Abstract
Glycogen synthase kinase-3 (GSK-3) plays a critical role in neuronal apoptosis. The two mammalian isoforms of the kinase, GSK-3α and GSK-3β, are inhibited by phosphorylation at Ser-21 and Ser-9, respectively. Depolarization, which is vital for neuronal survival, causes both an increase in Ser-21/9 phosphorylation and an inhibition of GSK-3α/β. However, the role of GSK-3 phosphorylation in depolarization-dependent neuron survival and the signaling pathway contributing to GSK-3 phosphorylation during depolarization remain largely unknown. Using several approaches, we showed that both isoforms of GSK-3 are important for mediating neuronal apoptosis. Nonphosphorylatable GSK-3α/β mutants (S21A/S9A) promoted apoptosis, whereas a peptide encompassing Ser-9 of GSK-3β protected neurons in a phosphorylation-dependent manner; these results indicate a critical role for Ser-21/9 phosphorylation on depolarization-dependent neuron survival. We found that Ser-21/9 phosphorylation of GSK-3 was mediated by Ca2+/calmodulin-dependent protein kinase II (CaMKII) but not by Akt/PKB, PKA, or p90RSK. CaMKII associated with and phosphorylated GSK-3α/β. Furthermore, the pro-survival effect of CaMKII was mediated by GSK-3 phosphorylation and inactivation. These findings identify a novel Ca2+/calmodulin/CaMKII/GSK-3 pathway that couples depolarization to neuronal survival.
Keywords: Apoptosis, Calcium Calmodulin-dependent Protein Kinase (CaMK), Glycogen Synthase Kinase 3, Neuron, Protein Phosphorylation, Depolarization
Introduction
The survival or death of neurons is critical for the establishment of appropriate neural circuitry during brain development (1, 2). Considerable evidence supports that electrical activity plays a crucial role in neuronal survival (3, 4). For example, pharmacological blockade of electrical activity in rat brain induces extensive apoptotic neurodegeneration (5, 6). Deafferentiation of the cerebellar granule layer in adult rats resulted in massive and typical apoptosis of cerebellar granule neurons (CGNs),3 suggesting the importance of afferent input-related factors for survival of CGNs in vivo (7). In culture, survival of rat CGNs can be maintained by electrical activity, which is effected by depolarizing concentrations of extracellular potassium [KCl]o = 25 mm KCl ((25 K) or potassium depolarization) (8, 9). Lowering [KCl]o to 5 mm KCl ((5 K) or potassium deprivation) triggers typical apoptosis (10). Presumably, this recapitulates the naturally occurring neuronal death that takes place in the newborn rat cerebellum (11). These characteristics, along with an abundant neuronal population and up to 98% homogeneity, make cultured CGNs an excellent and extensively studied model for deciphering the signaling mechanisms that underlie depolarization-dependent neuron survival (4).
It has been well documented that depolarizing conditions (such as elevated [KCl]o) sustain neuronal survival by causing the influx of Ca2+ through L-type Ca2+ channels (8, 12, 13), implicating Ca2+ as a necessary second messenger for survival signaling. When activated by elevated Ca2+, Ca2+/calmodulin-dependent protein kinase II (CaMKII) has been reported to mediate the depolarization-dependent survival of many types of neurons (12, 14, 15), including CGNs (16, 17). However, the potential substrates of CaMKII that account for its survival-promoting effect in response to depolarization remain to be elucidated.
GSK-3 is a ubiquitous serine/threonine kinase composed of two distinct isoforms, GSK-3α and GSK-3β (18). GSK-3 is inhibited by N-terminal serine phosphorylation (Ser-21 in GSK-3α and Ser-9 in GSK-3β) (19). Multiple kinases, including Akt/PKB (20), PKA (21, 22), cGMP-dependent protein kinase 1 (23), integrin-linked kinase (24), and p90RSK (25), have been reported to phosphorylate and inactivate GSK-3. Various studies have demonstrated that GSK-3 promotes neuronal apoptosis that is induced by a diverse array of insults (21, 26–30), and dysregulated GSK-3 activity has been implicated in cell death during the pathogenesis of both Parkinson (31, 32) and Alzheimer disease (33). Therefore, pro-survival signaling is likely to involve the inhibition of GSK-3. Although depolarization induces the inhibitory phosphorylation of GSK-3 (24, 29), the role of GSK-3 phosphorylation and the signaling pathway contributing to GSK-3 phosphorylation in depolarization-dependent neuron survival remain largely unknown.
A functional correlation, if any, between CaMKII and GSK-3 in cell survival pathways has yet to be identified. Here, we found that depolarization activates CaMKII, which then directly phosphorylates GSK-3α/β at Ser-21/9, causing inactivation of GSK-3. Furthermore, our results demonstrate that CaMKII-mediated phosphorylation of GSK-3 plays a critical role in depolarization-dependent neuron survival.
EXPERIMENTAL PROCEDURES
Reagents
AR-A014418, U0126, PD98059, KN62, wortmannin, LY294002, CaMKIINtide (calmodulin kinase IINtide), myr-CaMKIINtide (myristoylated calmodulin kinase IINtide), and protein A plus protein G-agarose were obtained from EMD Biosciences. PKI and STO-609 were purchased from Tocris; forskolin and SB415286 were purchased from Sigma. Purified recombinant CaMKIIβ and GSK-3β protein were purchased from Invitrogen and Sigma, respectively. Calmodulin and GSK-3α protein were purchased from Millipore. Myr-Ser(P)-9-tide (RPRTTpSFAESC, corresponding to residues 4–14 of GSK-3β, pS is phosphoserine) and myr-Ser-9-tide (RPRTTSFAESC) were synthesized by Genemed Synthesis, Inc.
CGN Cultures and Depolarization Treatment
Rat CGNs were prepared from 7- or 8-day-old Sprague-Dawley rat pups as described previously (21, 34–36). Briefly, neurons were dissociated from freshly dissected cerebella by mechanical disruption in the presence of trypsin and DNase and then seeded at a density of 1.5 × 106 cells/ml in basal modified Eagle's medium containing 10% fetal bovine serum and 25 mm KCl (a concentration that causes membrane depolarization). After 7 days, neurons maintained in serum plus 25 mm KCl were rinsed three times with serum-free medium and simultaneously switched to serum-free medium containing 5 or 25 mm KCl (5 K or 25 K). The 5 K treatment was used as a control for studying the mechanism of depolarization. Serum-free medium was used in all experiments to isolate the survival-promoting activity of potassium alone, because in CGNs serum provides a survival-promoting activity of unknown origin (37), thereby complicating the results. For inhibitor treatment, CGNs were cultured in the 25 or 5 mm KCl medium that contained the inhibitor for 12 h. Cells that were not exposed to the inhibitors were exposed to the vehicle as control. Human embryonic kidney 293A (HEK293A) cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum.
