Abstract
The DELTA like-4 ligand (DLL4) belongs to the highly conserved NOTCH family and is specifically expressed in the endothelium. DLL4 regulates crucial processes in vascular growth, including endothelial cell (EC) sprouting and arterial specification. Its expression is increased by VEGF-A. In the present study, we show that VEGF-induced DLL4 expression depends on NOTCH activation. VEGF-induced DLL4 expression was prevented by the blockage of NOTCH signaling with γ-secretase or ADAM inhibitors in human cardiac microvascular ECs. Similar to VEGF-A, recombinant DLL4 itself stimulated NOTCH signaling and resulted in up-regulation of DLL4, suggesting a positive feed-forward mechanism. These effects were abrogated by NOTCH inhibitors but not by inhibition of VEGF signaling. NOTCH activation alone suffices to induce DLL4 expression as illustrated by the positive effect of NOTCH intracellular domain (NICD)-1 or -4 overexpression. To discriminate between NICD/RBP-Jκ and FOXC2-regulated DLL4 expression, DLL4 promoter activity was assessed in promoter deletion experiments. NICD induced promoter activity was dependent on RBP-Jκ site but independent of the FOXC2 binding site. Accordingly, constitutively active FOXC2 did not affect DLL4 expression. The notion that the positive feed-forward mechanism might propagate NOTCH activation to neighboring ECs was supported by our observation that DLL4-eGFP-transfected ECs induced DLL4 expression in nontransfected cells in their vicinity. In summary, our data provide evidence for a mechanism by which VEGF or ligand-induced NOTCH signaling up-regulates DLL4 through a positive feed-forward mechanism. By this mechanism, DLL4 could propagate its own expression and enable synchronization of NOTCH expression and signaling between ECs.
Keywords: Cell-Cell Interaction, Cytokine, Endothelium, NOTCH Pathway, Signal Transduction
Introduction
The NOTCH family encompasses a fundamental signaling pathway involving four receptors (NOTCH-1, -2, -3, and -4) and, in vertebrates, five cognate ligands (DLL-1, -3, and -4, and JAGGED-1 and -2). Cell-cell contact is a prerequisite for NOTCH signaling as all members are membrane bound. The first activation step upon receptor-ligand interaction involves cleavage of the extracellular domain of the NOTCH receptor by ADAM (a disintegrin and metalloprotease). Subsequently, the γ-secretase complex instigates a second proteolytic cleavage resulting in the release of the NOTCH intracellular domain (NICD)2 into the cytoplasm. Next, the NICD translocates to the nucleus, where it associates with the Recombinant signal binding protein for immunoglobulin kappa J region (RBP-Jκ) CBF1/RBP-Jκ, Su(H), Lag1 (CSL) transcription factor to initiate the transcription of its downstream targets basic helix-loop-helix proteins HES (hairy/enhancer of split) and hairy related transcription factors (HRT, HEY, and HERP) (1).
Among the ligands, DLL4 is specifically expressed in the arterial endothelium and plays a major role during embryonic vascular development. Dll4 haploinsufficiency is embryonically lethal due to severe vascular abnormalities related to a loss of arterial and venous specification (2–7). DLL4 overexpression on the other hand induces arterialization even in the venous compartment that typically lacks NOTCH expression (8).
NOTCH signaling is important for the development of endothelial cell sprouting. In this process, VEGF induces the formation of new sprouts, whereas DLL4 signaling appears to reduce their formation (9, 10). Because DLL4 is up-regulated by VEGF (11), this signaling pathway might serve as a negative feedback loop with respect to endothelial sprouting. This feedback could be bidirectional as NOTCH reduces VEGF responsiveness through down-regulation of VEGFR-2 (12) and subsequently suppresses the sprouting phenotype in the adjacent stalk cell, thus limiting the number of sprouts. Accordingly, loss of DLL4 function promotes excessive endothelial cell sprouting and endothelial cell migration (13). VEGF-A has also been reported to induce the activation of ADAM-10 and -17 (14), which are the proteases required for the first step in NOTCH activation. This interrelation between VEGF-A and NOTCH signaling suggests that VEGF not only induces the expression of DLL4 but also stimulates NOTCH signaling. However, alternative pathways have been suggested for VEGF-induced DLL4 expression. Up-regulation of DLL4 by VEGF has been considered the result of activated Forkhead box C (FOXC) transcription factors by a PI3K and ERK/MAPK-dependent pathway (15). FOXC2 has been shown to activate the DLL4 and HEY2 promoters, which both contain a forkhead binding element (FBE) (15, 16). In particular, FOXC2 functionally interacts with RBP-Jκ and NICD to activate the HEY2 promoter (15). Similarly to HEY2, the DLL4 promoter presents with multiple RBP-Jκ binding sites (17), suggesting that NOTCH might regulate DLL4 expression as well. Altogether, these observations prompted us to speculate that NOTCH signaling could play a role in up-regulating the expression of its own ligand DLL4, which in turn would activate NOTCH signal transduction, thus creating a positive feed-forward signaling that propagates the activation to adjacent cells.
In the present study, we show that in endothelial cells, VEGF-induced DLL4 up-regulation is NOTCH activation-dependent. VEGF positively affects DLL4 expression and NOTCH signaling mainly by activating VEGFR-2, whereas inhibition of NOTCH signaling abrogated VEGF-induced DLL4 expression. Consistently, immobilized recombinant DLL4 also induced endogenous DLL4 expression in a NOTCH-dependent manner unrelated to VEGF signaling, indicating a feed-forward mechanism downstream of NOTCH. The increase in DLL4, along with transactivation of the DLL4 promoter by NICD-1 and NICD-4 confirmed the role of NOTCH signaling in this process, further suggesting that NOTCH activation is sufficient for DLL4 induction. In this endothelial system, we found no evidence for FOXC2-dependent DLL4 expression by VEGF. Finally, we show that DLL4 transfected endothelial cells were able to increase DLL4 expression of surrounding cells providing a mechanism by which NOTCH signaling is distributed between communicating endothelial cells.
