Abstract
Phagocytosis by macrophages is essential for host defense, i.e. preventing invasion of pathogens and foreign materials. Macrophages engulf immunoglobulin G (IgG)-opsonized particles through the action of the receptors for the Fc of IgG (FcγRs). Leukotriene B4 (LTB4) is a classical lipid chemoattractant derived from arachidonic acid. Leukotriene B4 receptor 1 (BLT1), a high affinity LTB4 receptor, is expressed in a variety of immune cells such as neutrophils, macrophages, and dendritic cells. Although LTB4 has been shown to enhance macrophage phagocytosis, few studies have investigated the intracellular mechanisms involved in this in detail. Furthermore, there have been no reports of the direct cross-talk between LTB4-BLT1 and IgG-FcγRs signaling. Here, we show that FcγRs-dependent phagocytosis was attenuated in BLT1-deficient macrophages as compared with wild-type (WT) cells. Moreover, cross-talk between LTB4-BLT1 and IgG-FcγRs signaling was identified at the level of phosphatidylinositol 3-OH kinase (PI3K) and Rac, downstream of Syk. In addition, the trimeric Gi protein (Gi) was found to be essential for BLT1-dependent phagocytosis. Surprisingly, we found that LTB4-BLT1 signaling restores phagocytosis in the absence of FcγRs signaling. These data indicate that LTB4-BLT1 signaling plays a pivotal role in macrophage phagocytosis and innate immunity.
Keywords: Arachidonic Acid, G Protein-coupled Receptors, Gene Knockout, Retrovirus, Signal Transduction
Introduction
Phagocytes such as macrophages prevent invasion of microorganisms and foreign materials by engulfment. Phagocytosis by macrophages is an important component of innate immunity. Efficient phagocytosis is achieved by opsonization of the targets by Ig and complement proteins. FcγRs3 on phagocytes play an essential role in recognition of opsonized materials (1, 2). Binding of IgG-containing immune complexes to FcγRs induces cross-linking of FcγRs and subsequent phosphorylation of tyrosine residues in the immunoreceptor tyrosine-based activation motif (ITAM) of the FcR common γ-chain (FcRγ) by Src family kinases, such as Lyn and Hck. The phosphorylated ITAM then serves as docking sites for the Src homology 2 (SH2) domain of Syk kinase, subsequently triggering phosphorylation of Syk by Src family kinases (3). Activation of Syk triggers stimulation of the downstream effectors phospholipase Cγ, PI3K, and GTPases of the Rho/Rac family. Activation of these downstream effectors results in various cellular events such as phagocytosis, cytokine/chemokine production, oxidative burst, and antibody-dependent cellular cytotoxicity (4). However, the precise downstream pathways of Syk via FcγR are still unclear. For example, a variety of Syk substrates that are phosphorylated may account for the diverse cellular functions following FcγR ligation. Syk (5), PI3K (6), and the Rho family proteins (Rho (7) and Rac and Cdc42 (8–10)) are all required for FcγRs-dependent phagocytosis.
LTB4 is a classical lipid chemoattractant derived from arachidonic acid by the actions of 5-lipoxygenase, 5-lipoxygenase-activating protein (FLAP), and LTA4 hydrolase. LTB4 attracts neutrophils and eosinophils by the binding to the LTB4-specific G-protein-coupled receptor, BLT1. BLT1, originally identified in our laboratory (11), is expressed in a variety of immune cells, including dendritic cells (12), differentiated T cells (13, 14), and mast cells (15). Analysis of BLT1-deficient mice revealed the importance in macrophage biology, as atherogenesis was markedly attenuated in BLT1-deficient mice in an apolipoprotein E (apoE)-null background (16). However, although LTB4 has been shown to activate macrophage phagocytosis, details of the intracellular mechanism of LTB4-dependent activation of phagocytosis remain to be elucidated. Recently, Serezani et al. (17) showed that FcγRI engagement results in tyrosine phosphorylation of BLT1 by Src and subsequent formation of a molecular complex of FcγRI and BLT1 within lipid rafts that drives phagocytic functions in rat alveolar macrophages. However, it remains to be determined whether there is direct cross-talk between LTB4-BLT1 and IgG-FcγRs signaling pathways.
In this study, we investigated the function of BLT1 in macrophage phagocytosis using BLT1-deficient mice. BLT1 was necessary for FcγR-dependent macrophage phagocytosis. More importantly, we identified cross-talk between LTB4-BLT1 and IgG-FcγRs signaling pathways at the level of PI3K and Rac, downstream of Syk. Finally, using FcRγ-deficient mice and retroviral gene transfer techniques, we showed that LTB4 stimulation alone is able to induce phagocytosis in macrophages that are deficient in FcγRs signaling.
EXPERIMENTAL PROCEDURES
Mice and BMMφ
BLT1-deficient mice (18) and FcRγ-deficient mice (19) in a C57BL/6J background were previously described. All animal studies and procedures were approved by the Ethics Committees for Animal Experiments of Kyushu University. BMMφ were prepared from bone marrow suspensions from mouse femurs and tibias as described previously (20). Bone marrow cells were cultured in RPMI 1640 medium (WAKO, Osaka, Japan) containing 10% fetal bovine serum (FBS; Invitrogen), penicillin and streptomycin (Nacalai Tesque, Kyoto, Japan), and 30% L929 culture supernatants containing macrophage-colony stimulating factor (M-CSF) for 5 days and then used for the experiments (21).