Constructs and Transfection
The plasmid pCMV-3×FLAG-GSK-3α WT or pcDNA3.1-V5-GSK-3α WT was cloned from rat GSK-3α cDNA and incorporated into a pCMV-3×FLAG or a pcDNA3.1-V5 expression vector. The plasmid pcDNA3.1-V5-GSK-3α S21A was constructed from pcDNA3.1-V5-GSK-3α WT, and the mutated site was constructed from TCG to GCG. The CDS of GSK-3α K148A was described previously (38) and was subcloned into a pcDNA3.1-V5 expression vector to generate pcDNA3.1-V5-GSK-3α K148A. The plasmid pCMV-3×FLAG-GSK-3β WT was cloned from rat cDNA and incorporated into a pCMV-3×FLAG expression vector. The plasmids pGEX-4T-1-GSK-3α and pGEX-4T-1-GSK-3β were cloned from rat GSK-3α or GSK-3β cDNA and incorporated into a pGEX-4T-1 prokaryotic expression vector. The plasmids pcDNA3.1-V5-GSK-3β WT, V5-GSK-3β K85R, and V5-GSK-3β S9A were kind gifts from Dr. Thilo Hagen. The GFP-CaMKIIN was a gift from Dr. Thomas R. Soderling. Dr. Yasunori Hayashi kindly provided the GFP-CaMKIIβ, which was used to construct the constitutively active CaMKIIβ T287D (from ACT to GAC). The mutations were introduced using overlap extension PCR. All the constructs were confirmed using DNA sequencing. For gene transfection, at days in vitro (DIV) 5 or 6, CGNs were transfected using a calcium phosphate transfection method as described previously (21, 34). HEK293A cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Western Blotting and Antibodies
Western blot analysis was performed as described previously (34, 36). Briefly, lysates were separated using SDS-PAGE and electrophoretically transferred to a polyvinylidene difluoride membrane. Membranes were blocked in Tris-buffered saline with 5% milk and 0.05% Tween and probed with primary antibodies at 4 °C overnight. Antibodies against phospho-GSK-3α/β (Ser-21/9), phospho-GSK-3α (Ser-21), phospho-GSK-3β (Ser-9), phospho-CRMP2 (Thr-514), CRMP2, phospho-Akt (Ser-473), phospho-Akt (Thr-308), phospho-FOXO3a (Thr-32), phospho-ERK1/2, phospho- p90RSK, Akt, and caspase-3 were obtained from Cell Signaling Technology; GSK-3α/β and phospho-CaMKII (Thr-286/Thr-287) were from Millipore; CaMKII (clone M-176), phospho-CaMKIV (Thr-196), and GSK-3β (clone H-76) were from Santa Cruz Biotechnology; CaMKIIβ was from Zymed Laboratories Inc.; phospho-GSK-3β (Tyr-216) and GSK-3β were from BD Transduction Laboratories; GSK-3α and GFP were from Abcam; FLAG and tubulin were from Sigma; and V5 was from Serotec. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit secondary antibodies (Jackson ImmunoResearch) and visualized using the ECL reagents.
Immunoprecipitation
Immunoprecipitation (IP) assays were performed as described previously (35). For CGN immunoprecipitation, neuronal extracts composed of 6.0 × 106 cells were prepared by solubilization in 400 μl of cell lysis buffer (1% Triton X-100, 150 mm NaCl, 20 mm Tris-Cl (pH 7.4), 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 2.5 mm pyrophosphate, 1 mm glycerol phosphate, and protease inhibitor mixture) for 10 min at 4 °C. After a brief sonication, the lysates were cleared by centrifugation at 15,000 × g for 10 min at 4 °C, and the cell extract was immunoprecipitated with 4 μg of antibodies against CaMKIIβ (Zymed Laboratories Inc.) or GSK-3β (Santa Cruz Biotechnology) and incubated with 60 μl of protein G plus protein A-agarose for 16 h at 4 °C by continuous inversion. Immunocomplexes were pelleted and washed three times. The precipitated immunocomplexes were then boiled in Laemmli buffer and subjected to Western blot analysis using anti-GSK-3α, anti-GSK-3β, or anti-CaMKIIβ antibody. For HEK293A cell immunoprecipitation, 2.5 μg of GFP-CaMKIIβ and 2.5 μg of V5-GSK-3α or 2.5 μg of V5-GSK-3β were co-transfected into HEK293A cells. Twenty four hours after transfection, cells were lysed and immunoprecipitated with 2 μg of either GFP (Abcam) or V5 (Serotec) antibody. The precipitated immunocomplexes were assayed using Western blot analysis with antibodies against either GFP or V5.
RNA Interference
Two 19-nucleotide GSK-3α siRNAs (siGSK-3α-a and siGSK-3α-b) were designed to target the sequences 5′-GCUCAUUCGGAGUAGUGUA-3′ and 5′-GCUUUAACUGAGACUCAGA-3′ of GSK-3α mRNA (NCBI accession number NM_017344). Two GSK-3β siRNAs, siGSK-3β-a and siGSK-3β-b, targeted the sequences 5′-GAAAGUUAGCAGAGAUAAA-3′ and 5′-GGACCCAAAUGUCAAACUA-3′ of GSK-3β mRNA (NCBI accession number: NM_032080). The targeted regions showed no significant homology with any other genes using BLAST searches. A nontargeting siRNA was used as a negative control (NC) for all siRNA transfection experiments. All siRNAs were synthesized by Shanghai GenePharma Co., Ltd.
To determine the efficacy and specificity of siRNAs, co-transfection of NC, siGSK-3α-a, siGSK-3α-b, siGSK-3β-a, or siGSK-3β-b, together with rat FLAG-GSK-3α or FLAG-GSK-3β plasmids into HEK293A cells, was performed. The expressions of FLAG-GSK-3α or FLAG-GSK-3β protein were examined by Western blot analysis using a FLAG antibody.