EXPERIMENTAL PROCEDURES
Reagents
Human cardiac microvascular endothelial cells (HCMvECs) (Lonza, Vervies, Belgium) were cultured in EGM-2MV, with 5% FBS (Lonza). Recombinant VEGF-A165 was purchased from RELIATech GmbH (Brauschweig, Germany); DLL4 (rDLL4) extracellular domain was from R&D Systems (Abingdon, Oxfordshire, UK); L685,458 (γ-secretase inhibitor) from Calbiochem (Nottingham, UK); and IMC-1121b (human VEGFR-2 inhibitor) and IMC-18F1 (human VEGFR-1 inhibitor) (18, 19) were both kindly provided by ImClone Systems Corp. (New York, NY). ADAM inhibitors GI250423X (ADAM-10 inhibitors) and GW28026X (ADAM-10/17 inhibitor) (20) were both kindly provided by GlaxoSmithKline.
Cell Culture and Cell Treatment
HCMvECs were cultured according to the manufacturer's protocol (Lonza). Cells were cultured in EGM-2MV medium without basic Fibroblast Growth Factor (bFGF) and VEGF-A165. Cells were stimulated for 24 h with 100 ng/ml of human recombinant VEGF-A165. For rDLL4 stimulation experiments, HCMvECs were grown for 48 h on 0.2% gelatin-coated plates containing 1 μg/ml rDLL4 or BSA as control (21). For inhibitor treatment, HCMvECs were incubated for 16 h with 5 μm L685,458, GI250423X, GW28026X, or 20 μg/ml IMC-1121b or IMC-18F1 antibodies prior to stimulation with VEGF-A165. Porcine aortic endothelial cells (PAECs), VEGFR-1/PAECs, and VEGFR-2/PAECs (22) were cultured in EGM-2MV medium without bFGF and VEGF-A165 (overnight) and subsequently stimulated with 100 ng/ml VEGF-A165 for 24 h. Cells were subcultured according to the manufacturer's protocol (Lonza), and a viable cell count was determined using trypan blue staining and the Countess Automated Cell Counter (Invitrogen).
Plasmids and Transfection
Mouse Notch-ICD-1 and human NOTCH-ICD-4 were both cloned in pcDNA expression vectors. The pIRES2-EGFP (Clontech) containing the full-length human DLL4 coding region, DLL4 promoter constructs pGL3-hDLL4 (6 kb), the pGL3-hDLL4 (−2616), the pGL3-hDLL4 SacI (−1517), and the pGL3-hDLL4 MscI (−931) were kindly provided by Professor Manfred Gessler and have been described previously in Ref. 17. To obtain a minimal DLL4 promoter fragment lacking any putative RPB-Jκ binding site the pGL3-hDLL4 MscI (−931) was digested with NruI and PstI to remove the RBP-Jκ binding site and then blunted and religated. Constitutively active FOXC2-VP16 pCB6 expressing vector was kindly provided by Professor Ormond A. MacDougald and has been described previously (23). All transfection experiments were carried out in triplicate. HCMvECs were grown on 0.2% gelatin-coated plates. Transfections were performed with Lipofectamine LTX and PLUS reagent (Invitrogen). After 24 or 48 h of culture, RNA or proteins were isolated and analyzed by RT-qPCR or Western blotting, respectively.
Luciferase Reporter Assay
HCMvECs seeded on 0.2% gelatin in PBS-coated plates were transiently transfected with 0.6 μg of either inducible RBP-Jκ responsive firefly luciferase construct (SuperArray Bioscience Corp., Frederick, MD). After 24 h of stimulation with VEGF-A165 or inhibitors, the cells were harvested using Dual Glo luciferase assay system (Promega, Leiden, the Netherlands), and reporter activity was measured on a PE Victor3 plate reader (PerkinElmer Life Sciences). PAECs were transiently transfected with pcDNA NICD-1, NICD-4, or vector alone along with the 6-kb DLL4 promoter firefly luciferase construct or with the deletional constructs of this promoter (−2616, −1587, −931, or −931-Deletion (D), respectively). The Renilla luciferase reporter plasmid was cotransfected as internal control for defining transfection efficiency. After 24 h of transfection, cells were harvested, and luciferase activity was assessed as reported above.