Phagocytosis Assay
Zymosan-Alexa488 and an opsonizing reagent were purchased from Molecular Probes (Eugene, OR). Opsonization of zymosan-Alexa488 was performed according to the manufacturer's protocol. BMMφ were seeded on 35-mm glass bottom dishes and then cultured overnight. After incubation with opsonized-zymosan particles for 4 h at 37 °C, BMMφ were washed with PBS, fixed with 4% paraformaldehyde/PBS, and then observed using a confocal laser scanning microscope (LSM510 META, Carl Zeiss, Germany). Where indicated, LTB4 (final concentration of 100 nm) (Cayman Chemicals, Ann Arbor, MI) was added along with opsonized zymosan particles. For the inhibition assay, BMMφ were pretreated with the following reagents and times prior to incubation with zymosan particles: CP105696 (1 μm, 30 min), PP2 (5 μm, 30 min) (Calbiochem), Piceatannol (20 μm, 30 min) (Calbiochem), LY294002 (50 μm, 16 h) (Calbiochem), Rac1 inhibitor (NSC23766; 200 μm, 16 h) (Calbiochem), pertussis toxin (PTX; 100 ng/ml, 16 h) (List Biological Laboratories, Campbell, CA), and YM-254890 (10 μm, 30 min), a kind gift from Dr. J. Takasaki, Astellas Pharmaceutical (22).
Generation of IgG Beads
Yellow-Green carboxylate beads (1.0 μm, Polysciences, Warrington, PA) were opsonized with mouse IgG (Jackson ImmunoResearch, West Grove, PA) by using a PolyLink protein coupling kit (Polysciences).
Flow Cytometry
BMMφ were incubated with 0.5 μg/ml PE-anti-mCD64 antibody (BioLegend, San Diego), 0.5 μg/ml PE-anti-mCD16/CD32 antibody (BD Biosciences), or 2.5 μg/ml mIgG2a-Alexa488 (eBioscience, San Diego) in PBS containing 2% FBS and 0.1% sodium azide. For the binding assay, BMMφ were pretreated with cytochalasin D (1 or 10 μm, Calbiochem) for 30 min prior to incubation with opsonized zymosan particles or IgG beads. Cells were analyzed using a FACSCalibur with CellQuest (BD Biosciences) and FlowJo software (TreeStar, Ashland, OR).
Syk Activation Assay
BMMφ were incubated with IgG beads and LTB4 for various times and then were solubilized in lysis buffer (20 mm Tris, pH 7.4, 150 mm sodium chloride, 1% Nonidet P-40, 1 mm EDTA, 1.5 mm magnesium chloride, 1 mm sodium fluoride, 1 mm sodium orthovanadate, 10 mm β-glycerophosphate, and a protease inhibitor mixture (Nacalai Tesque)). Cell lysates were resolved by SDS-PAGE (12%) and then transferred to an Immobilon-P PVDF membrane (Millipore, Bedford, MA). The membrane was incubated with primary antibody, either anti-phospho-Syk (Cell Signaling Technology, Danvers, MA) or anti-Syk (Santa Cruz Biotechnology, Santa Cruz, CA) antibody, followed by horseradish peroxidase (HRP)-labeled secondary antibody. Immunoreactive proteins were detected by enhanced chemiluminescence (PerkinElmer Life Sciences and Millipore) and LAS 4000 (Fuji Films, Tokyo, Japan).
Rac Pulldown Assay
A cDNA encoding the Rac-binding domain of human PAK2 (PAK2-RBD; amino acids 74–131) in pGEX-4T (23, 24) was introduced into Escherichia coli BL21 (DE3), and GST fusion protein (GST-PAK2-RBD) was expressed. The fusion protein was purified by glutathione-Sepharose 4B (GE Healthcare). For the in vitro pulldown assays, BMMφ cell lysates were mixed with GST-PAK2-RBD bound to glutathione-Sepharose 4B and allowed to incubate for 30 min at 4 °C. After washing three times with lysis buffer, bound proteins were eluted with 10 mm reduced glutathione in 50 mm Tris, pH 8.0. The eluates were resolved by SDS-PAGE (12%), transferred to an Immobilon-P PVDF membrane, and then analyzed by immunoblotting using anti-Rac (BD Biosciences) and anti-GST (Nacalai Tesque) primary antibodies.
Retroviral Transfection
cDNAs encoding WT and mutant (YF) FcRγ in pMX-IRES-rCD2 were transiently transfected into the packaging cell line, Phoenix (25, 26). The cultured media were collected and used as viral suspensions. Bone marrow cells from FcRγ-deficient mice were cultured for 3 days with these viral suspensions and then differentiated into BMMφ as described above. FcRγ-expressing cells were isolated using MACS cell separators (Miltenyi Biotec, Germany) after incubation with biotin-conjugated anti-rCD2 antibody (Cedarlane, Hornby, Canada) and streptavidin microbeads (Miltenyi Biotec). Viral suspensions for the expression of WT Rac and dominant-negative Rac (DN Rac, T17N) were prepared in a same way using pMXs-WT Rac-IRES-DsRed and pMXs-DN Rac-IRES-DsRed (kindly provided by Dr. Y. Fukui, Kyushu University) (27) and used for introduction into bone marrow cells from C57BL/6J mice.