Transfections of siRNAs in CGNs were conducted using a calcium phosphate protocol as described previously (34). Briefly, cell media were replaced with basal modified Eagle's medium, and original conditioned media were saved at 37 °C in an incubator. For transfecting 24-well plates, 100 pmol of siRNAs were combined with 37 μl of a 2 m CaCl2 solution in sterile, deionized water to a final volume of 300 μl and then mixed well with 300 μl of 2× HEPES-buffered saline. The mixtures were vortexed and incubated at 25 °C for approximately 4 min. The 30 μl mixture was added dropwise to the cells in each well and allowed to incubate for another 25 min. After washing cells two times with basal modified Eagle's medium, the original medium was added back to cultures. Forty eight hours following transfection, CGNs were treated with 25 or 5 K medium for 12 h. Subsequently, an apoptosis assay or an immunofluorescence assay was performed as described below. A GFP expression plasmid was co-transfected with siRNAs to mark the transfected cells. Transfection efficiency in CGNs was determined by calculating the percentages of GFP-positive cells out of the total cell numbers. The total number of neurons counted for each treatment group was more than 500. Efficiency of the siRNA transfection was ∼1% in our study.
Immunofluorescence
CGNs were grown on cover glasses (Fisher) and processed for immunofluorescence according to the standard protocol described previously (35). CGNs were fixed using freshly prepared 4% paraformaldehyde followed by permeabilization using 0.1% Triton X-100 in TBS. Cover glasses were incubated in primary antibodies, including anti-phospho-GSK-3α/β (Ser-21/9) (Cell Signaling Technology), anti-GSK-3α (Abcam), anti-GSK-3β (Santa Cruz Biotechnology), and anti-CaMKIIβ (Zymed Laboratories Inc.) at a dilution of 1:500. Phospho-GSK-3α/β, GSK-3α, or GSK-3β was detected with anti-rabbit secondary antibody conjugated to Alexa Fluor 555 (Invitrogen). CaMKIIβ was detected with anti-mouse secondary antibody conjugated to Alexa Fluor 488 (Invitrogen). Fluorescent staining was visualized using a 40× NA 1.3 oil objective on a confocal microscope (LSM 510 Meta; Carl Zeiss, Inc.). Images were recorded with sequential acquisition settings at a resolution of 512 × 512 pixels with 12-bit depth. In addition, a 420–480-nm bandpass emission filter was used for the blue channel; a 560-nm long pass filter was used for the red channel, and a 505–530-nm bandpass emission filter was used for the green channel. The confocal settings remained fixed for the duration of the experiments to allow easy comparison of the fluorescence intensities. Images were processed with LSM 510 software (Carl Zeiss, Inc.) before import into Photoshop CS2 (Adobe) for orientation and cropping. Co-localization of CaMKIIβ with GSK-3α or GSK-3β was estimated for individual neurons as the Pearson coefficient of correlation (where full co-localization is 1.0) using Image Pro Plus software.
Kinase Assay
To measure the GSK-3β activity of CGNs, GSK-3β activity assays were performed as described previously (21). Briefly, GSK-3β was immunoprecipitated from CGN lysate using the monoclonal anti-GSK-3β antibody (BD Transduction Laboratories) and subjected to a total volume of 40 μl that contained 50 mm Tris-HCl (pH 7.4), 10 mm MgCl2, 5 mm DTT, and 100 μm 2B-SP (Tocris). To evaluate the inhibitory effects of myr-Ser(P)-9-tide and myr-Ser-9-tide, recombinant GSK-3β (Sigma) was preincubated with myr-Ser(P)-9-tide or myr-Ser-9-tide for 5 min at the concentration indicated, and the GSK-3β kinase activity was then determined as described. Akt activity was measured as described previously (21) using the Akt activity assay kit (Cell Signaling Technology). PKA activity was determined using the PepTag® assay according to the manufacturer's instructions (Promega). For the in vitro CaMKIIβ kinase assay, CaMKIIβ was preactivated in a reaction containing calcium, calmodulin, and ATP with or without CaMKIINtide for 10 min at 30 °C. Recombinant GSK-3α (Millipore) or GSK-3β (Sigma) protein was added for 5 min and quenched with Laemmli buffer. Phosphorylation of GSK-3α (Ser-21) or GSK-3β (Ser-9) was examined using Western blot analysis.
Kinetic assays were performed as described previously by us (21). Briefly, GST-GSK-3α or GSK-3β was expressed in Escherichia coli BL21 strain and purified as substrate. 100 μm [γ-32P]ATP (2 μCi, PerkinElmer Life Sciences) was added to the reaction mixtures. At the end of the phosphorylation reactions, these samples were solubilized in Laemmli sample buffer and analyzed by SDS-PAGE. Gels were dried and subjected to autoradiography. Then radioactivity was assessed by Cerenkov counting of excised SDS-polyacrylamide gel slices. The substrate concentration at half-maximal velocity (Km) and at maximal velocity (Vmax) was determined using a nonlinear regression analysis of the data plotted by Michaelis-Menten kinetics.
Quantification of Neuronal Apoptosis
CGN apoptosis was quantified as described previously (34–36). In brief, CGNs were cultured in 24-well plates and incubated in 25 or 5 K medium with or without various inhibitors as described above. After a 12-h incubation, CGNs were stained with the DNA dye Hoechst 33258 (5 μg/ml) to visualize nuclear morphology. Apoptosis was quantified by scoring the percentage of cells in the neuron population with condensed or fragmented nuclei.
For quantification of apoptosis in transfected neurons, CGNs were co-transfected with respective plasmids and GFP to mark the transfected cells. Thirty six hours after transfection, the neurons were switched to 25 or 5 K medium for 12 h and then stained with Hoechst 33258 to visualize nuclear morphology and propidium iodide (0.25 μg/ml) to detect membrane damage, as described previously (34). Photographs were taken using a fluorescence microscope (at a magnification of 200×). GFP-positive neurons with intact nuclei showing diffuse Hoechst staining and no propidium iodide staining were scored as viable, and dying or dead neurons displayed condensed nuclei and fragmented nuclei with stronger fluorescence of Hoechst and propidium iodide. Apoptotic rates are represented as the percentage of apoptotic cells out of the total number of GFP-positive cells, as described previously (21, 34, 35). The numbers of neurons were counted from three randomly chosen microscopic fields per well. To obtain unbiased results, experiments were performed in a blinded manner, and cells were scored without knowledge of their prior treatments. All experiments were repeated at least three times, and the total number of neurons counted for each treatment group was more than 500.