RNA Isolation and RT-qPCR and Protein Extraction
After each experiment, RNA was isolated by using RNeasy micro-kit Qiagen (Qiagen, GmbH, Hilden, Germany). A total of 100 ng RNA per sample was subjected to Reverse Transcriptase (RT). qPCR was performed using SuperscriptIIITM Platinum Two-step qRT-PCR kit with SYBR green (Invitrogen) and a primer concentration of 10 μm according to Van den Akker et al. (24). The PCR primers used were as follows: human β-actin sense, 5′-ATCCTCACCCTGAAGTACCC-3′; β-actin antisense, 5′-CACGCAGCTCATTGTAGAAG-3′; GADPH sense, 5′-GCCTCAAGATCATCAGCAAT-3′ and GADPH antisense, 5′-GGACTGTGGTCATGAGTCCT-3′; DLL4 sense, 5′-ACAACTTGTCGGACTTCCAG-3′ and DLL4 antisense, 5′-CAGCTCCTTCTTCTGGTTTG-3′; HES-1 sense, 5′-CCAAAGACAGCATCTGAGCA-3′; HES-1 antisense, 5′-GCCGCGAGCTATCTTTCTT-3′; porcine β-actin sense, 5′-gcatcctgaccctcaagtac-3; β-actin antisense, 5′-cacgcagctcgttgtagaag-3′; GADPH sense, 5′-gtgtcggttgtggatctga-3′ and GADPH antisense, 5′-cctgcttcaccaccttctt-3′; and DLL-4 sense, 5′-tgtcgcaatggaggtagctgcaag-3′ and DLL-4 antisense, 5′-aaggtgctatgttcgcagtgcagg-3′. Primers were designed with OligoPerfectTM Designer (Invitrogen), Primer 3 and Mfold and were synthesized by Eurogentec (Seraing, Belgium). qPCR reactions were run on a Bio-Rad MiQ real-time PCR detection System (Bio-Rad). Proteins were isolated using radioimmune precipitation assay lysis buffer supplemented with PMSF, protease inhibitor mixture, and sodium orthovanadate (Santa Cruz Biotechnology). The protein concentration was measured using micro BCA (Pierce). For Western blot analysis, XT designation reducing agent, and sample buffer (Bio-Rad) were added to the total cell lysates and samples and were heated at 95 °C for 5 min, loaded on a 4–12% gradient Criterion XT Bis-Tris gel, and were subsequently subjected to electrophoresis in MOPS running buffer (Bio-Rad) at 200 V for 55 min. Proteins were transferred to a PVDF membrane (Bio-Rad) at 30 V overnight using Tris-glycine transfer buffer. Nonspecific binding of the antibodies was blocked by incubating with 3% milk powder in TBS supplemented with Tween 20 (TBS-T) for 1 h. The membranes were exposed to anti-NOTCH-1 (Santa Cruz Biotechnology, catalog no. sc-6014) or anti-NOTCH-4 (Santa Cruz Biotechnology, catalog no. sc-5594), or anti-DLL4 (R&D Systems, catalog no. MAB1389) antibodies and were subsequently incubated with biotinylated goat anti-rabbit IgG (Vector Labs, catalog no. BA-1000) or biotinylated rabbit anti-rat (Vector BA-9001) secondary antibody. The detection was performed by using SuperSignal West Femto or Pico substrate (Pierce) and was visualized with the ChemiDoc XRS System (Bio-Rad).
Immunofluorescence Staining for DLL4 and eGFP
HCMVECs (Lonza, Switzerland) were cultured on four-well slides (BD Biosciences) until confluence and transfected with pIRES2-EGFP or pDLL4IRES2-EGFP. After 48 h, cells were incubated for 1 h with the primary rabbit anti-human DLL4 antibody (R&D Systems, catalog no. MAB1389). Slides were washed and incubated with 2% rabbit serum and secondary antibody Alexa Fluor 594 rabbit anti-rat (Invitrogen). Photographs were taken using a Leica DFC350 FX digital camera.
Statistical Analysis
Statistical significance was determined by applying one-way ANOVA/Tukey (SigmaStat Software) testing. Values are expressed as mean ± S.E. of three experiments. Probability values of < 0.05 were considered significant.
RESULTS
VEGFR2-mediated DLL4 Expression Involves NOTCH Signaling in Primary Endothelial Cells
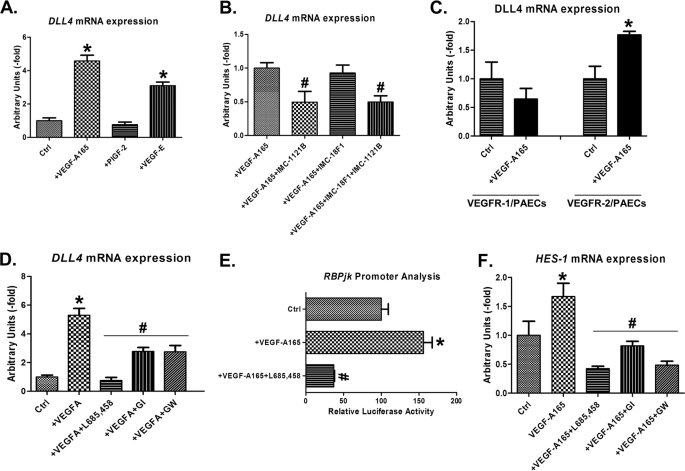
To investigate the effect of VEGFR-1 and VEGFR-2 signaling on DLL4 expression, HCMvECs were exposed to receptor specific ligands, i.e. Placenta Growth Factor-2 (PlGF-2) for VEGFR-1, VEGF-E for VEGFR-2, and VEGF-A165 for both receptors (25–27). Both VEGF-A165 and VEGF-E increased DLL4 expression, whereas PlGF-2 had no significant effect, suggesting a predominant involvement of VEGFR-2 (Fig. 1A). In accordance, specific inhibition of VEGFR-2 by the IMC-1121b antibody (Fig. 1B) prevented VEGF-A165 induced DLL4 expression, whereas VEGFR-1 inhibition with IMC-18F1 had no effect (Fig. 1B). Final confirmation for the dominance of VEGFR-2 signaling came from PAEC cell lines that overexpress either the human VEGFR-1 or VEGFR-2 (22). In line with the HCMvEC results, VEGF-A165 induced DLL4 only in VEGFR-2 transfected but not in VEGFR-1-transfected PAECs (Fig. 1C).
FIGURE 1.
VEGF-A165-induced DLL4 expression is VEGFR-2- and NOTCH signaling-mediated. A, DLL4 mRNA expression in HCMvECs treated with VEGF-A165, VEGF-E or PlGF-2 compared with the expression of nontreated cells as control. B, DLL4 mRNA expression in HCMvECs treated with VEGF-A165 or VEGF-A165 along with IMC-1121b (hVEGFR-2 inhibitor), or IMC-18F1 (hVEGFR-1 inhibitor) or IMC-1121b and IMC-18F1. C, DLL4 mRNA expression in VEGFR-2/PAECs (right) and VEGFR-1/PAECs (left) upon stimulation with or without VEGF-A165. D, DLL4 mRNA expression in HCMvECs stimulated with or without VEGF-A165 and VEGF-A165 along with L685,458 (γ-secretase inhibitor), GI254023X (ADAM-10 inhibitor) or GW280264X (GW; ADAM-10/ADAM-17 inhibitor). E, luciferase activity of HCMvECs cell lysates transiently transfected with RBP-Jκ -luciferase reporter construct and cultured with or without VEGF-A165 or VEGF-A165 along with L685,458 (γ-secretase inhibitor). F, HES-1 mRNA expression in HCMvECs stimulated with or without VEGF-A165 and VEGF-A165 along with L685,458 (γ-secretase inhibitor), GI254023X (ADAM-10 inhibitor), or GW280264X (ADAM-10/ADAM-17 inhibitor). Data represent mean ± SE (n = 3). One-way ANOVA test was used. *, significantly different from control p < 0.05; #, significantly different from VEGF-A165; p < 0.05. Ctrl, control.
