Abstract
Vasopressin-regulated water reabsorption through the water channel aquaporin-2 (AQP2) in renal collecting ducts maintains body water homeostasis. Vasopressin activates PKA, which phosphorylates AQP2, and this phosphorylation event is required to increase the water permeability and water reabsorption of the collecting duct cells. It has been established that the phosphorylation of AQP2 induces its apical membrane insertion, rendering the cell water-permeable. However, whether this phosphorylation regulates the water permeability of this channel still remains unclear. To clarify the role of AQP2 phosphorylation in water permeability, we expressed recombinant human AQP2 in Escherichia coli, purified it, and reconstituted it into proteoliposomes. AQP2 proteins not reconstituted into liposomes were removed by fractionating on density step gradients. AQP2-reconstituted liposomes were then extruded through polycarbonate filters to obtain unilamellar vesicles. PKA phosphorylation significantly increased the osmotic water permeability of AQP2-reconstituted liposomes. We then examined the roles of AQP2 phosphorylation at Ser-256 and Ser-261 in the regulation of water permeability using phosphorylation mutants reconstituted into proteoliposomes. The water permeability of the non-phosphorylation-mimicking mutant S256A-AQP2 and non-phosphorylated WT-AQP2 was similar, and that of the phosphorylation-mimicking mutant S256D-AQP2 and phosphorylated WT-AQP2 was similar. The water permeability of S261A-AQP2 and S261D-AQP2 was similar to that of non-phosphorylated WT-AQP2. This study shows that PKA phosphorylation of AQP2 at Ser-256 enhances its water permeability.
Keywords: Kidney, Membrane Proteins, Protein Kinase A (PKA), Protein Phosphorylation, Water Channel, Aquaporin, Water Permeability
Introduction
Body water homeostasis is essential for the survival of mammals and is regulated by the renal collecting duct. Key components in the regulation of collecting duct water permeability are the vasopressin receptor and the water channel aquaporin-2 (AQP2)2 (1–6). Mutations in the vasopressin V2 receptor and AQP2 cause congenital nephrogenic diabetes insipidus, a disease characterized by a massive loss of water through the kidney (7, 8). The binding of the antidiuretic hormone vasopressin to vasopressin V2 receptors on renal principal cells stimulates cAMP synthesis via activation of adenylate cyclase. The subsequent activation of PKA leads to phosphorylation of AQP2 at Ser-256, and this phosphorylation event is required to increase the water permeability and water reabsorption of renal principal cells. It has been established that the phosphorylation of AQP2 induces its apical membrane insertion, rendering the cell water-permeable. We showed recently that AQP2 binds to a multiprotein “motor” complex and that phosphorylation-dependent reciprocal interaction with G-actin and tropomyosin 5b directs AQP2 to the apical membrane (9–13). However, whether this phosphorylation regulates the water permeability of individual AQP2 proteins still remains unclear.
Kuwahara et al. (14) showed that cAMP stimulation increased the water permeability of AQP2 expressed in Xenopus oocytes, although this stimulation did not increase the amount of AQP2 on the oocyte membrane. This finding suggests that cAMP-mediated phosphorylation of AQP2 increases the water permeability of individual AQP2 proteins. On the other hand, Lande et al. (15) purified endosomes derived from the apical membrane of rat inner medullary collecting duct cells that were highly enriched for AQP2 and showed that their water permeability was not changed by AQP2 phosphorylation. However, it was not excluded that other proteins contained in oocytes or endosomes prepared from inner medullary collecting duct cells may have affected these responses. For example, basal phosphorylation levels of AQP2 determined by endogenous kinase or phosphatase activities may affect the results in these experimental systems. To clarify whether the water permeability of AQP2 is regulated by its phosphorylation event alone, an experimental system that does not contain other regulatory proteins is required.
In this study, we performed large-scale expression of full-length recombinant human AQP2, purified it, reconstituted it into proteoliposomes, and examined the protein function. Here, we show the direct evidence that the water permeability of AQP2 is regulated by its phosphorylation at Ser-256 by PKA.
EXPERIMENTAL PROCEDURES
Expression and Purification of Recombinant AQP2
Human AQP2 cDNA was subcloned into pET32 (Novagen, Madison, WI) to generate His-tagged thioredoxin (Trx)-fused AQP2 (AQP2/Trx). AQP2 mutants were generated by PCR using a QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the following sense primers: I, 5′-GTGCGACGGCGGCAGGCGGTGGAGCTGCACTCG-3′; II, 5′-GTGCGACGGCGGCAGGACGTGGAGCTGCACTCG-3′; III, 5′-TCGGTGGAGCTGCACGCGCCGCAGAGCCTGCCA-3′; IV, 5′-TCGGTGGAGCTGCACGACCCGCAGAGCCTGCCA-3′; V, 5′-CTGCACTCGCCGCAGGCGCTGCCACGGGGTACC-3′; VI, 5′-GCGGTGGAGCTGCACGCGCCGCAGAGCCTGCCA-3′; VII, 5′-CTGCACGCGCCGCAGGCGCTGCCACGGGGTACC-3′; and VIII, 5′-CTGCCACGGGGTGCGAAGGCCTGAAAGCTT-3′. The S256A and S256D mutants were generated from WT-AQP2 using primers I and II, respectively. The S261A and S261D mutants were generated from WT-AQP2 using primers III and IV, respectively. The S256A/S261A/S264A mutant was generated using primer VII from S256A/S261A, which was generated from S256A using primer VI. The S256A/S261A/S264A/T269A mutant was generated from S256A/S261A/S264A using primer VIII. The S256A/S261A/T269A mutant was generated from S256A/S261A using primer VIII. The S256A/S264A/T269A mutant was generated using primer VIII from S256A/S264A, which was generated from S256A using primer V. The S261A/S264A/T269A mutant was generated using primer VIII from S261A/S264A, which was generated from S261A using primer VII.