RESULTS
BLT1 Is Required for Macrophage Phagocytosis via FcγRs
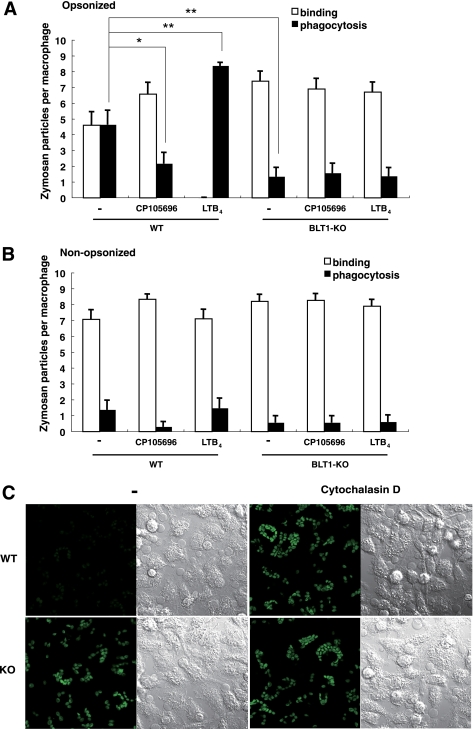
LTB4 is a known potent chemoattractant for neutrophils, whereas the role of BLT1 in activating macrophages has not been clearly defined. To analyze the function of BLT1 in macrophages, we compared phagocytosis of opsonized zymosan in WT and BLT1-deficient BMMφ. After 4 h of incubation with opsonized zymosan, WT BMMφ phagocytosed 4.63 zymosan particles per Mφ, and this increased to 8.37 upon stimulation of the cells with 100 nm LTB4 (Fig. 1A, left panel). Inhibition of BLT1 using the BLT1 antagonist CP105696 resulted in a decrease in phagocytosis (to 2.17) (Fig. 1A, left panel). BLT1-deficient BMMφ phagocytosed 1.33 opsonized zymosan particles per Mφ, which was significantly lower than WT BMMφ (Fig. 1A, right panel). Neither stimulation of BLT1 by LTB4 nor inhibition by CP105696 affected phagocytosis of opsonized zymosan in BLT1-deficient BMMφ.
FIGURE 1.
BLT1 is required for FcγR-dependent phagocytosis in macrophages. A and B, Phagocytosis of opsonized zymosan particles (A) and nonopsonized particles (B) in BMMφ from wild-type (WT) and BLT1-deficient (KO) mice is shown. Number of bound or phagocytosed zymosan particles per macrophage was counted under a confocal laser scanning microscopy. Data represent the means ± S.E. *, p < 0.05; **, p < 0.001. C, engulfment of opsonized zymosan particles by BMMφ was observed using a confocal laser scanning microscope. Phagocytosed zymosan particles were observed as less bright spots (WT, −) than bright particles that represent zymosan particles bound on the cell surface. Differential interference contrast images are also presented to show similar numbers of the macrophages in each field. Representative photos are shown.
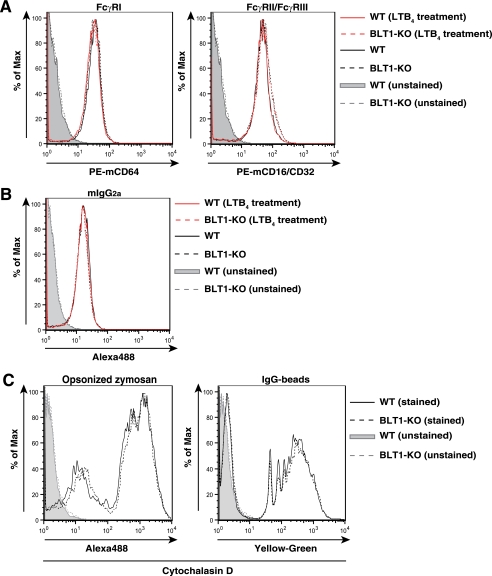
Invading microorganisms are recognized not only by FcγRs but also by pattern-recognition receptors, which recognize highly conserved invariant molecular patterns such as lipopolysaccharide (LPS) and peptideglycan. Pattern-recognition receptors of zymosan have been reported to be Dectin-1, TLR2, and TLR6 (28). Phagocytosis of nonopsonized zymosan by WT BMMφ was much lower than that of opsonized particles and was comparable with phagocytosis by BLT1-deficient BMMφ (Fig. 1B). These results indicated that the effects of Dectin-1, TLR2, and TLR6 in the phagocytosis of opsonized zymosan by BMMφ were negligible and that the signaling pathways involved in the engulfment of opsonized particles was mediated mainly via FcγRs. Because the phagocytosis of opsonized zymosan by macrophages requires FcγRs (FcγRI, FcγRII, and FcγRIII), we measured cell surface expression of FcγRs in WT and BLT1-deficient BMMφ. Flow cytometric analysis showed that the expression of FcγRI and FcγRII/III was similar between WT and BLT1-deficient BMMφ (Fig. 2A). Furthermore, expression of functional FcγRs examined by the binding of labeled IgG1, IgG2b (data not shown), and IgG2a was similar between WT and BLT1-deficient BMMφ (Fig. 2B). We also confirmed that LTB4 did not up-regulate the expression of FcγRs (Fig. 2, A and B). We also measured the binding of opsonized zymosan or IgG beads to BMMφ in the presence of cytochalasin D, which blocks internalization of bound particles by inhibiting actin polymerization. Similar levels of binding were observed between WT and BLT1-deficient BMMφ (Fig. 2C), which indicated that the attenuated phagocytosis observed in BLT1-deficient BMMφ was not due to the reduced cell surface expression of FcγRs and binding of opsonized particles.
FIGURE 2.
LTB4-BLT1 signaling does not affect the expression of FcγRs. A–C, flow cytometric analysis of cell surface expression of FcγRs. BMMφ were incubated with or without PE-anti-mCD64 antibody (A, left panel), PE-anti-mCD16/CD32 antibody (A, right panel), mIgG2a-Alexa488 (B), opsonized zymosan-Alexa488 (C, left panel) and IgG-yellow-green beads (C, right panel). Where indicated, BMMφ were pretreated with LTB4 (100 nm, 30 min) prior to incubation with labeled antibodies (A and B).