VEGFA-induced DLL4 expression in HCMvECs was abrogated by blockage of NOTCH signaling with γ-secretase and ADAM inhibitors (Fig. 1D), suggesting a role for NOTCH signaling. To further substantiate that VEGF-A165 activated NOTCH-signaling, RBP-Jκ luciferase activity and NOTCH target gene HES-1 expression were assessed. VEGF-A165 induced RPB-Jκ promoter activity and HES-1 expression (Fig. 1, E and F), an effect that was prevented by NOTCH inhibition (Fig. 1, E and F).
Activated NOTCH Signaling Induces DLL4 Expression by Activating DLL4 Promoter
NOTCH-1 and NOTCH-4 are the main mediators of NOTCH signaling in the endothelium (28, 29). To define the direct effect of NOTCH signaling on DLL4 expression, HCMvECs were transfected with the intracellular domain of NOTCH-1 and -4 (NICD-1 and NICD-4, respectively) or empty vector. Both NICD-1 and -4 induced DLL4 mRNA and protein expression (Fig. 2, A and C, lower panel). Endogenous NOTCH signaling was not required for this stimulation as NICD-1 and -4 overexpression had the same effect in the presence of γ-secretase inhibition (Fig. 2B).
FIGURE 2.
NICD-1- and NICD-4-induced DLL4 expression in HMvECs. A and B, DLL4 mRNA expression in HCMvECs transiently transfected with NICD-1, NICD-4, or pcDNA empty vector as control with or without L685,458. Data represent mean ± SE (n = 3). One-way ANOVA test was used. *, significantly different from control (mock) p < 0.05. C, Western blot analysis of protein extracts from HCMvECs transfected with NICD-1 and NICD-4. Membranes were probed with anti-NOTCH-1 (upper left panel), anti-NICD-4 (upper right panel), or anti-DLL4 (lower panel).
The DLL4 promoter has previously been described to contain a FBE and DLL4 expression was reported to be induced by VEGFA in a FOXC-mediated manner (15, 16). Furthermore, the DLL4 promoter sequence also contains several RBP-Jκ binding sites (17), suggesting that NOTCH signaling might regulate DLL4 expression as well.
To discriminate between FOXC and NICD/RBP-Jκ-regulated DLL4 expression, several deletional DLL4 promoter fragments were analyzed for their transactivation activity upon NICD-1 and -4 activation in PAECs. These promoter fragments lacked the FBE site and contained either three (−2616), two (−1587), one (−931), or no (−931-D) RBP-Jκ binding site, respectively (17). The 6-kb DLL4 promoter fragment was significantly stimulated when co-transfected with NICD-1 or NICD-4 (Fig. 3A). FBE deletion in the presence of the three RBP-Jκ sites resulted in NICD-1 transactivation that was comparable with the full-length promoter. Significant transactivation was also apparent after NICD-4 overexpression, although it was less effective than NICD-1 (Fig. 3A).
FIGURE 3.
Induction of DLL4 promoter activity by NICD-1 and NICD-4. A, luciferase assay activity from PAE cells contransfected with a 6-kb DLL4 promoter luciferase reporter or shortened fragments of the 6-kb promoter containing three (−2616), two (−1587), one (−931), or none (−931-D) RBP-Jκ binding site along with NICD-1, NICD-4, or empty pcDNA (mock) vector. Schematic drawing of the 6-kb DLL4 promoter and of the shortened fragments −2616, −1587, −931, and −931-D (relative to the transcriptional initiation site) with potential RBP-Jκ (R) sites indicated by red bars, FBE is indicated by a green bar, and deletion of (R) is indicated by an X. B, DLL4 mRNA expression in PAECs transiently transfected with NICD-1 and NICD-4 and pcDNA as control. Data represent mean ± SE (n = 3). One-way ANOVA test was used. *, significantly different from control (mock), p < 0.05. C, Western blot analysis of protein extracts from PAECs transfected with NICD-1 (upper panel) and NICD-4 (lower panel). Results are from one representative experiment. D, DLL4 mRNA expression in HCMvECs (left panel) and PAECs (right panel) transiently transfected with constitutively active FOXC2 (caFOXC2) and empty vector as control.
The shorter −1587 and −931 constructs, containing two and one RBP-Jκ binding sites, respectively, showed reduced but still significant induction by NICD-1. Although NICD-4 transactivated the −1587 fragment that contained two RBP-Jκ binding sites, NICD-4 was unable to induce promoter activity in the −931 DLL4 promoter fragment that presented with only one RBP-Jκ binding site. As expected, no NICD transactivation effect was observed for the −931-D fragment that lacked both the FBE and all RBP-Jκ binding sites (Fig. 3A).
Like in HCMvECs, NICD-1 and -4 overexpression (Fig. 3C) up-regulated DLL4 mRNA expression (Fig. 3B) in PAECs. Transfection of both HCMvECs and PAECs with a constitutively active FOXC2 expressing vector did not alter DLL4 expression (Fig. 3D), indicating that in endothelial cells, the FOXC2 pathway seems redundant for NOTCH-stimulated DLL4 expression.