WT-AQP2/Trx and Mutants were expressed in Escherichia coli BL21 (Novagen) by induction with 1 mm isopropyl-β-d-thiogalactopyranoside for 4 h at 30 °C. The bacterial pellets were incubated with lysozyme, sonicated, and centrifuged at 10,000 × g. For AQP2 purification, the pellets were suspended with 6 m urea in PBS and centrifuged. The supernatants were diluted to a concentration of 2 m urea and applied to a column of TALON resin (Clontech). The column was washed and eluted by buffer A (50 mm K-HEPES (pH 7.6), 100 mm KCl, and 10 mm MgCl2) containing 2% octyl glucoside and 150 mm imidazole. AQP2 purification was confirmed by Coomassie Blue staining, silver staining, and immunoblotting using anti-AQP2 antibody, which was generated against a synthetic peptide corresponding to 15 C-terminal amino acid residues (positions 257–271) of AQP2 (12, 16). For PKA phosphorylation, AQP2 protein was added with 1 mm ATP and 200,000 units/ml cAMP-dependent PKA (New England Biolabs, Beverly, MA) and incubated at 30 °C for 1 h. The phosphorylation was confirmed by immunoblotting using antibodies for AQP2 phosphorylated at Ser-256 (12, 17). Antibodies for AQP2 phosphorylated at Ser-256 were generated against a synthetic peptide corresponding to amino acids 253–262 of human AQP2, with the addition of a cysteine residue at the C terminus and a glycine residue at the N terminus, which was phosphorylated at Ser-256, as described previously (18, 19).
Measurement of the Sites and Efficiency of Phosphorylation by PKA in Recombinant Human AQP2
The phosphorylation sites and efficiency were measured as described previously (20, 21) with the following modifications. A synthetic phosphopeptide corresponding to the C terminus of AQP2 (residues 231–271) that was phosphorylated at Ser-256 was used as a standard. As shown in Fig. 3D, WT-AQP2 and mutant proteins and the phosphopeptide standard were immunoblotted using antibodies for phosphoserine (Sigma) to examine their phosphorylation efficiencies. A dot blot was prepared by spotting 25 pmol (2 μl) of each AQP2 protein and the phosphopeptide standard onto a nitrocellulose membrane. The membrane was then processed as for Western blot analysis. Coomassie Blue staining was performed to confirm that the loaded molar amounts were equal among WT-AQP2 and mutant proteins.
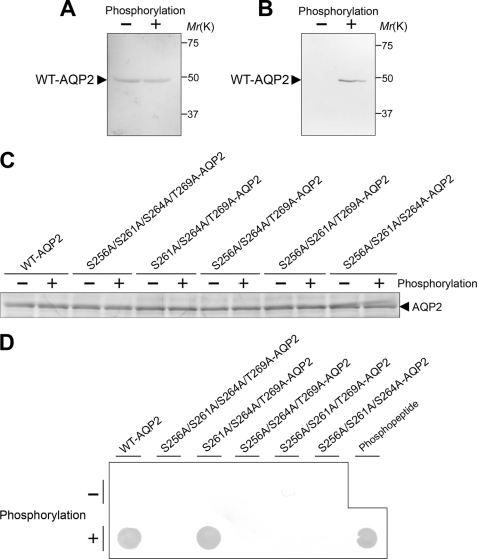
FIGURE 3.
In vitro phosphorylation of AQP2 by PKA. A and B, WT-AQP2 with or without phosphorylation was immunoblotted using anti-AQP2 antibody (A) and anti-phosphorylated AQP2 antibody (B). Anti-AQP2 antibody was generated against a synthetic peptide corresponding to 15 C-terminal amino acid residues (positions 257–271) of AQP2. Anti-phosphorylated AQP2 antibody was generated against a synthetic peptide corresponding to amino acids 253–262 of AQP2 with phosphorylation at Ser-256. Details are provided under “Experimental Procedures.” C and D, sites and efficiency of phosphorylation by PKA in AQP2. C, WT-AQP2 and mutant proteins (25 pmol each), with or without phosphorylation by PKA, were analyzed by SDS-PAGE and Coomassie Blue staining. D, WT-AQP2 and mutant proteins (25 pmol each), with or without phosphorylation by PKA, and the phosphopeptide standard were subjected to dot blot analysis using antibodies for phosphoserine.