Statistical Analysis
All the statistical data in the graphs represent the means of at least three independent experiments with ± S.E. bars. The groups were compared using Student's t test with control values. The significance is indicated as follows: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
RESULTS
Depolarization Induces Inhibitory Phosphorylation of GSK-3α (Ser-21) and GSK-3β (Ser-9)
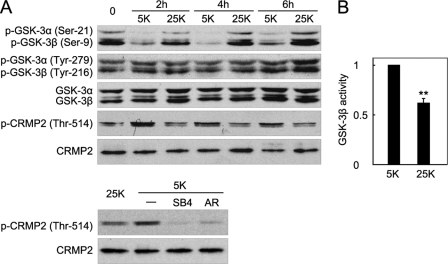
Studies have reported increased Ser-21/9 phosphorylation of GSK-3α/β following KCl depolarization (24, 29). To confirm and expand upon previous observations, DIV7 neurons that were maintained in serum plus 25 mm KCl (0 h) were simultaneously shifted to serum-free medium containing 5 mm KCl (5 K or potassium deprivation) or 25 mm KCl (25 K or potassium depolarization) at the times indicated, and whole-cell extracts were prepared for Western blotting with a phospho-GSK-3α/β (Ser-21/9) antibody. After switching to non-serum medium, potassium depolarization (25 K), as compared with 5 K, maintained an increase of the Ser-21/9 phosphorylation level of both isoforms throughout the time course (Fig. 1A, top).
FIGURE 1.
Depolarization induces phosphorylation of GSK-3α (Ser-21) and GSK-3β (Ser-9) and thus inactivates GSK-3α/β. A, DIV7 CGNs were maintained in serum plus 25 mm KCl (0 h) and then switched to 5 or 25 K medium in the absence of serum for the indicated time (2, 4, or 6 h) (top panel). Cell lysates of CGNs were analyzed using a Western blot with the indicated antibodies. Neurons were maintained in 5 K medium with or without 40 μm SB415286 (SB4) or 10 μm AR-A104418 (AR) for 4 h and then analyzed using a Western blot (bottom panel). B, reduction in GSK-3β activity upon depolarization. After incubation in 5 or 25 K conditions for 4 h, CGN lysates were immunoprecipitated by GSK-3β antibody, and GSK-3β activity was determined using 2B-SP as the substrate. The data represent the mean ± S.E. of four independent experiments, ** denotes that p < 0.01.
In addition to serine phosphorylation, GSK-3 activity is also positively regulated by tyrosine phosphorylation at Tyr-279 and Tyr-216 of GSK-3α and GSK-3β (39). However, the level of phospho-Tyr-279/216 was insensitive to the potassium concentration and unchanged by the treatments (Fig. 1A, top panel). Additionally, the protein levels of both isoforms were also unaffected by the 25 and 5 K treatments (Fig. 1A, top panel).
Because Ser-21/9 phosphorylation has been well documented to autoinhibit GSK-3 kinase activity via a pseudosubstrate mechanism (40, 41), the above observations suggest that depolarization leads to inactivation of GSK-3. As expected, the phosphorylation level of a well characterized GSK-3 substrate (collapsin response mediator protein 2, CRMP2) at Thr-514 (42, 43) was decreased by depolarization, whereas the total CRMP2 protein level remained unchanged (Fig. 1A, top panel), indicating that endogenous GSK-3 activity was reduced by depolarization. This CRMP2 phosphorylation was abolished by two structurally unrelated GSK-3 inhibitors, SB415286 (44) and AR-A014418 (32, 45), indicating that the increased Thr-514 phosphorylation specifically results from GSK-3 activation (Fig. 1A, bottom panel). Furthermore, the precipitates following immunoprecipitation of GSK-3β from CGNs in 25 K medium showed decreased GSK-3β activity compared with that in 5 K medium (Fig. 1B). From these findings, we conclude that depolarization promotes GSK-3 phosphorylation and thus inactivates it.
Not Only GSK-3β but Also GSK-3α Mediates Neuronal Apoptosis Induced by Potassium Deprivation
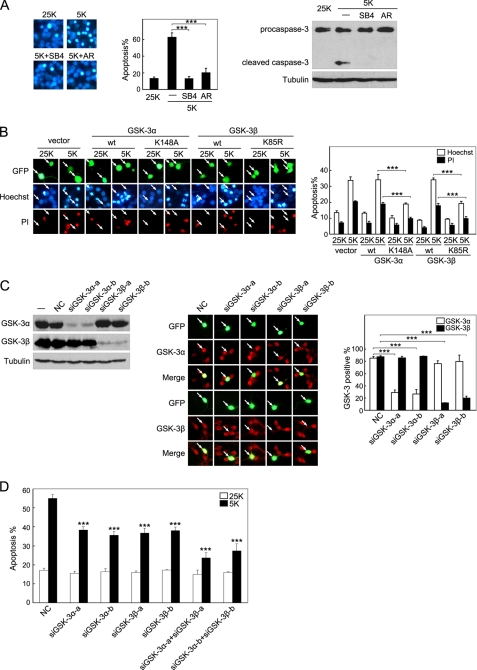
If inactivation of GSK-3 is a critical downstream checkpoint in the depolarization-dependent survival pathways of CGNs, GSK-3 activity should be required for potassium deprivation-induced apoptosis. As shown in Fig. 2A and described by other studies (9, 10), cultures of CGNs switched to medium containing 5 mm KCl manifested the hallmarks of apoptosis, including increased pyknotic nuclei and the appearance of active caspase-3. These changes were blocked by treatment with the two GSK-3 inhibitors, SB415286 and AR-A014418 (Fig. 2A), indicating that GSK-3 activity is critical for apoptosis. Because pharmacological inhibitors can be promiscuous (46), we employed GSK-3α K148A (a dominant-negative GSK-3α mutant) (38) and found that GSK-3α K148A exerted a significant protection from apoptosis (Fig. 2B). In contrast, neurons transfected with wild-type (WT) GSK-3α displayed strong staining and condensed nuclei, characteristic of apoptotic cells. Transfection of GSK-3β K85R (47), a dominant-negative GSK-3β mutant, also protected CGNs from apoptosis, whereas WT GSK-3β did not (Fig. 2B). These results are consistent with previous studies (21, 27). Thus, GSK-3 activity plays a crucial role in potassium deprivation-induced neuron apoptosis.