LTB4 Potentiates FcγR-dependent Rac Activation
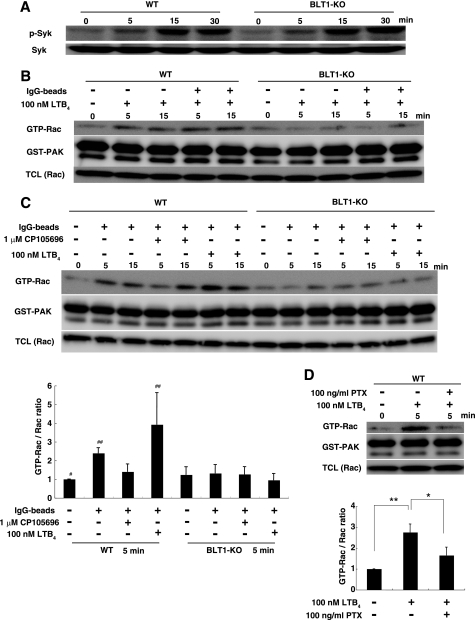
To determine whether the cross-talk between the LTB4-BLT1 and IgG-FcγR signaling pathways resulted in enhanced phagocytosis in macrophages, we examined the activation of Syk induced by incubation with IgG beads and LTB4. Syk phosphorylation is one of the initial events induced by FcγR cross-linking by opsonized particles (5). Syk was phosphorylated following the addition of IgG beads and LTB4 in a time-dependent manner, and there was no difference between WT and BLT1-deficient BMMφ (Fig. 3A). Thus, reduced phagocytosis in BLT1-deficient BMMφ was most likely not due to alternation in Syk phosphorylation. Rac is intimately involved in macrophage phagocytosis and functions downstream of Gi-coupled G-protein-coupled receptors, including BLT1 (29). LTB4 activated Rac in WT BMMφ but not in BLT1-deficient BMMφ (Fig. 3B). The addition of IgG beads alone induced the activation of Rac in WT BMMφ, and there was a synergistic increase in Rac activation upon co-treatment with LTB4 (Fig. 3C, left panel). In contrast, IgG beads-dependent activation of Rac was greatly attenuated in BLT1-deficient BMMφ, and there was no synergistic activation by LTB4 (Fig. 3C, right panel). Furthermore, treatment of WT BMMφ with the BLT1 antagonist CP105696 attenuated Rac activation by IgG beads, and this effect was not apparent in BLT1-deficient BMMφ (Fig. 3C). These data suggested that LTB4 was either included in the culture media or produced by macrophages during phagocytosis and activated BLT1 in macrophages. Quantification of LTB4 in the media during phagocytosis by ELISA and LC-MS/MS (30) revealed trace amounts of LTB4 15 min after the addition of IgG beads that were below the limit of detection (5 pg/ml) (data not shown). In addition, Rac activation induced by LTB4 was inhibited by an inhibitor of Gi protein, PTX (Fig. 3D). This result indicates that BLT1-dependent Rac activation is mediated by Gi.
FIGURE 3.
LTB4 potentiates Rac activation during FcγR-dependent phagocytosis. A, effect of BLT1 deficiency on Syk phosphorylation in BMMφ stimulated with IgG beads and LTB4. BMMφ from WT and BLT1-deficient mice were incubated with IgG beads and 100 nm LTB4 for the indicated periods of time. Cell extracts were separated by SDS-PAGE and immunoblotted with the indicated antibodies. B and C, effect of BLT1 deficiency on Rac activation. BMMφ from WT and BLT1-deficient mice were stimulated with IgG beads in the presence or absence of LTB4 (B and C) and BLT1 antagonist CP105696 (C). Activated Rac (GTP-Rac) was pulled down with GST-PAK followed by immunoblotting. TCL, total cell lysates. GTP-Rac/Rac ratio was normalized to 1 for the WT (unstimulated) (C and D). Data represents the means ± S.D. (n = 3). ##, p < 0.05 versus #. D, effect of PTX on BLT1-dependent Rac activation in WT BMMφ. Cells were stimulated with LTB4 in the presence or absence of PTX. Rac pulldown assay was performed as described above. Data represent the means± S.D. (n = 3). *, p < 0.05; **, p < 0.01.
LTB4 Enhances Macrophage Phagocytosis
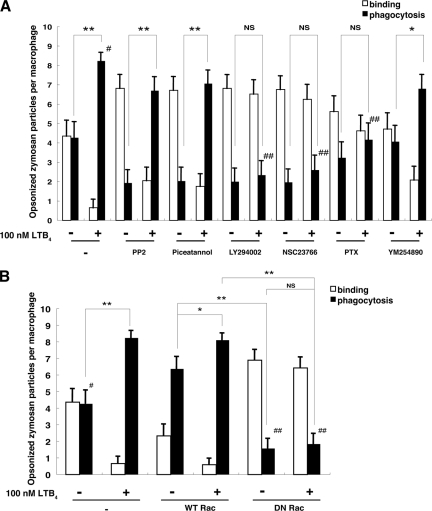
As shown in Fig. 1, LTB4 enhanced phagocytosis in WT BMMφ. To investigate the signaling molecules engaged in phagocytosis that were affected by BLT1 deficiency, we examined the effects of various signal transduction inhibitors on the binding and phagocytosis of opsonized zymosan in WT macrophages. Src family kinase inhibitor (PP2), Syk inhibitor (Piceatannol), PI3K inhibitor (LY294002), and Rac inhibitor (NSC23766) all prevented phagocytosis in the absence of LTB4 (Fig. 4A, −). PTX had a partial effect, and the Gq inhibitor (YM-254890) had no effect on phagocytosis (Fig. 4A). Under conditions of 100 nm LTB4 stimulation, inhibition of Src family kinases and Syk did not inhibit phagocytosis of opsonized zymosan (Fig. 4A, +). Thus, LTB4 abrogated the inhibitory effects of PP2 and Piceatannol. In contrast, in the presence of LTB4, LY294002, NSC23766, and PTX were all effective in inhibiting phagocytosis. These data suggested that there is cross-talk between the LTB4-BLT1 and IgG-FcγRs signaling pathways at a specific step downstream of Syk. To verify the function of Rac in phagocytosis, we retrovirally introduced WT and dominant-negative (DN) form (T17N) of Rac into WT macrophages, and we then examined the binding and phagocytosis of opsonized zymosan in the presence or absence of LTB4. Infection of WT Rac greatly increased phagocytosis in the absence of LTB4, and this was further augmented by LTB4 (Fig. 4B, WT Rac). In cells that expressed DN Rac, phagocytosis was greatly reduced and was not activated by LTB4 (Fig. 4B, DN Rac).