Reconstitution of Recombinant AQP2 into Liposomes
Lipids (Sigma) were mixed in chloroform/methanol (2:1, v/v) to yield phosphatidylcholine (molar ratio of 1) and cholesterol (molar ratio of 1.6). After drying under N2 gas, they were resuspended in buffer A containing 2% octyl glucoside to a total lipid concentration of 7.5 mm, mixed with an equal volume of recombinant AQP2 (0.7 mg/ml), and dialyzed against 50 mm Tris-HCl (pH 7.5) and 10 mm MgCl2. The proteoliposomes were overlaid with step gradients of 30 and 40% OptiPrep (Axis-Shield, Oslo, Norway) and centrifuged using an SW41Ti rotor (Beckman Coulter, Fullerton, CA) at 39,000 rpm for 4 h. The proteoliposomes were collected at the 0–30% interface and extruded through polycarbonate filters with 50-nm pores using a LiposoFast extruder (Avestin, Ottawa, Canada) to obtain unilamellar vesicles (22).
Measurement of Osmotic Water Permeability
The stopped-flow experiments were performed on an Applied Photophysics SX18.MV stopped-flow apparatus. The proteoliposomes suspended in 50 mm Tris-HCl (pH 7.5) containing 1 mm DTT were abruptly mixed with 50 mm Tris-HCl (pH 7.5) containing 600 mm mannitol, and the liposomes were imposed with a 300 mm inwardly directed osmotic gradient. To inhibit the water transport of AQP2, AQP2-incorporated liposomes (AQP2-liposomes) were treated with 1 mm HgCl2 for 15 min. A decrease in the liposome volume due to osmotic water efflux driven by the osmotic gradient was monitored as the time-dependent increase in 90° scattered light intensity monitored at 466 nm. The data were fitted to a double exponential function. The osmotic water permeability (Pf) was calculated from the initial rate of volume change by the relation dV(t)/dt = Pf·SAV·VW(Osmout − Osmin), where V(t) is liposome volume at time t, SAV is initial surface area to volume ratio (calculated from the liposome diameter examined by electron microscopy), VW is the molar volume of water (18 × 10−3 liters/mol), and Osmout − Osmin is the difference in external and internal osmolarity.
Statistical Analysis
All values are expressed as means ± S.E. Data were analyzed by one-way analysis of variance, followed by the Bonferroni test for multiple comparisons, and p < 0.05 was considered to be statistically significant. All statistical procedures were carried out using SPSS Version 11.5 (SPSS Inc., Chicago, IL).
RESULTS
Expression and Purification of Recombinant WT-AQP2 and Mutants
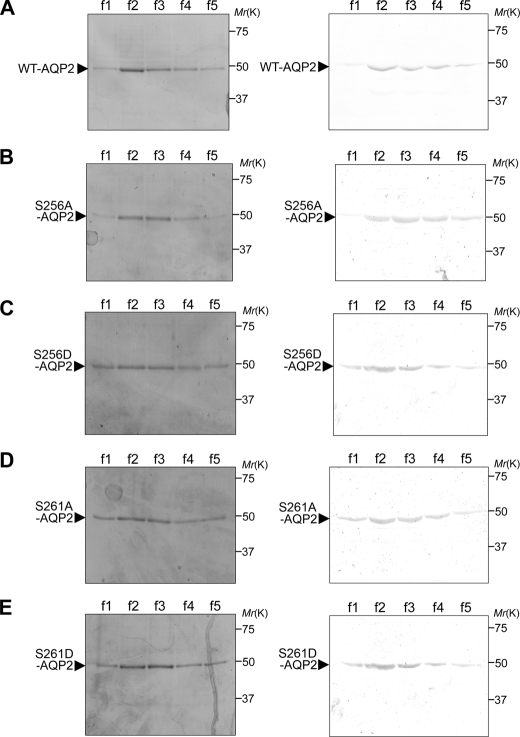
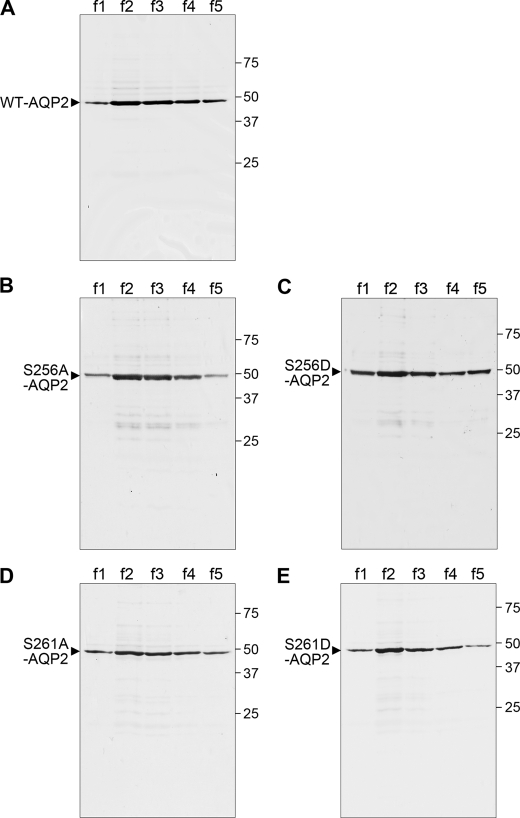
To examine the regulation of the water permeability of AQP2 by its phosphorylation without the effects of other regulatory proteins, we performed large-scale expression of full-length recombinant human AQP2 fused to Trx and purified it (Fig. 1A). A single band corresponding to the theoretical size of 49 kDa was recognized by both Coomassie Blue staining and immunoblotting using anti-AQP2 antibody, confirming that the purified recombinant protein preparation was indeed AQP2. To further confirm the purity of this protein, silver staining was performed (Fig. 2A). There were faint nonspecific protein bands. Densitometric analysis showed that the purity of AQP2 protein was >95%. To examine the roles of phosphorylation at Ser-256 and Ser-261, we also expressed and purified mutants mimicking the phosphorylated (S256D and S261D) and non-phosphorylated (S256A and S261A) states. In addition to Ser-256, phosphorylation at Ser-261 is also regulated by vasopressin, and its roles have been examined (17, 23–25). Coomassie Blue staining and immunoblotting using anti-AQP2 antibody showed the purification of these mutants (Fig. 1, B–E). Silver staining further confirmed that these mutant proteins were of high purity (Fig. 2, B–E).
