to refine the medical approach to patient risk management.
Acknowledgments
We thank Janice A. Taylor and James R. Talaska (Confocal Laser Scanning Microscope Core Facility, University of Nebraska Medical Center) for providing assistance with confocal microscopy and the Nebraska Research Initiative and the Eppley Cancer Center for support of the Core Facility.
This work was supported by National Scientist Development Grant-American Heart Association Grant 07030210N and the National Institutes of Health Student Loan Repayment Program (to D. R. A.) and by the Division of Cardiology, Department of Internal Medicine, at the University of Nebraska Medical Center.
- PESDA
- perfluorocarbon-exposed sonicated dextrose albumin
- SRA
- scavenger receptor A
- SRB-1
- scavenger receptor B-1
- TLR
- Toll-like receptor
- SEM
- scanning electron microscopy
- ox-LDL
- oxidized LDL
- MAA-LDL
- malondialdehyde-acetaldehyde-modified LDL
- IHC
- immunohistochemistry.
REFERENCES
- 1.Wilson P. W., D'Agostino R. B., Levy D., Belanger A. M., Silbershatz H., Kannel W. B. (1998) Circulation 97, 1837–1847 [DOI] [PubMed] [Google Scholar]
- 2.Karim R., Hodis H. N., Detrano R., Liu C. R., Liu C. H., Mack W. J. (2008) Am. J. Cardiol. 102, 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathiresan S., Larson M. G., Keyes M. J., Polak J. F., Wolf P. A., D'Agostino R. B., Jaffer F. A., Clouse M. E., Levy D., Manning W. J., O'Donnell C. J. (2007) Am. J. Cardiol. 99, 310–314 [DOI] [PubMed] [Google Scholar]
- 4.Anderson D. R., Tsutsui J. M., Xie F., Radio S. J., Porter T. R. (2007) Cardiovasc. Res. 73, 597–606 [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui J. M., Xie F., Cano M., Chomas J., Phillips P., Radio S. J., Lof J., Porter T. R. (2004) J. Am. Coll. Cardiol. 44, 1036–1046 [DOI] [PubMed] [Google Scholar]
- 6.Rasband W. S., Magelhaes P. J., Ram S. J. (2001) Biophotonics Int. 11, 36–42 [Google Scholar]
- 7.Acton S. L., Scherer P. E., Lodish H. F., Krieger M. (1994) J. Biol. Chem. 269, 21003–21009 [PubMed] [Google Scholar]
- 8.Kataoka H., Kume N., Miyamoto S., Minami M., Murase T., Sawamura T., Masaki T., Hashimoto N., Kita T. (2000) J. Biol. Chem. 275, 6573–6579 [DOI] [PubMed] [Google Scholar]
- 9.Lee K. D., Pitas R. E., Papahadjopoulos D. (1992) Biochim. Biophys. Acta 1111, 1–6 [DOI] [PubMed] [Google Scholar]
- 10.Miller Y. I., Viriyakosol S., Binder C. J., Feramisco J. R., Kirkland T. N., Witztum J. L. (2003) J. Biol. Chem. 278, 1561–1568 [DOI] [PubMed] [Google Scholar]
- 11.Lindner J. R., Coggins M. P., Kaul S., Klibanov A. L., Brandenburger G. H., Ley K. (2000) Circulation 101, 668–675 [DOI] [PubMed] [Google Scholar]
- 12.Lindner J. R., Song J., Xu F., Klibanov A. L., Singbartl K., Ley K., Kaul S. (2000) Circulation 102, 2745–2750 [DOI] [PubMed] [Google Scholar]
- 13.Hansson G. K., Libby P., Schönbeck U., Yan Z. Q. (2002) Circ. Res. 91, 281–291 [DOI] [PubMed] [Google Scholar]
- 14.Libby P., Ridker P. M. (2004) Am. J. Med. 116, Suppl. 6A, 9S–16S [DOI] [PubMed] [Google Scholar]
- 15.Naghavi M., Libby P., Falk E., Casscells S. W., Litovsky S., Rumberger J., Badimon J. J., Stefanadis C., Moreno P., Pasterkamp G., Fayad Z., Stone P. H., Waxman S., Raggi P., Madjid M., Zarrabi A., Burke A., Yuan C., Fitzgerald P. J., Siscovick D. S., de Korte C. L., Aikawa M., Airaksinen K. E., Assmann G., Becker C. R., Chesebro J. H., Farb A., Galis Z. S., Jackson C., Jang I. K., Koenig W., Lodder R. A., March K., Demirovic J., Navab M., Priori S. G., Rekhter M. D., Bahr R., Grundy S. M., Mehran R., Colombo A., Boerwinkle E., Ballantyne C., Insull W., Jr., Schwartz R. S., Vogel R., Serruys P. W., Hansson G. K., Faxon D. P., Kaul S., Drexler H., Greenland P., Muller J. E., Virmani R., Ridker P. M., Zipes D. P., Shah P. K., Willerson J. T. (2003) Circulation 108, 1772–1778 [DOI] [PubMed] [Google Scholar]
- 16.Wickelgren I. (2006) Science 312, 184–187 [DOI] [PubMed] [Google Scholar]
- 17.Li H., Sun B. (2007) J. Cell. Mol. Med. 11, 88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieuwdorp M., Meuwese M. C., Mooij H. L., van Lieshout M. H., Hayden A., Levi M., Meijers J. C., Ince C., Kastelein J. J., Vink H., Stroes E. S. (2009) Atherosclerosis 202, 296–303 [DOI] [PubMed] [Google Scholar]
- 19.Boekholdt S. M., Agema W. R., Peters R. J., Zwinderman A. H., van der Wall E. E., Reitsma P. H., Kastelein J. J., Jukema J. W. (2003) Circulation 107, 2416–2421 [DOI] [PubMed] [Google Scholar]
- 20.Edfeldt K., Bennet A. M., Eriksson P., Frostegård J., Wiman B., Hamsten A., Hansson G. K., de Faire U., Yan Z. Q. (2004) Eur. Heart J. 25, 1447–1453 [DOI] [PubMed] [Google Scholar]
- 21.Manning-Tobin J. J., Moore K. J., Seimon T. A., Bell S. A., Sharuk M., Alvarez-Leite J. I., de Winther M. P., Tabas I., Freeman M. W. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michelsen K. S., Wong M. H., Shah P. K., Zhang W., Yano J., Doherty T. M., Akira S., Rajavashisth T. B., Arditi M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsui K., Inoue N., Kobayashi S., Shiraki R., Honjo T., Takahashi M., Hirata K., Kawashima S., Yokoyama M. (2007) Heart Vessels 22, 416–422 [DOI] [PubMed] [Google Scholar]
- 24.Erickson B., Sperber K., Frishman W. H. (2008) Cardiol. Rev. 16, 273–279 [DOI] [PubMed] [Google Scholar]
- 25.Buono C., Come C. E., Witztum J. L., Maguire G. F., Connelly P. W., Carroll M., Lichtman A. H. (2002) Circulation 105, 3025–3031 [DOI] [PubMed] [Google Scholar]
- 26.Hart M. L., Walsh M. C., Stahl G. L. (2004) Mol. Immunol. 41, 165–171 [DOI] [PubMed] [Google Scholar]
- 27.Lindner J. R., Ismail S., Spotnitz W. D., Skyba D. M., Jayaweera A. R., Kaul S. (1998) Circulation 98, 2187–2194 [DOI] [PubMed] [Google Scholar]
- 28.Hajishengallis G., Lambris J. D. (2010) Trends Immunol. 31, 154–163 [DOI] [PMC free article] [PubMed] [Google Scholar]