FIGURE 2.
Not only GSK-3β but also GSK-3α mediate neuronal apoptosis induced by potassium deprivation. A, pharmacological inhibition of GSK-3 suppresses apoptosis. Representative images of CGNs incubated in media containing 25 K, 5 K, 5 K plus 40 μm SB415286 (SB4), or 10 μm AR-A104418 (AR) for 12 h are shown (left panel). Neurons were stained with Hoechst 33258 to visualize nuclei. Apoptosis was quantified by scoring the percentage of neuron population with pyknotic nuclei (middle panel). CGNs with the same treatments were subjected to Western blot analysis (right panel). B, dominant-negative (DN) GSK-3α and GSK-3β mutants attenuate apoptosis. DIV5 CGNs were co-transfected with the indicated plasmid along with GFP to mark transfected neurons. After incubation in 25 or 5 K conditions for 12 h, CGNs were doubly stained by Hoechst 33258 (blue) and propidium iodide (PI) (red) (left panel). Apoptotic rates of CGNs were quantified as described under “Experimental Procedures” (right panel). C, efficacy and specificity of siGSK-3α and siGSK-3β are shown. HEK293A cells were co-transfected with the indicated siRNAs together with rat pCMV-3×FLAG-GSK-3α or pCMV-3×FLAG-GSK-3β plasmid, respectively (left panel). Expression of rat GSK-3α or GSK-3β was monitored using Western blotting. DIV5 CGNs were transfected with the same siRNAs, and typical images of the GSK-3α or GSK-3β immunostaining are shown (middle panel). A nontargeting siRNA was used as negative control (NC). The percentages of GSK-3α or GSK-3β-positive neurons under indicated treatments were quantified (right panel). D, knockdown of GSK-3α, GSK-3β, or combination of both isoforms reduces apoptosis. DIV5 CGNs were co-transfected with the indicated siRNAs along with GFP for 48 h. Then CGNs were incubated in either 25 or 5 K medium for 12 h, and apoptosis was quantified. Data in B–D are the mean ± S.E. of more than three independent experiments; *** denotes p < 0.001.
Because the peptide sequences used as the antigen of the GSK-3 total antibody or Tyr-216 antibody are exactly identical between the two isoforms, the results shown in Fig. 1A, top panel, indicate that both GSK-3α and GSK-3β are expressed with almost equal abundance in CGNs. This result is consistent with another study (48). Furthermore, both isoforms underwent Ser-21/9 phosphorylation in response to depolarization (Fig. 1A, top panel). Thus, the role of GSK-3α cannot be ignored. However, previous literature has primarily focused on GSK-3β (rather than GSK-3α) in neuronal apoptosis (26, 48), and GSK-3 inhibitors and mutants do not discriminate between the two isoforms. To specifically address the role of each GSK-3 isoform in neuronal apoptosis, siRNAs that separately targeted either GSK-3α or GSK-3β were used to independently reduce protein levels of the individual isoforms. HEK293A cells were co-transfected with rat FLAG-GSK-3α or rat FLAG-GSK-3β together with siRNAs specific for rat GSK-3α or rat GSK-3β. As shown in Fig. 2C, left panel, transfection with the GSK-3α-targeted siRNAs or GSK-3β-targeted siRNAs specifically suppressed the expression of their respective rat proteins, whereas the level of the nontargeted GSK-3 isoform or of an unrelated gene (like tubulin) was unaffected. These results verify a high selectivity and efficacy of the siRNAs tested. In CGNs, these siRNAs also resulted in a 65–80% knockdown of endogenous GSK-3α or GSK-3β in an isoform-specific manner (Fig. 2C, middle and right panels). The silencing of the endogenous expression of either GSK-3α or GSK-3β independently through the use of isoform-specific siRNAs provided significant protection from 5 K-induced death, whereas there was no significant effect of the RNA interference technique on the viability of CGNs (as revealed by the NC siRNA, Fig. 2D). Furthermore, combining the siRNAs to knock down both isoforms yielded additive effects against neuronal apoptosis (Fig. 2D). These data indicate that both GSK-3β and GSK-3α are critical players in neuronal apoptosis induced by potassium deprivation.
Phosphorylation of GSK-3 Is Critical for Depolarization-dependent Neuron Survival
Phosphorylation at Ser-21/9 is the most important mechanism for the inhibition of GSK-3 activity (40, 41). Having established that inhibition of GSK-3 can protect CGNs from potassium deprivation-induced apoptosis, we then asked whether inhibitory phosphorylation of GSK-3 at Ser-21/9 was critical for neuronal survival. CGNs were transfected with GSK-3α S21A or GSK-3β S9A, the constitutively active GSK-3α/β mutants that cannot be phosphorylated at Ser-21/9. Expression of GSK-3α S21A (rather than WT GSK-3α) promoted apoptosis in 25 K (Fig. 3A), indicating that dephosphorylation of GSK-3α Ser-21 is sufficient to induce apoptosis in depolarizing conditions. Similarly, expression of GSK-3β S9A (the constitutively active GSK-3β mutant) also enhanced neuronal death during depolarization. In contrast, neither GSK-3α S21A nor GSK-3β S9A promoted apoptosis in the presence of serum plus 25 mm KCl treatment (Fig. 3A), which is in accordance with a recent study showing that DIV7 CGNs isolated from GSK-3α/β S21A/S9A knock-in mice survived normally in serum plus a depolarization condition (48). This suggests that serum may contain some survival-promoting factors that can overcome the pro-apoptotic role of GSK-3. However, our findings in the absence of serum substantiate that Ser-21/9 phosphorylation of GSK-3α/β is specifically critical for depolarization-supporting neuronal survival.
FIGURE 3.