FIGURE 1.
Purification of recombinant WT-AQP2 and mutants. Full-length human WT-AQP2 (A), S256A-AQP2 (B), S256D-AQP2 (C), S261A-AQP2 (D), and S261D-AQP2 (E) were fused to Trx; expressed in E. coli BL21; purified using a TALON metal affinity column; separated by SDS-PAGE; and subjected to Coomassie Blue staining (left panels) and immunoblotting using anti-AQP2 antibody (right panels). Lanes f1–f5 indicate consecutive serial elution fractions (5 × 500 μl) of 1 ml of the TALON matrix column.
FIGURE 2.
Silver staining of recombinant WT-AQP2 and mutants. Purified WT-AQP2 (A), S256A-AQP2 (B), S256D-AQP2 (C), S261A-AQP2 (D), and S261D-AQP2 (E) were separated by SDS-PAGE and subjected to silver staining. Lanes f1–f5 indicate consecutive serial elution fractions (5 × 500 μl), and 35 μl from each fraction was loaded.
Phosphorylation of AQP2 by PKA
WT-AQP2 was then subjected to in vitro PKA phosphorylation. Immunoblotting for total AQP2 and phosphorylated AQP2 confirmed AQP2 phosphorylation (Fig. 3, A and B).
We then examined the sites and efficiencies of phosphorylation of recombinant human AQP2 by PKA (Fig. 3, C and D). Recent phosphoproteome analyses have shown that three other sites are also phosphorylated in addition to Ser-256, although Ser-256 is phosphorylated predominantly in rat renal inner medullary collecting duct cells (20, 23). Ser-256 phosphorylation is most likely mediated by PKA (3, 26), whereas the kinases acting at the other sites have not been reported. To confirm the sites phosphorylated by PKA in recombinant human AQP2, we expressed and purified non-phosphorylation-mimicking mutants at plausible phosphorylation sites: Ser-256, Ser-261, Ser-264, and Thr-269. Coomassie Blue staining of 25 pmol each of WT-AQP2 and mutants, with or without phosphorylation by PKA, is shown in Fig. 3C. The band densities were equal, confirming that the loaded molar amounts were equal. In the immunoblot for phosphoserine, the signal of WT-AQP2 phosphorylated by PKA was similar to that of an equimolar amount of phosphopeptide, indicating that almost all WT-AQP2 was phosphorylated (Fig. 3D). Furthermore, the signal of the phosphorylated S261A/S264A/T269A-AQP2 mutant, in which Ser-256 was intact, was equal to those of phosphorylated WT-AQP2 and the phosphopeptide. On the other hand, positive signals were not detected for any mutants that included the S256A mutation. These findings indicate that only Ser-256 in recombinant AQP2 is phosphorylated by PKA.