FIGURE 4.
LTB4-dependent phagocytosis requires PI3K and Rac activation. A, effect of various inhibitors on phagocytosis in the presence or absence of LTB4 in WT BMMφ. Cells were treated with inhibitors prior to the addition of LTB4 and opsonized zymosan. Number of bound and phagocytosed zymosan was counted under a confocal laser scanning microscopy. Data represent the means ± S.E. *, p < 0.05; **, p < 0.001; ##, p < 0.001 versus #. B, effect of retroviral transfection of WT Rac or DN Rac on phagocytosis. Data represent the means ± S.E. *, p < 0.05; **, p < 0.001; ##, p < 0.001 versus #.
LTB4 Signaling Is Sufficient to Induce Macrophage Phagocytosis in the Absence of FcγRs Signaling
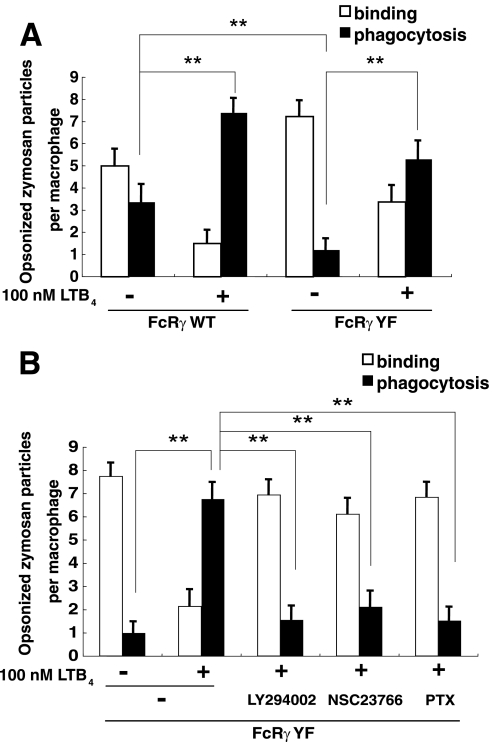
FcRγ is important for phagocytosis not only in cell activation but also in the efficient assembly and cell surface expression of FcγRI and FcγRIII, which capture opsonized particles (4). To determine whether LTB4-BLT1 signaling was sufficient to induce macrophage phagocytosis in the absence of FcγRs signaling, we investigated phagocytosis in FcRγ-deficient macrophages retrovirally transfected with WT FcRγ or a mutant form of FcRγ (YF) in which two tyrosine residues in the ITAM were replaced by phenylalanine. The expression levels of retrovirally transfected WT and YF FcRγ are shown in supplemental Fig. 1. The YF mutant is deficient in transducing the signals induced by FcRγ in mast cells and basophils, but surface expression of FcRs remains intact (25, 26). Phagocytosis in cells expressing WT FcRγ was readily apparent and was further enhanced by LTB4 (Fig. 5A, WT), similar to WT macrophages (Fig. 4A, −). Phagocytosis in cells expressing FcRγ YF was reduced as expected, and interestingly, LTB4 stimulation restored the levels of phagocytosis in FcRγ YF-infected cells (Fig. 5A, YF). To verify these observed effects of LTB4 on phagocytosis in FcRγ YF cells, we treated cells with LY294002, NSC23766, and PTX. All of the inhibitors suppressed LTB4-dependent phagocytosis of opsonized zymosan (Fig. 5B). Taken together, these data indicated that LTB4-BLT1 signaling is sufficient to induce phagocytosis when opsonized particles bound to FcγRs.
FIGURE 5.
LTB4 signaling is sufficient to induce macrophage phagocytosis in the absence of FcγR signaling. A, effect of LTB4 stimulation on phagocytosis in FcRγ-deficient BMMφ retrovirally transfected with rCD2-fused WT or mutant (YF) FcRγ. BMMφ expressing WT or mutant FcRγs were separated by MACS using a biotin anti-rCD2 antibody and subjected to phagocytosis assay. Data represent the means ± S.E. **, p < 0.001. B, effect of various inhibitors on LTB4-dependent recovery of phagocytosis in FcRγ-deficient cells expressing FcRγ YF. Data represent the means ± S.E. **, p < 0.001.
DISCUSSION
In the host defense mechanism against pathogens, phagocytosis by macrophages plays an essential role. Macrophages engulf IgG-opsonized particles via recognition and binding by FcγRs. FcRγ is particularly important for phagocytosis; FcRγ is a common subunit of FcγRs and is required for the efficient assembly and cell surface expression of FcγRs. FcRγ-deficient mice are more susceptible to infection due to defects in the phagocytosis of IgG-coated particles (31). The intracellular signaling pathways involved in FcγRs-dependent phagocytosis have been studied in detail. Binding of opsonized particles to FcγRs and subsequent cross-linking of the receptors induces ITAM phosphorylation of FcRγ by Src family kinases. Syk is then recruited to the phosphorylated ITAM and activated by Src family kinases. The downstream events of Syk activation trigger stimulation of PI3K and the Rac GTPase, resulting in phagocytosis (4).