Phosphorylation of GSK-3 is critical for depolarization-dependent neuron survival. A, nonphosphorylatable GSK-3α or GSK-3β mutants promote apoptosis. DIV5 CGNs were co-transfected with the indicated plasmids along with GFP for 36 h. Then CGNs were maintained in serum plus 25 K or in 25 K alone for 24 h, and apoptotic rates of transfected cells were quantified. Data are the mean ± S.E. of nine independent experiments; ** denotes p < 0.01. B, Myr-Ser(P)-9-tide, but not myr-Ser-9-tide, inhibits GSK-3β activity and protects CGNs against apoptosis. Recombinant GSK-3β was preincubated with the indicated concentration of myr-Ser(P)-9-tide or myr-Ser-9-tide for 5 min, and GSK-3β activity was determined using 2B-SP as the substrate (left panel). CGNs incubated in 5 K medium were treated with myr-Ser(P)-9-tide or myr-Ser-9-tide for 12 h at the concentration indicated, and apoptosis was quantified (right panel). Data represent the mean ± S.E. of at least three determinations. For GSK-3β activity, p < 0.05 at 10, 20, and 40 μm; for CGN apoptosis, p < 0.05 at 10 and 15 μm and p < 0.001 at 20 μm.
Crystal structural analysis of GSK-3β has revealed that the N-terminal region containing phosphorylated Ser-9 acts as a competitive pseudosubstrate that binds to the substrate-binding cleft of GSK-3, suppressing its activity (40, 41, 49). These studies also yielded a peptide encompassing Ser-9 (4–14 amino acids) of GSK-3β that can effectively repress GSK-3β in a Ser-9 phosphorylation-dependent manner. To evaluate further the role of Ser-21/9 phosphorylation in neuronal survival, we generated the same peptide, Ser(P)-9-tide (RPRTTpSFAESC, 4–14 amino acids of GSK-3β, where pS is phosphoserine), and introduced N-myristoylation to Ser(P)-9-tide to promote cell permeability for use in intact cell experiments. The myr-Ser(P)-9-tide inhibited GSK-3β activity in a dose-dependent manner, whereas an unphosphorylated version of this peptide (myr-Ser-9-tide) caused no inhibition (Fig. 3B, left). This result indicates that myr-Ser(P)-9-tide (but not myr-Ser-9-tide) was able to suppress GSK-3β and that the introduction of N-myristoylation did not interfere with the specificity of Ser(P)-9-tide. Therefore, the above data, together with previous reports (40), reveal that myr-Ser(P)-9-tide can mimic the phosphorylated N terminus of GSK-3 and be used as a cell-permeable GSK-3β inhibitor. In CGNs, treatment with myr-Ser(P)-9-tide, but not the unphosphorylated myr-Ser-9-tide, resulted in a significant and dose-dependent protection against potassium deprivation-induced apoptosis (Fig. 3B, right panel), underscoring the pivotal role of Ser-21/9 phosphorylation in depolarization-maintained neuronal viability.
Inhibitory Phosphorylation of GSK-3 during Depolarization Is Independent of Akt, PKA, and p90RSK
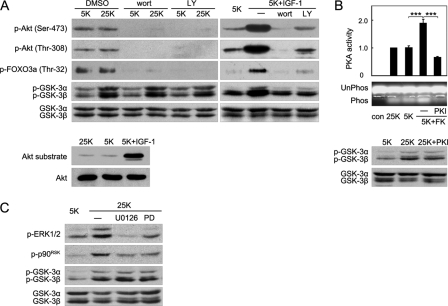
After establishing the crucial role of Ser-21/9 phosphorylation in neuron survival, we next tested which kinase is responsible for GSK-3 phosphorylation in depolarized neurons. PI3K-Akt/PKB is a major pathway mediating the phosphorylation and inactivation of GSK-3 induced by neurotrophins such as insulin-like growth factor-1 (20, 50). However, no significant difference in Akt phosphorylation (both Ser-473 and Thr-308) or Akt activity, represented by phosphorylation of the well documented substrate FOXO3a in vivo and by Akt substrate in kinase assay, was found between 25 K- and 5 K-treated CGNs (Fig. 4A), demonstrating that Akt was not stimulated by depolarization. In contrast, robust GSK-3 phosphorylation was detected in response to depolarization, indicating that depolarization-maintained GSK-3 phosphorylation occurred independently from Akt (Fig. 4A). Although wortmannin and LY294002, two PI3K pathway inhibitors, effectively inhibited IGF-1-induced Akt phosphorylation, FOXO3a phosphorylation, and GSK-3 phosphorylation as positive controls (20, 50), neither wortmannin nor LY294002 erased the depolarization-induced phosphorylation of GSK-3. This result further strengthens the notion that 25 K-induced GSK-3 phosphorylation was independent of the PI3K-Akt pathway (Fig. 4A). Together, these results indicate that the PI3K-Akt pathway is not activated by depolarization and exclude the possibility that Akt phosphorylates GSK-3 during depolarization.
FIGURE 4.
Inhibitory phosphorylation of GSK-3 during depolarization is independent of Akt, PKA, and p90RSK. A, DIV7 CGNs maintained in media containing 25 or 5 K in the presence of DMSO, 200 nm wortmannin (wort), or 10 μm LY294002 (LY) were assayed using Western blotting. DIV7 CGNs in 5 K medium were treated using 200 ng/ml IGF-1, IGF-1 plus 200 nm wortmannin, or IGF-1 plus 10 μm LY294002 for 6 h and were analyzed using Western blotting (top panel). CGNs maintained in 25 K, 5 K, or 5 K plus 200 ng/ml IGF-1 were processed for an Akt activity assay using a GSK-3 fusion protein as the substrate (bottom panel). B, a representative image of the PKA phosphorylation of the PepTag® A1 peptide is shown (top panel). PKA activity was determined using CGN extracts in media containing 25 K, 5 K, 5 K with 10 μm forskolin (FK), or 5 K with 10 μm FK plus 10 μm PKI, respectively. The data represent the mean ± S.E. bars for five independent experiments, *** denotes p < 0.001. A Western blot of the indicated protein extracts was assayed (bottom panel). Unphos, unphosphorylated; Phos, phosphorylation; con, control. C, CGNs were cultured in 5 or 25 K conditions in the absence or presence of 10 μm U0126 or 50 μm PD98059 (PD) for 6 h, and protein extracts were analyzed using Western blotting.