Reconstitution of AQP2 into Liposomes
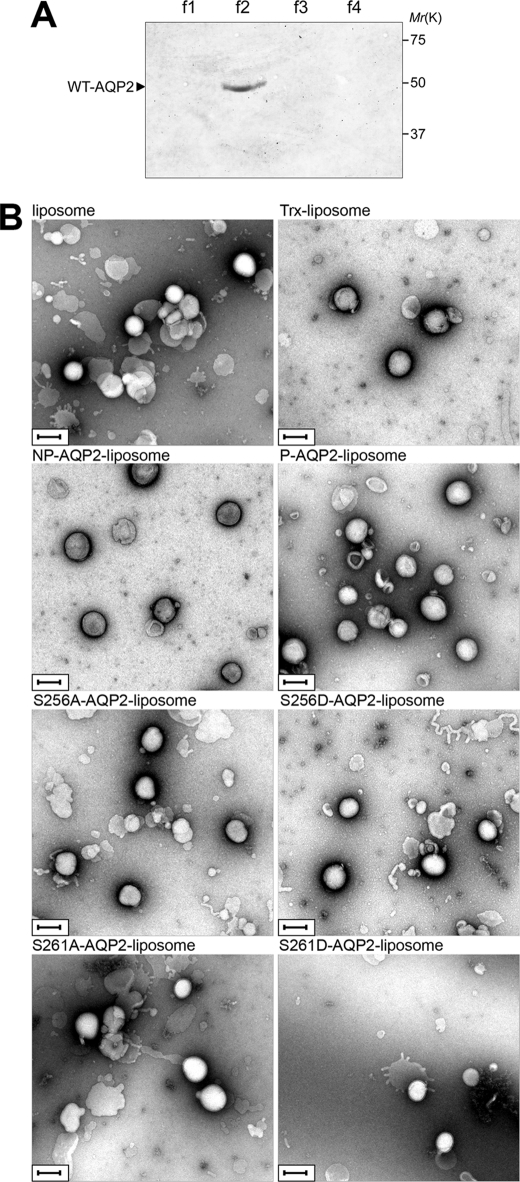
WT-AQP2 and mutants were reconstituted into proteoliposomes as described under “Experimental Procedures.” Proteins that were not reconstituted into liposomes were removed by fractionating on OptiPrep step gradients (Fig. 4A). The proteoliposomes were extruded through polycarbonate filters to obtain unilamellar vesicles (22). The size of the proteoliposomes was examined by negative stain electron microscopy (Fig. 4B). The diameter was similar in different kinds of liposomes: liposomes without protein reconstitution, 185 ± 4 nm; Trx-liposomes, 193 ± 5 nm; non-phosphorylated WT-AQP2-liposomes, 200 ± 4 nm; phosphorylated WT-AQP2-liposomes, 199 ± 5 nm; S256A-AQP2-liposomes, 189 ± 4 nm; S256D-AQP2-liposomes, 190 ± 4 nm; S261A-AQP2-liposomes, 200 ± 4 nm; and S261D-AQP2-liposomes, 196 ± 6 nm.
FIGURE 4.
Reconstitution of AQP2 into liposomes. A, WT-AQP2 reconstituted in proteoliposomes was fractionated on OptiPrep step gradients, separated by SDS-PAGE, and immunoblotted using anti-AQP2 antibody. The OptiPrep concentrations in each fraction were 0% (f1), 0–30% interface (f2), 30% (f3), and 40% (f4). Proteoliposomes existed in the 0–30% OptiPrep interface. B, negative stain electron microscopy of liposome without protein incorporation, Trx-liposome, non-phosphorylated WT-AQP2-liposome (NP-AQP2-liposome), phosphorylated WT-AQP2-liposome (P-AQP2-liposome), S256A-AQP2-liposome, S256D-AQP2-liposome, S261A-AQP2-liposome, and S261D-AQP2-liposome, which were derived from the f2 fraction. Scale bars = 200 nm.
PKA Phosphorylation of AQP2 Enhances Its Water Permeability
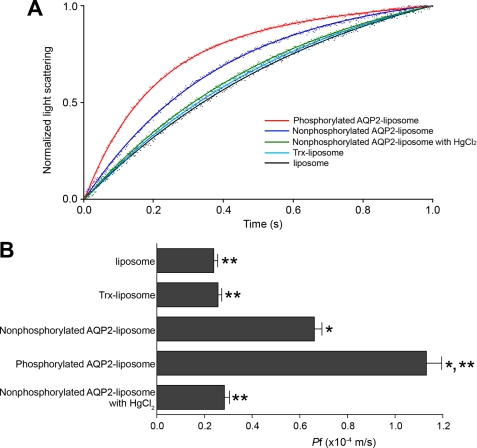
We then examined the Pf of WT-AQP2-liposomes, Trx-liposomes, and liposomes without protein reconstitution (Fig. 5). The Pf of non-phosphorylated WT-AQP2-liposomes was significantly greater than that of the control substances, which were Trx-liposomes and liposomes without protein incorporation. Treatment of WT-AQP2-liposomes with HgCl2 significantly decreased their Pf to the control levels. This finding indicates that the increased Pf of AQP2-liposomes is mediated by the AQP2 function of water transport. PKA phosphorylation significantly increased the water permeability of WT-AQP2-liposomes. These findings indicate that the water permeability of AQP2 is enhanced by its phosphorylation.
FIGURE 5.
PKA phosphorylation of AQP2 enhances its water permeability. A, stopped-flow analysis. Proteoliposomes or liposomes without protein incorporation were abruptly imposed to an inwardly directed osmotic gradient, and the vesicle shrinkage was monitored by measuring the increase in scattered light. The dots are measured values, and the solid lines are their fitted curves. Red, phosphorylated WT-AQP2-liposome; dark blue, non-phosphorylated WT-AQP2-liposome; green, non-phosphorylated WT-AQP2-liposome treated with HgCl2; light blue, Trx-liposome; black, liposome without protein incorporation. B, summary of osmotic water permeability (Pf). Data represent means ± S.E. from 15–20 independent experiments. *, p < 0.001 versus Trx-liposome; **, p < 0.001 versus non-phosphorylated AQP2-liposome.