DLL4-induced NOTCH Signaling Up-regulates DLL4 Expression
To address a potential DLL4-driven positive feed-forward loop, rDLL4 protein was used to stimulate the NOTCH pathway in HCMvECs. Similar to VEGFA, rDLL4 was able to increase the expression of DLL4. NOTCH signaling blockage by γ-secretase and ADAMs inhibitors prevented rDLL4-induced DLL4 expression (Fig. 4A). The activation of NOTCH signaling by rDLL4 was further assessed by RBP-Jκ luciferase promoter analysis. rDLL4 stimulation induced transactivation of RBP-Jκ promoter, which was prevented by NOTCH inhibitors (Fig. 4B). To investigate whether rDLL4-induced DLL4 expression depends on VEGFR-2 signaling, we cultured HMCvECs on rDLL4 along with specific VEGFR-2 or/and VEGFR-1 inhibitors and analyzed DLL4 expression. The inhibition of VEGFR-2, VEGFR-1, or of both receptors did not affect rDLL4-induced DLL4 expression suggesting that VEGF signaling is not required for the positive feed-forward circuit (Fig. 4C).
FIGURE 4.
rDLL4 activated NOTCH signaling induced DLL4 expression. A, DLL4 mRNA expression in HCMvECs stimulated with BSA as control, rDLL4, or rDLL4 along with L685,458 (γ-secretase inhibitor), or GI254023X (ADAM-10 inhibitors) or GW280264X (ADAM-10 and ADAM-17). B, luciferase activity of HCMvEC cell lysates transiently transfected with RBP-Jκ-luciferase reporter construct and stimulated with BSA as control, rDLL4 protein or with rDLL4 and L685,458. C, DLL4 mRNA expression in HCMvECs stimulated with BSA as control, rDLL4 or rDLL4 along with IMC-1121b (hVEGFR-2 inhibitor), or IMC-18F1 (hVEGFR-1 inhibitor) or IMC-1121b and IMC-18F1. Data represent mean ± SE (n = 3). One-way ANOVA test was used. *, significantly different from control (BSA) p < 0.05; #, significantly different from rDLL4.
DLL4 Induces Propagation of Endogenous DLL4 Expression in Adjacent Cells
As NOTCH is an intercellular signaling pathway, we hypothesized that NOTCH-induced DLL4 expression taking place in one specific cell would eventually induce NOTCH signaling and subsequently DLL4 expression in its neighboring cell. To test this hypothesis, HCMvECs were transfected with pIRES2-eGFP or pDLL4IRES2-eGFP with or without NOTCH signaling inhibitor (L685,458), and DLL4 expression was analyzed by immunofluorescence staining. The eGFP (green) signal allows to discriminate between transfected and nontransfected cells (Fig. 5, A–C, GFP). pIRES2-eGFP-transfected control cells presented relatively weak DLL4 expression (red staining) in their surrounding cells (Fig. 5A, DLL4). In contrast, the pDLL4IRES2-eGFP-transfected cells revealed high DLL4 levels, and prominent DLL4 staining was also detected in the nontransfected surrounding cells (Fig. 5B, DLL4). The pDLL4IRES2-eGFP expressing cells treated with L685,458 had high DLL4 expression; however, no DLL4 was detected in the surrounding nontransfected cells (Fig. 5C, DLL4) indicating that inhibition of NOTCH signaling was sufficient to prevent the DLL4-induced positive feed-forward loop.
FIGURE 5.
DLL4-overexpressing cells induce endogenous DLL4 expression in the neighboring and adjacent cells DLL4 immunofluorescent labeling in pIRES-eGFP transfected HCMvECs versus pIRES-DLL4-eGFP transfected HCMvECs with or without L685,458. eGFP (green) signal allows to discriminate between transfected and nontransfected cells (A–C; GFP). pIRES-DLL4-eGFP transfected ECs revealed higher DLL4 staining (red) (B and C; DLL4) compared with pIRES-eGFP transfected cells (A; DLL4). Cells surrounding pIRES-eGFP-DLL4 transfected cells also showed increased DLL4 expression (B; DLL4) compare with cells surrounding pIRES-eGFP transfected cells (A; DLL4). This effect was prevented by adding the NOTCH signaling inhibitor L685,458 (C, DLL4). Merged images between eGFP and DLL4 staining (A–C; Merge) and phase contrast microscopy (PCI) images (A–C; PCI) are also reported. Scale bars, 50 μm.
DISCUSSION
The VEGF and NOTCH signaling pathways and their intricate interactions are indispensable for regulating blood vessel formation. VEGF-A has been reported to modulate the expression of NOTCH members in arterial endothelial cells. VEGFR-2 acts as the main receptor in mediating VEGF signaling in vascular endothelial cells (22, 30, 31) and likewise has been suggested to positively regulate NOTCH/DELTA expression (10, 11, 21, 24, 32). Also a reciprocal effect of NOTCH signaling on the expression of VEGFR-2 has been reported, i.e. a reduction in VEGFR-2 and an increase in VEGFR-1 expression with subsequent drop in VEGFA responsiveness (32).
Accordingly, we show that in primary adult HCMvECs, the expression of DLL4 is stimulated by the VEGFR-2 acting ligands VEGF-A165 and VEGF-E, and not by PlGF-2. Intriguingly, no other NOTCH ligands beside DLL4 were affected by VEGF-A-related endothelial signaling (data not shown) suggesting a selectivity of VEGF-A-related signaling in primary adult microvascular ECs. The essential role of VEGFR-2 was confirmed in porcine aortic endothelial cells expressing only one of the receptors and with a specific VEGFR-2 inhibitor. The presented data clearly show that up-regulation of DLL4 by VEGF-A is NOTCH signaling-dependent. VEGF-A failed to induce DLL4 expression when NOTCH signaling was inhibited, indicating the requirement of activated NOTCH for this process. In addition, NOTCH signaling alone was sufficient to induce DLL4 expression as shown by NICD-1 and NICD-4 overexpression. A comparable effect of VEGF-A-induced DLL4 expression was described for embryonic (33) and human bone marrow-derived mesenchymal stem cells (4).