We and another group have identified PKA as an upstream kinase of GSK-3 when intracellular cAMP increases (21, 22). Thus, a PKA kinase assay was employed to examine whether PKA was activated by depolarization. Compared with the 5 K medium, the depolarization medium (25 K) had no detectable effect on PKA activity (Fig. 4B, top panel). In contrast, forskolin, an activator of adenylate cyclase that is known to elevate the intracellular cAMP levels, which leads to activation of PKA, dramatically stimulated PKA. However, PKI, a specific PKA inhibitor, drastically blocked PKA activity as expected (Fig. 4B, top panel). Western blot analysis revealed that PKI was unable to repress GSK-3 phosphorylation during depolarization, indicating that GSK-3 phosphorylation was not associated with PKA activity (Fig. 4B, bottom panel). Therefore, like Akt, PKA is not involved in GSK-3 phosphorylation following depolarization.
It has been shown previously that p90RSK phosphorylates GSK-3 following stimulation by EGF (25). However, U0126 and PD98059 (51, 52), two structurally distinct MEK inhibitors that block the MEK/ERK1/2/p90RSK pathway, failed to affect GSK-3 phosphorylation (Fig. 4C); this evidence excludes p90RSK as an upstream kinase of GSK-3 in CGN depolarization.
Together, these findings provide direct evidence that depolarization-induced GSK-3 phosphorylation is independent of Akt, PKA, and p90RSK. Furthermore, the lack of a correlation between these three known kinases and GSK-3 phosphorylation in CGNs argues in favor of a previously unidentified route for GSK-3 phosphorylation and inactivation in response to depolarization.
CaMKII Phosphorylates GSK-3α/β at Ser-21/9
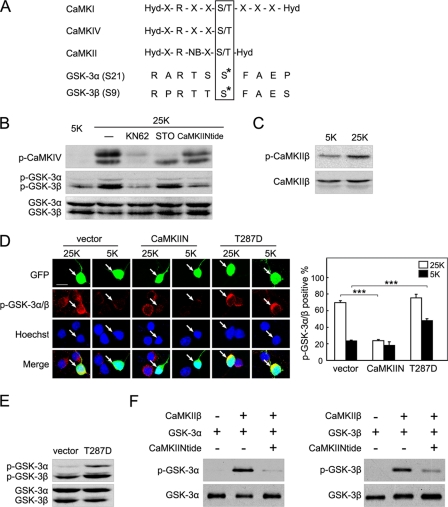
As we attempted to identify the upstream kinase of GSK-3 in depolarized CGNs, we noticed that the sequences flanking Ser-21/9 of GSK-3α/β corresponded closely to the optimal substrate recognition motifs of CaMKs (Fig. 5A) (53).
FIGURE 5.
CaMKII phosphorylates GSK-3α (Ser-21) and GSK-3β (Ser-9). A, a comparison between the substrate phosphorylation motif of CaMKs and the sequences encompassing Ser-21/9 of GSK-3α/β. Hyd represents hydrophobic, X represents any, and NB represents a non-basic amino acid residue. B, CGNs were incubated in 25 or 5 K medium with or without 10 μm KN62, 15 μm STO-609 (STO), or 15 μm myr-CaMKIINtide (CaMKIINtide) for 6 h and then subjected to Western blotting. C, depolarization activates CaMKIIβ. Cell lysates of CGNs incubated in 25 or 5 K medium for 6 h were analyzed using Western blotting. D, confocal images of phospho-GSK-3α/β (Ser-21/9) immunofluorescence upon activation or inhibition of CaMKII are shown (left panel). DIV5 CGNs were transfected with GFP-tagged CaMKIIN, CaMKIIβ T287D (T287D), or vector for 36 h and then incubated in 25 or 5 K medium for 4 h. The scale bar, 10 μm. Percentages of phospho-GSK-3α/β-positive cells in transfected neurons were scored (right panel). Mean values from four independent experiments are presented ± S.E.; *** denotes p < 0.001. E, CaMKIIβ activation results in GSK-3α/β phosphorylation in vivo. HEK293A cells were transfected with vector or T287D and analyzed using Western blotting. F, CaMKIIβ phosphorylates GSK-3α/β in vitro. After an in vitro CaMKIIβ kinase assay, levels of phospho-GSK-3α (Ser-21) and GSK-3α were analyzed using Western blotting (left panel). A similar assay was performed for GSK-3β (right panel).
CaMKs are crucial mediators of the physiological effects of depolarization-induced elevations of the intracellular Ca2+ level (12), and the family is mainly composed of CaMKK, CaMKI, CaMKII, and CaMKIV (54). To test whether CaMKs mediate GSK-3 phosphorylation, KN62 (55), a pan-inhibitor of CaMKs, was used to treat depolarized CGNs. Western blot analysis revealed that KN62 eliminated depolarization-stimulated GSK-3 phosphorylation (Fig. 5B). To further investigate which subtype of the CaMK family was involved, we used STO-609 (56), a specific inhibitor of CaMKK, which is the upstream kinase of CaMKI and CaMKIV. Whereas the STO-609 dramatically attenuated the phosphorylation of CaMKIV, a well recognized CaMKK substrate (57), the GSK-3 phosphorylation levels remained unaltered in the presence of STO-609 (Fig. 5B), excluding the possibility that CaMKK, CaMKI, and CaMKIV phosphorylated GSK-3 during depolarization. Next, we used myr-CaMKIINtide to show that GSK-3 phosphorylation was from CaMKII. Myr-CaMKIINtide is a cell-permeable CaMKII inhibitor derived from CaMKIIN, an endogenous inhibitory protein of CaMKII (58). Myr-CaMKIINtide has been well characterized as a highly selective and potent inhibitor of CaMKII with no effect on CaMKI, CaMKIV, or CaMKK. Treatment of CGNs with myr-CaMKIINtide decreased endogenous GSK-3 phosphorylation (Fig. 5B). Collectively, depolarization-induced GSK-3 phosphorylation depends on CaMKII, rather than CaMKK, CaMKI, or CaMKIV.
It is noteworthy that Yano et al. (59) reported CaMKK-stimulated phosphorylation of Akt at Thr-308 mediated neuronal activity-dependent survival in the NG108 neuroblastoma cell line. However, Akt phosphorylation at Thr-308 was unchanged following depolarization in our data (Fig. 4A). Although different cellular models may account for the discrepancy, this finding also suggests that CaMKK activation following depolarization in CGNs may lead to phosphorylation and activation of CaMKIV but not of Akt. Subsequently, CaMKIV may promote survival by other mechanisms, including stimulating cAMP-response element-binding protein-dependent gene expression (60, 61).