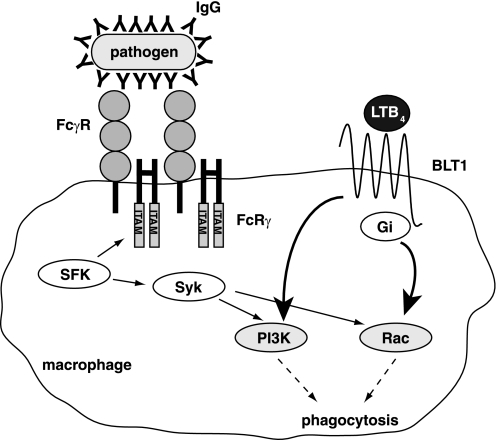
In addition to FcγRs, certain lipid-derived mediators are known to modulate phagocytosis. Among them, the classical lipid mediator LTB4 is a potent activator of phagocytes (32). Although LTB4 has been shown to activate phagocytosis in macrophages, the molecular details of the underlying intracellular mechanism are lacking. Furthermore, there have been no reports of the direct cross-talk between LTB4-BLT1 and IgG-FcγRs signaling pathways. In this study, analysis of the role of BLT1 on FcγRs-dependent phagocytosis using BLT1-deficient macrophages showed that LTB4-BLT1 signaling plays a pivotal role in the engulfment of opsonized particles by macrophages. Flow cytometric analysis showed that the attenuated phagocytosis in BLT1-deficient macrophages was not due to the reduced expression of FcγRs on the cell surface or impaired binding of opsonized particles. These results suggested that there may be cross-talk between LTB4-BLT1 and IgG-FcγRs signaling pathways. Immunoblotting and inhibition assays showed that LTB4-BLT1 signaling intersects with IgG-FcγRs signaling at the level of PI3K and Rac, downstream of Syk. Based on these results, we explored whether LTB4-BLT1 signaling was sufficient to restore phagocytosis in the absence of FcγRs signaling. Because FcRγ-deficient macrophages lack surface expression of FcγRs, we adopted a relatively complex experiment in which WT or mutated (YF) FcRγ was retrovirally introduced into FcRγ-deficient macrophages. FcRγ YF lacks key tyrosine residues in the ITAM and does not transduce FcRs signals; however, in FcRγ YF-expressing cells, expression of the receptors for the Fc of IgE (FcϵRs) on the cell surface is intact (25). Thus, the FcRγ YF mutant does not affect the expression of FcRs. This method enabled us to analyze the roles of LTB4-BLT1 signaling in phagocytosis in the absence of FcγRs-dependent intracellular signaling. Phagocytosis in FcRγ YF-reconstituted macrophages was reduced, as expected. Interestingly LTB4 stimulation restored this defect in phagocytosis. Based on these results, we propose a model in which cross-talk between IgG-FcγRs and LTB4-BLT1 signaling regulates phagocytosis in macrophages (Fig. 6). The data presented here support a novel mechanism whereby G-protein-coupled receptor stimulation restores macrophage phagocytosis in the absence of FcγRs signaling in the following manner. LTB4-BLT1 signaling induces both Rac activation through Gi protein and PI3K activation, which in turn enhance phagocytosis synergistically with FcγR signaling.
FIGURE 6.
Model for the regulation of phagocytosis by cross-talk between LTB4-BLT1 and IgG-FcγRs signaling pathways. Binding of IgG-bound pathogens to FcγRs induces cross-linking of FcγRs and subsequent phosphorylation of tyrosine residues in the ITAM of FcRγ by Src family kinases (SFKs). Phosphorylated ITAM then serves as docking sites for the SH2 domain of Syk kinase. Docking of Syk triggers its phosphorylation by SFKs. Syk activation triggers stimulation of PI3K and a GTPase Rac, resulting in an acceleration of phagocytosis. PI3K and Rac also play a pivotal role in phagocytosis downstream of BLT1. LTB4-BLT1 signaling induces both Rac activation through Gi protein and PI3K activation, and these activations lead to an augmentation of phagocytosis.
One remaining question from this study is the source of LTB4 during phagocytosis. Macrophages as well as neutrophils express all of the enzymes required for LTB4 biosynthesis (cytosolic phospholipase A2, 5-lipoxygenase, FLAP, and LTA4 hydrolase) and produce LTB4 in response to increased intracellular calcium (33). We attempted to quantify LTB4 production in macrophages during phagocytosis by ELISA and LC-MS/MS techniques (30), but we were only able to detect trace amounts of LTB4 that were below the detection limit of the assays (5 pg/ml). Although there are no reports on LTB4 production in response to FcγRs signaling, it is reasonable to consider that phospholipase Cγ-dependent calcium mobilization leads to LTB4 production upon cross-linking of FcγRs by immune complexes. Macrophages are known to express CYP4F18, an LTB4-oxidizing cytochrome P450 enzyme (34). Thus, LTB4 produced during phagocytosis might be oxidized rapidly by this enzyme under our experimental conditions. Recently, transcellular LTB4 production is intensively studied. In this case, LTA4 predominantly produced in phagocytic cells is transcellularly transferred to a variety of cells that express LTA4 hydrolase, where it is converted to a large amount of LTB4 (35). Macrophages are adherent cells, and it is reasonable to consider that more LTB4 is produced upon phagocytosis in vivo.