We show that both NICD-1 and NICD-4 were able to transactivate the DLL4 promoter. Previous reports (15, 16) indicated that the FOXC2 transcription factor can serve as a mediator for VEGF-A-induced DLL4 expression by interacting with a FBE located in the DLL4 promoter. Besides the FBE, multiple RBP-Jκ binding sites are present in the DLL4 promoter sequence, providing the potential molecular cues for NOTCH-induced expression. Indeed, NICD-1 effectively transactivated both the full-length DLL4 promoter and FBE-deleted DLL4 promoter sequences, providing evidence that the FOX transcription factor pathway does not mediate VEGF-induced NOTCH activation in the endothelium. Accordingly, overexpression of a constitutively active FOXC2 transcript did not increase DLL4 expression. The FBE lacking −2616, −1587, and −931 promoter constructs showed a transactivation profile that suggests dependence on the number of RBP-Jκ binding sites. As expected, both NICD-1 and -4 were unable to stimulate the −931 DLL4 promoter fragment that lacked any RBP-Jκ binding site. NICD-4 had a similar profile of transactivation as NICD-1 with respect to −2616 and −1587 fragments but, in accordance with previous studies, at lower levels (34). This difference likely relates to the presence of a C-terminal autonomous transactivation domain in the NICD-1, which acts independently of RBP-Jκ. Such a transactivation domain sequence is absent in the NICD-4, and consequently, it requires RBP-Jκ to initiate transcription (35). Therefore, the reduced induction capacity of NICD-4 is likely related to the number of RBP-Jκ that are present in the shortened −2616 and −1587 DLL4 promoter fragments. Accordingly, no effect was shown by NICD-4 for the −931 DLL4 promoter fragment that contained only one RBP-Jκ binding site, whereas NICD-1 could still effectively transactivate this promoter.
NOTCH is typically activated by one its ligands, including membrane-bound DLL4. Therefore, similar to VEGF-A, NOTCH activation by surface-bound recombinant DLL4 induced DLL4 expression in HCMvECs. Inhibition of NOTCH signaling, but not of VEGF signaling, was sufficient to prevent this induction. These data indicate that activated NOTCH can induce DLL4 expression and transactivate the DLL4 promoter in absence of FOXC transcription factors and support the observation that NOTCH can induce DLL4 expression independently of FOXC2-mediated VEGF signaling. To our knowledge, these results show for the first time that DLL4 induces its own expression in endothelial cells, most likely through direct NICD-mediated transcriptional regulation. VEGF signaling also induced the up-regulation of HES-1 and RBP-Jκ transactivation, indicating that VEGF-A activates NOTCH signaling. This activation is likely not secondary to ligand overexpression, as NOTCH inhibition prevented the VEGF-A-induced expression of DLL4.
The link between VEGF-A and NOTCH signaling most likely relates to VEGFR2 increased ADAM expression and activity. In a previous study, we showed that ADAM expression and protease activity (i.e. sheddase) in HUVECs and VEGFR-2/PAEC was increased upon VEGF-A165 stimulation (36). VEGF-A has also been reported to induce the expression of ADAM-10 and to enhance shedding of VEGFR-2 and its co-receptor NRP1 by activating ADAM-10 and ADAM-17, respectively (14). Although this mechanism has been postulated to modulate VEGF-A signaling (14), VEGF-A increased ADAM sheddase activity might potentiate NOTCH signaling as well. In line with our observations, Hainaud et al. (37) showed an accumulation of cleaved NOTCH-4 in VEGF-A-treated HUVECs with concomitant up-regulation of the ADAM and presinilin (part of the γ-secretase complex) expression.
The involvement of ADAM-10 and ADAM-17 in NOTCH processing and signaling has been unequivocally established (38–42). ADAM-10 mutant mice phenotypically resemble Notch-1 null mutants and die at embryonic day 9.5, whereas Adam-17 mutants are viable (43). Among others Bozkulak and Weinmaster (41) showed that NOTCH-1 is a substrate for both ADAM-10 and ADAM-17 and, although ADAM-10 was essentially required for ligand induced NOTCH-1 signaling, ADAM-17 mediated ligand independent signaling. The importance of ADAM in NOTCH signaling was also reflected by our experiments showing that inhibition of ADAM activity abrogated the VEGF-A induced expression of DLL4 and the NOTCH transcription factor HES-1. In addition, rDLL4-induced DLL4 expression was depressed by blockage of ADAM activity as well. Altogether, these data indicate that VEGF-A activates ADAM, which is required for NOTCH signaling activation.
The ability of the activated NOTCH receptor to specifically induce the expression of its ligand would provide endothelial cells a mechanism by which NOTCH signaling is distributed and propagated between communicating cells with initially a limited amount of ligand. A comparable positive feed-forward mechanism has recently been described to regulate the interaction between endothelial and mural cells. JAGGED-1 ligand expressing endothelial cells induced NOTCH-3 signaling activation in mural cells (44), that in turn promote JAGGED-1 expression. In this way, mural cells can interact more efficiently with endothelial cells and can activate NOTCH signaling of neighboring cells, maintaining and distributing the signal to other mural cells. In the setting of membrane-bound ligands, the positive feed-forward mechanism is especially attractive to expand the signal to distant cells. The regulation of ligand expression by NOTCH receptors has already been described in Drosophila (45). NOTCH signaling was found to increase the expression of DELTA and SERRATE through a positive feedback loop during the regulation of the dorsoventral boundary of the developing wing (45). Likewise, in vertebrates, DELTA-like ligands are required during somitogenesis for the maintenance of somite borders and accumulate in a NOTCH-dependent manner (46–48).