Phosphorylation of AQP2 at Ser-256 Is Essential for PKA Regulation of Its Water Permeability
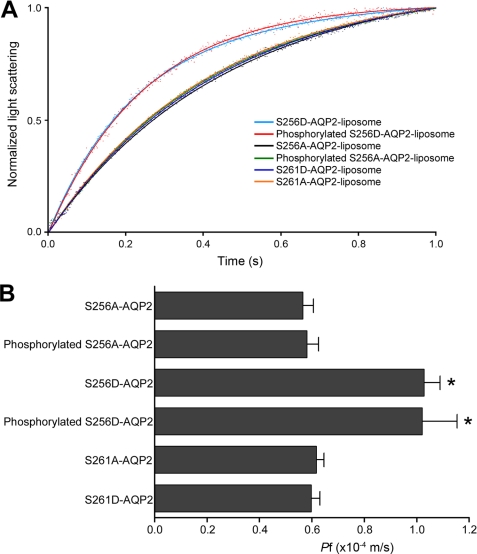
We then examined the role of AQP2 phosphorylation in the regulation of Pf using phosphorylation mutants of AQP2 reconstituted into proteoliposomes (Fig. 6). The Pf of non-phosphorylation-mimicking mutant S256A-AQP2-liposomes was similar to that of non-phosphorylated WT-AQP2-liposomes. On the other hand, the Pf of phosphorylation-mimicking mutant S256D-AQP2-liposomes was significantly increased up to the levels of phosphorylated WT-AQP2-liposomes. PKA phosphorylation did not alter the Pf of either S256A-AQP2 or S256D-AQP2. These findings indicate that phosphorylation of AQP2 at Ser-256 is essential for PKA regulation of its water permeability and that phosphorylation at other sites in AQP2 is not involved in this regulation. Furthermore, the Pf values of both S261A and S261D mutants were similar to those of non-phosphorylated WT-AQP2, indicating that phosphorylation at Ser-261 has no effect on Pf.
FIGURE 6.
Phosphorylation of AQP2 at Ser-256 is essential for PKA regulation of its water permeability. A, stopped-flow analysis of phosphorylation mutants of AQP2 reconstituted into liposomes. Light blue, S256D-AQP2-liposome; red, PKA-phosphorylated S256D-AQP2-liposome; black, S256A-AQP2-liposome; green, PKA-phosphorylated S256A-AQP2-liposome; dark blue, S261D-AQP2-liposome; orange, S261A-AQP2-liposome. B, summary of Pf. Data represent means ± S.E. from 15–25 independent experiments. *, p < 0.001 versus S256A-AQP2-liposome.
DISCUSSION
This study provides direct evidence for the regulation of the water transport activity of AQP2 by phosphorylation. AQP2-regulated water reabsorption in kidney collecting ducts is a key event for the maintenance of body water balance. In addition to the intracellular translocation of AQP2 to the luminal membrane, our findings indicate that the water transport activity of individual AQP2 proteins is important for the regulation of water reabsorption in kidney collecting ducts.
To date, several groups have examined the role of phosphorylation in Pf. Kuwahara et al. (14) examined the phosphorylation and Pf of AQP2 expressed in Xenopus oocytes. They showed that PKA phosphorylated AQP2 at Ser-256. cAMP stimulation increased the Pf of oocytes expressing AQP2, although this stimulation did not increase the amount of AQP2 on the oocyte surface, which was examined by immunoblotting of AQP2 using oocyte membranes. This finding suggested that the Pf of individual AQP2 proteins was increased by cAMP-mediated phosphorylation of AQP2. However, the possibility of phosphorylation-induced translocation of AQP2 could not be excluded in this experimental system. Recently, Moeller et al. (27) also examined the role of phosphorylation using AQP2 expressed in Xenopus oocytes. To evaluate the Pf of a single channel, Pf relative to the plasma membrane abundance was compared among WT-AQP2 and mutants. Both the Pf and plasma membrane abundance of the non-phosphorylation-mimicking mutant S256A-AQP2 were decreased compared with those of WT-AQP2, resulting in the Pf relative to the plasma membrane abundance being similar. This finding suggested that a lack of phosphorylation at this site had no effect on individual AQP2 proteins. However, the methods determining the plasma membrane abundance were semiquantitative, and this study could not exclude the possibility that the Pf of individual AQP2 proteins was altered by this mutation. On the other hand, Lande et al. (15) purified endosomes derived from the apical membrane of rat inner medullary collecting duct cells that were highly enriched for AQP2. These endosomes contained endogenous PKA and phosphatase activities that could phosphorylate and dephosphorylate AQP2. Therefore, these authors prepared two kinds of samples to detect the effect of phosphorylation on Pf. For phosphorylated AQP2, AQP2-endosomes were incubated with exogenous PKA catalytic subunit and ATP to maximize the phosphorylation levels. For non-phosphorylated AQP2, the above phosphorylated sample was then incubated with exogenous alkaline phosphatase, which was shown to remove 95% of the phosphate from AQP2. There was no significant difference in Pf between these two samples, suggesting that the Pf of AQP2 is not changed by its phosphorylation. However, the phosphorylation levels of AQP2-endosomes incubated with exogenous PKA and ATP progressively decreased over 20 min, which might have been caused by endogenous phosphatase activities. It is possible that the differences in Pf may have been underestimated due to the existence of endogenous PKA, phosphatase, and other regulatory proteins in AQP2-endosomes. Our experimental system using recombinant AQP2 reconstituted into liposomes did not contain any other proteins, and thus, the potential effects of other regulatory proteins were excluded. This provides direct evidence that phosphorylation of AQP2 regulates its water permeability.