LTB4 production is increased at the site of inflammation (36–43). In this study, we stimulated macrophages with LTB4 at a concentration of 100 nm. It has been reported that LTB4 in excess of 100 nm is produced in human neutrophils by stimulation with granulocyte macrophage colony-stimulating factor (GM-CSF) and formyl-methionyl-leucyl-phenylalanine (44) and that LTB4 levels reach 50 nm following stimulation of human monocytes with the calcium ionophore A23187 (45) or LPS (46). Thus, it is possible that macrophages are exposed to LTB4 above the level in vivo. When macrophages infiltrate into inflammatory sites, they are activated by lipid mediators, including LTB4. Activated macrophages engulf pathogens and release cytokines and chemokines to recruit neutrophils. LTB4 produced by recruited neutrophils results in further activation of macrophages. By this positive feedback loop, macrophage phagocytosis is further accelerated and plays an important role in the clearance of pathogens. The results of several recent studies suggested the importance of BLT1 in atherosclerosis. BLT1 deficiency resulted in reduced atherogenesis in an apoE-deficient background (16), and this effect was explained by reduced level of production of monocyte chemoattractant protein-1 (MCP-1) in BLT1-deficient macrophages (47). Future studies investigating the possible role of LTB4-BLT1 signaling, especially Rac activation, in MCP-1 production in macrophages would be of particular interest.
Supplementary Material
Acknowledgments
We thank the Support Center for Education and Research, Kyushu University, for technical support; M. Otsu, E. Koba, M. Yasutake, and T. Matsunobu for technical assistance; Dr. Y. Fukui (Kyushu University) for retroviral vectors; Dr. J. Takasaki (Astellas Pharmaceutical) for YM-254890; Dr. Y. Kita (University of Tokyo) for LTB4 measurement; and Dr. T. Okuno and our laboratory members for valuable advice and discussions. We also thank Dr. K. Taniguchi, Dr. T. Ayada, and M. Hashimoto for technical support and advice.
This work was supported by Grants-in-aid for Scientific Research 21390083, 22116001, and 22116002 (to T. Y.) and 22790320 (to K. S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and grants from the Takeda Science Foundation, Mitsubishi Foundation, Uehara Memorial Foundation, Sankyo Foundation of Life Science, and Japan Society for the Promotion of Science (Global COE Program) (to T. Y.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- FcγR
- receptors for the Fc of IgG
- BLT1
- leukotriene B4 receptor 1
- FcRγ
- Fc receptor common γ-chain
- ITAM
- immunoreceptor tyrosine-based activation motif
- LTB4
- leukotriene B4
- BMMφ
- bone marrow-derived macrophages
- PTX
- pertussis toxin
- DN
- dominant-negative
- PE
- phycoerythrin.
REFERENCES
- 1.Aderem A., Underhill D. M. (1999) Annu. Rev. Immunol. 17, 593–623 [DOI] [PubMed] [Google Scholar]
- 2.Flannagan R. S., Cosío G., Grinstein S. (2009) Nat. Rev. Microbiol. 7, 355–366 [DOI] [PubMed] [Google Scholar]
- 3.García-García E., Rosales C. (2002) J. Leukocyte Biol. 72, 1092–1108 [PubMed] [Google Scholar]
- 4.Takai T. (2002) Nat. Rev. Immunol. 2, 580–592 [DOI] [PubMed] [Google Scholar]
- 5.Crowley M. T., Costello P. S., Fitzer-Attas C. J., Turner M., Meng F., Lowell C., Tybulewicz V. L., DeFranco A. L. (1997) J. Exp. Med. 186, 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox D., Tseng C. C., Bjekic G., Greenberg S. (1999) J. Biol. Chem. 274, 1240–1247 [DOI] [PubMed] [Google Scholar]
- 7.Hackam D. J., Rotstein O. D., Schreiber A., Zhang W., Grinstein S. (1997) J. Exp. Med. 186, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massol P., Montcourrier P., Guillemot J. C., Chavrier P. (1998) EMBO J. 17, 6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. (1997) J. Exp. Med. 186, 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caron E., Hall A. (1998) Science 282, 1717–1721 [DOI] [PubMed] [Google Scholar]
- 11.Yokomizo T., Izumi T., Chang K., Takuwa Y., Shimizu T. (1997) Nature 387, 620–624 [DOI] [PubMed] [Google Scholar]
- 12.Toda A., Terawaki K., Yamazaki S., Saeki K., Shimizu T., Yokomizo T. (2010) Biochimie 92, 682–691 [DOI] [PubMed] [Google Scholar]
- 13.Goodarzi K., Goodarzi M., Tager A. M., Luster A. D., von Andrian U. H. (2003) Nat. Immunol. 4, 965–973 [DOI] [PubMed] [Google Scholar]
- 14.Ott V. L., Cambier J. C., Kappler J., Marrack P., Swanson B. J. (2003) Nat. Immunol. 4, 974–981 [DOI] [PubMed] [Google Scholar]
- 15.Weller C. L., Collington S. J., Brown J. K., Miller H. R., Al-Kashi A., Clark P., Jose P. J., Hartnell A., Williams T. J. (2005) J. Exp. Med. 201, 1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller E. A., Liu E., Tager A. M., Sinha S., Roberts J. D., Koehn S. L., Libby P., Aikawa E. R., Chen J. Q., Huang P., Freeman M. W., Moore K. J., Luster A. D., Gerszten R. E. (2005) Circulation 112, 578–586 [DOI] [PubMed] [Google Scholar]