The expression pattern of DLL4 ligand in arteries and sprouting vessels affects spatial distribution of NOTCH signaling in endothelial cells. During sprouting, tip endothelial cells will express high DLL4 levels and low NOTCH activity, whereas their neighbor stalk cells will have high NOTCH activity but low DLL4 expression. However, a static situation in which a subpopulation of cells presents high level of DLL4 and low NOTCH signaling is unlikely. NOTCH signaling is in fact required for arterial specification, and lack or reduction of NOTCH signaling would cause loss of arterial identity. Here, we show, that in contrast to eGFP-transfected cells, DLL4-eGFP ECs were able to induce DLL4 expression of nontransfected surrounding ECs, through a feed-forward mechanism, that was NOTCH signaling-dependent. Coordinated activation of NOTCH signaling would produce a wave of DLL4 expression that provides periodic NOTCH signaling activation in each cell (9). Such a mechanism could be important to maintain an arterial phenotype along a blood vessel and for regulating spatial patterning of branching as well (9, 49).
In summary, stimulation of VEGFR-2 by VEGF-A165 up-regulates DLL4 expression by activating NOTCH signaling in HCMvECs, independently of FOXC2. The NOTCH involvement in regulating DLL4 expression indicates the existence of a feed-forward mechanism by which NOTCH signaling can be propagated between adjacent endothelial cells.
Acknowledgments
We thank Dr. Bronek Pytowski from ImClone Systems Corporations for kindly providing the inhibitors IMC-18F1 and IMC-1121b and Dr. David Becherer from GlaxoSmithKline for the inhibitors GI254023X and GW280264X. We are grateful to Dr. Manfred Gessler for providing us with the pGL3-hDll4 (6 kb), the pGL3-hDll4 (−2616) and the pGL3-hDll4 SacI (−1587), pGL3-hDLL4 MscI (-931), and pDLL4-IRES-eGFP constructs and to Professor Ormond A. MacDougald for the FOXC2-VP16-pCB6 construct and Martin Köhler for technical support.
This work was supported by The Netherlands Heart Foundation Grant 2005B254 (to M. P.), the Dr. E. Dekker Grant 200T034 (to M. D.), and the Marie Curie FP6 Early Stage Researcher Training Grant MEST-CT-2005-020706 (to Cardiovascular Research Institute Maastricht).
- NICD
- NOTCH intracellular domain
- DLL4
- DELTA like-4 ligand
- FBE
- forkhead binding element
- VEGFR
- VEGF receptor
- HCMvEC
- human cardiac microvascular endothelial cell
- PAEC
- porcine aortic endothelial cell
- qPCR
- quantitative PCR
- MOPS
- 4-morpholinepropanesulfonic acid
- ANOVA
- analysis of variance
- NICD
- NOTCH intracellular domain
- EC
- endothelial cell
- FOXC
- Forkhead box C.
REFERENCES
- 1.Iso T., Kedes L., Hamamori Y. (2003) J. Cell. Physiol. 194, 237–255 [DOI] [PubMed] [Google Scholar]
- 2.Gale N. W., Dominguez M. G., Noguera I., Pan L., Hughes V., Valenzuela D. M., Murphy A. J., Adams N. C., Lin H. C., Holash J., Thurston G., Yancopoulos G. D. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 15949–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iso T., Maeno T., Oike Y., Yamazaki M., Doi H., Arai M., Kurabayashi M. (2006) Biochem. Biophys. Res. Commun. 341, 708–714 [DOI] [PubMed] [Google Scholar]
- 4.Zhang G., Zhou J., Fan Q., Zheng Z., Zhang F., Liu X., Hu S. (2008) FEBS Lett. 582, 2957–2964 [DOI] [PubMed] [Google Scholar]
- 5.Krebs L. T., Shutter J. R., Tanigaki K., Honjo T., Stark K. L., Gridley T. (2004) Genes Dev. 18, 2469–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y. H., Hu H., Guevara-Gallardo S., Lam M. T., Fong S. Y., Wang R. A. (2008) Development 135, 3755–3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aranguren X. L., Luttun A., Clavel C., Moreno C., Abizanda G., Barajas M. A., Pelacho B., Uriz M., Araña M., Echavarri A., Soriano M., Andreu E. J., Merino J., Garcia-Verdugo J. M., Verfaillie C. M., Prósper F. (2007) Blood 109, 2634–2642 [DOI] [PubMed] [Google Scholar]
- 8.Trindade A., Kumar S. R., Scehnet J. S., Lopes-da-Costa L., Becker J., Jiang W., Liu R., Gill P. S., Duarte A. (2008) Blood 112, 1720–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phng L. K., Gerhardt H. (2009) Dev. Cell 16, 196–208 [DOI] [PubMed] [Google Scholar]
- 10.Lobov I. B., Renard R. A., Papadopoulos N., Gale N. W., Thurston G., Yancopoulos G. D., Wiegand S. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3219–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z. J., Shirakawa T., Li Y., Soma A., Oka M., Dotto G. P., Fairman R. M., Velazquez O. C., Herlyn M. (2003) Mol. Cell. Biol. 23, 14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segarra M., Williams C. K., Sierra Mde L., Bernardo M., McCormick P. J., Maric D., Regino C., Choyke P., Tosato G. (2008) Blood 112, 1904–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedito R., Trindade A., Hirashima M., Henrique D., da Costa L. L., Rossant J., Gill P. S., Duarte A. (2008) BMC Dev. Biol. 8, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swendeman S., Mendelson K., Weskamp G., Horiuchi K., Deutsch U., Scherle P., Hooper A., Rafii S., Blobel C. P. (2008) Circ. Res. 103, 916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi H., Kume T. (2008) PLoS One 3, e2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo S., Fujita H., Nakano A., Kang M., Duarte A., Kume T. (2006) Dev. Biol. 294, 458–470 [DOI] [PubMed] [Google Scholar]