In this study, it is possible that not all AQP2 proteins were reconstituted in liposomes with the proper orientation and topology, which may have caused underestimation of their water transport activity. However, significant water transport via AQP2 was observed, demonstrating functional reconstitution into liposomes, as shown in Fig. 5. Moreover, the effects of PKA phosphorylation and mutations of AQP2 on Pf were clearly observed (Figs. 5 and 6).
There is increasing evidence that aquaporins are gated (28). The spinach aquaporin SoPIP2;1 is gated by phosphorylation at Ser-115 and Ser-274 (29). Structural studies using electron diffraction (30) and x-ray crystallography (31) proposed the molecular mechanism of this gating. In the closed conformation, loop D caps the pore of this channel from the cytoplasm, preventing the water passage. In the open conformation, loop D is displaced, thereby unblocking the cytoplasmic entrance of the water pore. Molecular dynamic simulations indicate that Ser-115 phosphorylation triggers this movement of loop D (31). Furthermore, x-ray structural analysis of phosphorylation mutants suggests that phosphorylation at Ser-188 may also produce an open channel, which was supported by an increased water transport activity of this mutant and also molecular dynamics simulations (32). These simulations have revealed interactions between phosphorylated Ser-188 and Lys-270 of the C terminus that lead to a conformational change in loop D and channel opening. This finding also indicates an important role for the C terminus of aquaporin in the gating mechanism.
AQP4 is the predominant water channel in the mammalian brain, and its water permeability is also regulated by its phosphorylation. The water permeability of AQP4 is decreased by its phosphorylation at Ser-180 by protein kinase C (33) and is enhanced by phosphorylation at Ser-111 by protein kinase G (34). Electron crystallography of double-layered, two-dimensional crystals suggests a mechanism that phosphorylated Ser-180 may bind to the C-terminal domain, which blocks the cytoplasmic entrance of this water channel (35).
The yeast aquaporin Aqy1 has also been shown to be a gated channel by x-ray structural analysis at 1.15 Å (36). The extended N terminus caps the water channel entrance. Furthermore, molecular dynamics simulations and functional studies have suggested that its water transport activity is regulated by mechanosensitivity and Ser-107 phosphorylation (36).
We have shown here that the water transport activity of AQP2 is regulated by phosphorylation at Ser-256, which is located in the cytoplasmic C-terminal domain. Although a crystal structure of AQP2 has not been reported to date, we speculate that this phosphorylation induces conformational changes in the C terminus that are important for channel gating. Structural analysis is required to further characterize the molecular details of AQP2 gating.
Acknowledgments
We thank Prof. Kaoru Mitsuoka and Drs. Nobuhiko Gyobu and Daisuke Kasuya (National Institute of Advanced Industrial Science and Technology) for teaching us the electron microscopic technique and observation.
This work was supported in part by Grants-in-aid 17GS0312 and 21591053 from the Japan Society for the Promotion of Science.
- AQP2
- aquaporin-2
- Trx
- thioredoxin.
REFERENCES
- 1.Fushimi K., Uchida S., Hara Y., Hirata Y., Marumo F., Sasaki S. (1993) Nature 361, 549–552 [DOI] [PubMed] [Google Scholar]
- 2.Nielsen S., Frøkiaer J., Marples D., Kwon T. H., Agre P., Knepper M. A. (2002) Physiol. Rev. 82, 205–244 [DOI] [PubMed] [Google Scholar]
- 3.Noda Y., Sasaki S. (2006) Biochim. Biophys. Acta 1758, 1117–1125 [DOI] [PubMed] [Google Scholar]
- 4.Boone M., Deen P. M. (2008) Pflugers Arch. 456, 1005–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown D., Breton S., Ausiello D. A., Marshansky V. (2009) Traffic 10, 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffert J. D., Chou C. L., Knepper M. A. (2009) J. Biol. Chem. 284, 14683–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loonen A. J., Knoers N. V., van Os C. H., Deen P. M. (2008) Semin. Nephrol. 28, 252–265 [DOI] [PubMed] [Google Scholar]
- 8.Noda Y., Sohara E., Ohta E., Sasaki S. (2010) Nat. Rev. Nephrol. 6, 168–178 [DOI] [PubMed] [Google Scholar]
- 9.Noda Y., Horikawa S., Katayama Y., Sasaki S. (2004) Biochem. Biophys. Res. Commun. 322, 740–745 [DOI] [PubMed] [Google Scholar]