- 17.Serezani C. H., Aronoff D. M., Sitrin R. G., Peters-Golden M. (2009) Blood 114, 3316–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terawaki K., Yokomizo T., Nagase T., Toda A., Taniguchi M., Hashizume K., Yagi T., Shimizu T. (2005) J. Immunol. 175, 4217–4225 [DOI] [PubMed] [Google Scholar]
- 19.Park S. Y., Ueda S., Ohno H., Hamano Y., Tanaka M., Shiratori T., Yamazaki T., Arase H., Arase N., Karasawa A., Sato S., Ledermann B., Kondo Y., Okumura K., Ra C., Saito T. (1998) J. Clin. Invest. 102, 1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 21.Pinto A. K., Jamieson A. M., Raulet D. H., Hill A. B. (2007) J. Virol. 81, 12564–12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takasaki J., Saito T., Taniguchi M., Kawasaki T., Moritani Y., Hayashi K., Kobori M. (2004) J. Biol. Chem. 279, 47438–47445 [DOI] [PubMed] [Google Scholar]
- 23.Akasaki T., Koga H., Sumimoto H. (1999) J. Biol. Chem. 274, 18055–18059 [DOI] [PubMed] [Google Scholar]
- 24.Daniels R. H., Zenke F. T., Bokoch G. M. (1999) J. Biol. Chem. 274, 6047–6050 [DOI] [PubMed] [Google Scholar]
- 25.Sakurai D., Yamasaki S., Arase K., Park S. Y., Arase H., Konno A., Saito T. (2004) J. Immunol. 172, 2374–2381 [DOI] [PubMed] [Google Scholar]
- 26.Hida S., Yamasaki S., Sakamoto Y., Takamoto M., Obata K., Takai T., Karasuyama H., Sugane K., Saito T., Taki S. (2009) Nat. Immunol. 10, 214–222 [DOI] [PubMed] [Google Scholar]
- 27.Gotoh K., Tanaka Y., Nishikimi A., Nakamura R., Yamada H., Maeda N., Ishikawa T., Hoshino K., Uruno T., Cao Q., Higashi S., Kawaguchi Y., Enjoji M., Takayanagi R., Kaisho T., Yoshikai Y., Fukui Y. (2010) J. Exp. Med. 207, 721–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dostert C., Tschopp J. (2007) Nat. Immunol. 8, 17–18 [DOI] [PubMed] [Google Scholar]
- 29.Kuniyeda K., Okuno T., Terawaki K., Miyano M., Yokomizo T., Shimizu T. (2007) J. Biol. Chem. 282, 3998–4006 [DOI] [PubMed] [Google Scholar]
- 30.Kita Y., Takahashi T., Uozumi N., Shimizu T. (2005) Anal. Biochem. 342, 134–143 [DOI] [PubMed] [Google Scholar]
- 31.Takai T., Li M., Sylvestre D., Clynes R., Ravetch J. V. (1994) Cell 76, 519–529 [DOI] [PubMed] [Google Scholar]
- 32.Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. (1987) Science 237, 1171–1176 [DOI] [PubMed] [Google Scholar]
- 33.Brink C., Dahlén S. E., Drazen J., Evans J. F., Hay D. W., Nicosia S., Serhan C. N., Shimizu T., Yokomizo T. (2003) Pharmacol. Rev. 55, 195–227 [DOI] [PubMed] [Google Scholar]
- 34.Christmas P., Tolentino K., Primo V., Berry K. Z., Murphy R. C., Chen M., Lee D. M., Soberman R. J. (2006) J. Biol. Chem. 281, 7189–7196 [DOI] [PubMed] [Google Scholar]
- 35.Gijón M. A., Zarini S., Murphy R. C. (2007) J. Lipid Res. 48, 716–725 [DOI] [PubMed] [Google Scholar]
- 36.Konstan M. W., Walenga R. W., Hilliard K. A., Hilliard J. B. (1993) Am. Rev. Respir. Dis. 148, 896–901 [DOI] [PubMed] [Google Scholar]
- 37.Crooks S. W., Bayley D. L., Hill S. L., Stockley R. A. (2000) Eur. Respir. J. 15, 274–280 [DOI] [PubMed] [Google Scholar]
- 38.Montuschi P., Barnes P. J. (2002) J. Allergy Clin. Immunol. 109, 615–620 [DOI] [PubMed] [Google Scholar]
- 39.Antonelli M., Bufi M., De Blasi R. A., Crimi G., Conti G., Mattia C., Vivino G., Lenti L., Lombardi D., Dotta A., et al. (1989) Intensive Care Med. 15, 296–301 [DOI] [PubMed] [Google Scholar]
- 40.Neu I., Mallinger J., Wildfeuer A., Mehlber L. (1992) Acta Neurol. Scand. 86, 586–587 [DOI] [PubMed] [Google Scholar]
- 41.Ahmadzadeh N., Shingu M., Nobunaga M., Tawara T. (1991) Inflammation 15, 497–503 [DOI] [PubMed] [Google Scholar]
- 42.Sharon P., Stenson W. F. (1984) Gastroenterology 86, 453–460 [PubMed] [Google Scholar]
- 43.Duell E. A., Ellis C. N., Voorhees J. J. (1988) J. Invest. Dermatol. 91, 446–450 [DOI] [PubMed] [Google Scholar]
- 44.Tedeschi A., Ciceri P., Zarini S., Lorini M., Di Donato M., Nicosia S., Miadonna A., Sala A. (2004) Biochem. Pharmacol. 67, 385–393 [DOI] [PubMed] [Google Scholar]
- 45.Conti P., Panara M. R., Barbacane R. C., Reale M., Bongrazio M., Dempsey R. A. (1992) Agents Actions, Spec. No C93–C95 [PubMed] [Google Scholar]
- 46.Conti P., Panara M. R., Barbacane R. C., Bongrazio M., Dempsey R. A., Reale M. (1993) Clin. Exp. Immunol. 91, 526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahluwalia N., Lin A. Y., Tager A. M., Pruitt I. E., Anderson T. J., Kristo F., Shen D., Cruz A. R., Aikawa M., Luster A. D., Gerszten R. E. (2007) J. Immunol. 179, 691–697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.