- 17.Diez H., Fischer A., Winkler A., Hu C. J., Hatzopoulos A. K., Breier G., Gessler M. (2007) Exp. Cell Res. 313, 1–9 [DOI] [PubMed] [Google Scholar]
- 18.Wu Y., Zhong Z., Huber J., Bassi R., Finnerty B., Corcoran E., Li H., Navarro E., Balderes P., Jimenez X., Koo H., Mangalampalli V. R., Ludwig D. L., Tonra J. R., Hicklin D. J. (2006) Clin. Cancer Res. 12, 6573–6584 [DOI] [PubMed] [Google Scholar]
- 19.Miao H. Q., Hu K., Jimenez X., Navarro E., Zhang H., Lu D., Ludwig D. L., Balderes P., Zhu Z. (2006) Biochem. Biophys. Res. Commun. 345, 438–445 [DOI] [PubMed] [Google Scholar]
- 20.Melenhorst W. B., Mulder G. M., Xi Q., Hoenderop J. G., Kimura K., Eguchi S., van Goor H. (2008) Hypertension 52, 987–993 [DOI] [PubMed] [Google Scholar]
- 21.Williams C. K., Li J. L., Murga M., Harris A. L., Tosato G. (2006) Blood 107, 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. (1994) J. Biol. Chem. 269, 26988–26995 [PubMed] [Google Scholar]
- 23.Gerin I., Bommer G. T., Lidell M. E., Cederberg A., Enerback S., Macdougald O. A. (2009) J. Biol. Chem. 284, 10755–10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Akker N. M., Caolo V., Wisse L. J., Peters P. P., Poelmann R. E., Carmeliet P., Molin D. G., Gittenberger-de Groot A. C. (2008) Cardiovasc. Res. 78, 366–375 [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N., Gerber H. P., LeCouter J. (2003) Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 26.Meyer M., Clauss M., Lepple-Wienhues A., Waltenberger J., Augustin H. G., Ziche M., Lanz C., Büttner M., Rziha H. J., Dehio C. (1999) EMBO J. 18, 363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsson A. K., Dimberg A., Kreuger J., Claesson-Welsh L. (2006) Nat. Rev. Mol. Cell Biol. 7, 359–371 [DOI] [PubMed] [Google Scholar]
- 28.Shawber C. J., Das I., Francisco E., Kitajewski J. (2003) Ann. N.Y. Acad. Sci. 995, 162–170 [DOI] [PubMed] [Google Scholar]
- 29.Iso T., Hamamori Y., Kedes L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 543–553 [DOI] [PubMed] [Google Scholar]
- 30.Hiratsuka S., Minowa O., Kuno J., Noda T., Shibuya M. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 9349–9354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalaby F., Rossant J., Yamaguchi T. P., Gertsenstein M., Wu X. F., Breitman M. L., Schuh A. C. (1995) Nature 376, 62–66 [DOI] [PubMed] [Google Scholar]
- 32.Harrington L. S., Sainson R. C., Williams C. K., Taylor J. M., Shi W., Li J. L., Harris A. L. (2008) Microvasc. Res. 75, 144–154 [DOI] [PubMed] [Google Scholar]
- 33.Lanner F., Sohl M., Farnebo F. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 487–493 [DOI] [PubMed] [Google Scholar]
- 34.Kurooka H., Kuroda K., Honjo T. (1998) Nucleic Acids Res. 26, 5448–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kato H., Sakai T., Tamura K., Minoguchi S., Shirayoshi Y., Hamada Y., Tsujimoto Y., Honjo T. (1996) FEBS Lett. 395, 221–224 [DOI] [PubMed] [Google Scholar]
- 36.Donners M. M., Wolfs I. M., Olieslagers S., Mohammadi-Motahhari Z., Tchaikovski V., Heeneman S., van Buul J. D., Caolo V., Molin D. G., Post M. J., Waltenberger J. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 2188–2195 [DOI] [PubMed] [Google Scholar]
- 37.Hainaud P., Contrerès J. O., Villemain A., Liu L. X., Plouët J., Tobelem G., Dupuy E. (2006) Cancer Res. 66, 8501–8510 [DOI] [PubMed] [Google Scholar]
- 38.Brou C., Logeat F., Gupta N., Bessia C., LeBail O., Doedens J. R., Cumano A., Roux P., Black R. A., Israël A. (2000) Mol. Cell 5, 207–216 [DOI] [PubMed] [Google Scholar]
- 39.Delwig A., Rand M. D. (2008) Cell Mol. Life Sci. 65, 2232–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian L., Wu X., Chi C., Han M., Xu T., Zhuang Y. (2008) Int. Immunol. 20, 1181–1187 [DOI] [PubMed] [Google Scholar]
- 41.Bozkulak E. C., Weinmaster G. (2009) Mol. Cell. Biol. 29, 5679–5695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Tetering G., van Diest P., Verlaan I., van der Wall E., Kopan R., Vooijs M. (2009) J. Biol. Chem. 284, 31018–31027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartmann D., de Strooper B., Serneels L., Craessaerts K., Herreman A., Annaert W., Umans L., Lübke T., Lena Illert A., von Figura K., Saftig P. (2002) Hum. Mol. Genet 11, 2615–2624 [DOI] [PubMed] [Google Scholar]
- 44.Liu H., Kennard S., Lilly B. (2009) Circ. Res. 104, 466–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Celis J. F., Bray S. (1997) Development 124, 3241–3251 [DOI] [PubMed] [Google Scholar]
- 46.Bettenhausen B., Hrabĕ de Angelis M., Simon D., Guénet J. L., Gossler A. (1995) Development 121, 2407–2418 [DOI] [PubMed] [Google Scholar]
- 47.Hrabĕ de Angelis M., McIntyre J., 2nd, Gossler A. (1997) Nature 386, 717–721 [DOI] [PubMed] [Google Scholar]
- 48.Jen W. C., Wettstein D., Turner D., Chitnis A., Kintner C. (1997) Development 124, 1169–1178 [DOI] [PubMed] [Google Scholar]
- 49.Gerhardt H. (2008) Organogenesis 4, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]