- 10.Noda Y., Horikawa S., Furukawa T., Hirai K., Katayama Y., Asai T., Kuwahara M., Katagiri K., Kinashi T., Hattori M., Minato N., Sasaki S. (2004) FEBS Lett. 568, 139–145 [DOI] [PubMed] [Google Scholar]
- 11.Noda Y., Horikawa S., Katayama Y., Sasaki S. (2005) Biochem. Biophys. Res. Commun. 330, 1041–1047 [DOI] [PubMed] [Google Scholar]
- 12.Noda Y., Horikawa S., Kanda E., Yamashita M., Meng H., Eto K., Li Y., Kuwahara M., Hirai K., Pack C., Kinjo M., Okabe S., Sasaki S. (2008) J. Cell Biol. 182, 587–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda Y., Sasaki S. (2008) Pflugers Arch. 456, 737–745 [DOI] [PubMed] [Google Scholar]
- 14.Kuwahara M., Fushimi K., Terada Y., Bai L., Marumo F., Sasaki S. (1995) J. Biol. Chem. 270, 10384–10387 [DOI] [PubMed] [Google Scholar]
- 15.Lande M. B., Jo I., Zeidel M. L., Somers M., Harris H. W., Jr. (1996) J. Biol. Chem. 271, 5552–5557 [DOI] [PubMed] [Google Scholar]
- 16.Yamashita Y., Hirai K., Katayama Y., Fushimi K., Sasaki S., Marumo F. (2000) Am. J. Physiol. Renal Physiol. 278, F395–F405 [DOI] [PubMed] [Google Scholar]
- 17.Li Y. H., Eto K., Horikawa S., Uchida S., Sasaki S., Li X. J., Noda Y. (2009) Int. J. Biochem. Cell Biol. 41, 2466–2476 [DOI] [PubMed] [Google Scholar]
- 18.Christensen B. M., Zelenina M., Aperia A., Nielsen S. (2000) Am. J. Physiol. Renal Physiol. 278, F29–F42 [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto G., Zelenina M., Li D., Yasui M., Aperia A., Nielsen S., Nairn A. C. (1999) Am. J. Physiol. Renal Physiol. 276, F254–F259 [DOI] [PubMed] [Google Scholar]
- 20.Xie L., Hoffert J. D., Chou C. L., Yu M. J., Pisitkun T., Knepper M. A., Fenton R. A. (2010) Am. J. Physiol. Renal Physiol. 298, F1018–F1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashimoto Y., Saito S., Tong X. H., Hong A., Sakaguchi K., Appella E., Anderson C. W. (2000) J. Biol. Chem. 275, 23199–23203 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. (1991) Biochim. Biophys. Acta 1061, 297–303 [DOI] [PubMed] [Google Scholar]
- 23.Hoffert J. D., Pisitkun T., Wang G., Shen R. F., Knepper M. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7159–7164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffert J. D., Nielsen J., Yu M. J., Pisitkun T., Schleicher S. M., Nielsen S., Knepper M. A. (2007) Am. J. Physiol. Renal Physiol. 292, F691–F700 [DOI] [PubMed] [Google Scholar]
- 25.Lu H. J., Matsuzaki T., Bouley R., Hasler U., Qin Q. H., Brown D. (2008) Am. J. Physiol. Renal Physiol. 295, F290–F294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fushimi K., Sasaki S., Marumo F. (1997) J. Biol. Chem. 272, 14800–14804 [DOI] [PubMed] [Google Scholar]
- 27.Moeller H. B., MacAulay N., Knepper M. A., Fenton R. A. (2009) Am. J. Physiol. Renal Physiol. 296, F649–F657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedfalk K., Törnroth-Horsefield S., Nyblom M., Johanson U., Kjellbom P., Neutze R. (2006) Curr. Opin. Struct. Biol. 16, 447–456 [DOI] [PubMed] [Google Scholar]
- 29.Johansson I., Karlsson M., Shukla V. K., Chrispeels M. J., Larsson C., Kjellbom P. (1998) Plant Cell 10, 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukulski W., Schenk A. D., Johanson U., Braun T., de Groot B. L., Fotiadis D., Kjellbom P., Engel A. (2005) J. Mol. Biol. 350, 611–616 [DOI] [PubMed] [Google Scholar]
- 31.Törnroth-Horsefield S., Wang Y., Hedfalk K., Johanson U., Karlsson M., Tajkhorshid E., Neutze R., Kjellbom P. (2006) Nature 439, 688–694 [DOI] [PubMed] [Google Scholar]
- 32.Nyblom M., Frick A., Wang Y., Ekvall M., Hallgren K., Hedfalk K., Neutze R., Tajkhorshid E., Törnroth-Horsefield S. (2009) J. Mol. Biol. 387, 653–668 [DOI] [PubMed] [Google Scholar]
- 33.Zelenina M., Zelenin S., Bondar A. A., Brismar H., Aperia A. (2002) Am. J. Physiol. Renal Physiol. 283, F309–F318 [DOI] [PubMed] [Google Scholar]
- 34.Gunnarson E., Zelenina M., Axehult G., Song Y., Bondar A., Krieger P., Brismar H., Zelenin S., Aperia A. (2008) Glia 56, 587–596 [DOI] [PubMed] [Google Scholar]
- 35.Hiroaki Y., Tani K., Kamegawa A., Gyobu N., Nishikawa K., Suzuki H., Walz T., Sasaki S., Mitsuoka K., Kimura K., Mizoguchi A., Fujiyoshi Y. (2006) J. Mol. Biol. 355, 628–639 [DOI] [PubMed] [Google Scholar]
- 36.Fischer G., Kosinska-Eriksson U., Aponte-Santamaría C., Palmgren M., Geijer C., Hedfalk K., Hohmann S., de Groot B. L., Neutze R., Lindkvist-Petersson K. (2009) PLoS Biol. 7, e1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]