CaMKII consists of several isoforms derived from at least the four genes α, β, γ, and δ (62, 63); CGNs predominantly express CaMKIIβ (64). It is well established that autophosphorylation at Thr-287 maintains CaMKIIβ in a Ca2+/calmodulin-independent state (54, 65). In CGNs, we detected increased Thr-287 phosphorylation of CaMKIIβ in 25 K medium when compared with the phosphorylation that occurred in 5 K medium (Fig. 5C). This was in the context of a constant level of CaMKIIβ protein, confirming that CaMKIIβ is activated by depolarization.
To examine whether the activity of CaMKIIβ is responsible for phosphorylation of GSK-3, CGNs were transfected with CaMKIIN, an endogenous inhibitory protein of CaMKII (58). Expression of CaMKIIN effectively reduced phospho-GSK-3α/β (Ser-21/9) immunostaining in the 25 K condition compared with immunostaining in the vector control (Fig. 5D), indicating that CaMKII is necessary for GSK-3 phosphorylation. To assess whether CaMKII activation is sufficient for GSK-3 phosphorylation, a constitutively active mutant, CaMKIIβ T287D, was used to mimic the autophosphorylation of CaMKIIβ at Thr-287. As shown in Fig. 5D, transfection of CaMKIIβ T287D into CGNs elevated phospho-GSK-3α/β (Ser-21/9) in 5 K medium. This result is further validated by evidence from HEK293A cells, in which CaMKIIβ T287D transfection also led to a marked enhancement of GSK-3 phosphorylation (Fig. 5E). These data support the contention that CaMKIIβ activity is required for GSK-3 phosphorylation in vivo.
To determine whether CaMKIIβ directly phosphorylates GSK-3, an in vitro kinase assay was performed using purified CaMKIIβ and recombinant GSK-3α or GSK-3β protein. The results showed that CaMKIIβ directly phosphorylated GSK-3α at Ser-21, whereas the addition of CaMKIINtide to the assay diminished GSK-3α phosphorylation (Fig. 5F, left panel). Similarly, CaMKIIβ directly and specifically phosphorylated GSK-3β at Ser-9 (Fig. 5F, right panel). Furthermore, kinetic assays, with CaMKIIβ as kinase and GSK-3α or GSK-3β as substrate, were conducted to assess the kinetics of phosphorylation reactions as described previously (21). The Km and Vmax values were 18.12 ± 1.65 μm and 40.45 ± 4.12 μmol·min−1·mg−1 with GSK-3α as substrate, respectively. For GSK-3β as substrate, the Km and Vm values were 16.12 ± 1.34 μm and 60.45 ± 5.14 μmol·min−1·mg−1, respectively. As compared with the reported data for CaMKII phosphorylation of autocamtide (66) and for PKA phosphorylation of GSK-3β (21), the relatively low Km value for the in vitro phosphorylation of GSK-3 by CaMKII is consistent with both GSK-3α and GSK-3β acting as physiological substrates of CaMKIIβ. We conclude from these data that CaMKIIβ phosphorylates GSK-3α and GSK-3β both in vivo and in vitro.
CaMKII Physically Interacts with GSK-3
To explore the possibility that CaMKIIβ may directly phosphorylate GSK-3 in vivo, we examined whether CaMKIIβ physically interacts with GSK-3 in CGNs. As shown by immunofluorescent staining in Fig. 6A, CaMKIIβ (green), GSK-3α, and GSK-3β (red) were all distributed mainly within the cytoplasm, which is consistent with a previous analysis of the distribution of GSK-3 (48). The degree of co-localization, represented by yellow staining, was quantified by Pearson correlation coefficients (mean ± S.E. of CaMKIIβ and GSK-3α was 0.79 ± 0.02, n = 35; mean ± S.E. of CaMKIIβ and GSK-3β was 0.80 ± 0.03, n = 36; full co-localization would be 1.0). This suggests general co-localization of CaMKIIβ and GSK-3 in intact cells.
FIGURE 6.
CaMKII associates with GSK-3. A, confocal images of CaMKIIβ co-localization with GSK-3α or GSK-3β. CGNs were labeled with antibodies against CaMKIIβ (green) and GSK-3α or GSK-3β (red) and then stained with Hoechst 33258 to visualize the nuclei (blue). Scale bar, 10 μm. B, CGN lysates were immunoprecipitated (IP) with CaMKIIβ antibody and processed for Western blot (WB) analysis using the indicated antibodies (left panel). CGN lysates were immunoprecipitated with GSK-3β antibody and processed for Western blot analysis to examine CaMKIIβ and GSK-3β (right panel). C, GFP-CaMKIIβ and V5-GSK-3α were co-transfected in HEK293A cells for 24 h. Cell extracts were immunoprecipitated with GFP antibody, and Western blot analyses were performed using anti-V5 and anti-GFP (left panel). Cell extracts were immunoprecipitated with V5 antibody, and Western blot analyses were performed using anti-GFP and anti-V5 (right panel). D, as described in C, and similar experiments were performed using V5-GSK-3β. All Input in this figure denotes 10% of total cell extracts.
To obtain biochemical evidence for an interaction between CaMKIIβ and GSK-3, co-immunoprecipitation experiments were carried out. CGN lysates were incubated with a CaMKIIβ antibody, and the immune complex was then purified, separated by SDS-PAGE, and immunoblotted with the GSK-3 antibody. We observed that both GSK-3α and GSK-3β were present in the complex immunoprecipitated by the CaMKIIβ antibody (Fig. 6B, left). In addition, CaMKIIβ was also present in the complex in a reciprocal immunoprecipitate using the antibody against GSK-3β (Fig. 6B, right panel). Neither CaMKII nor GSK-3β was detected in the immune complex associated with control IgG, validating the specificity of the observed co-association. These data indicate that CaMKIIβ physically interacts with GSK-3α and GSK-3β in CGNs.
Because there was no GSK-3α antibody available for immunoprecipitation, reciprocal co-expression/immunoprecipitation experiments were conducted with lysates of HEK293A cells that were co-transfected with V5-tagged GSK-3α (V5-GSK-3α) and GFP-tagged CaMKIIβ (GFP-CaMKIIβ). As expected, we detected that GSK-3α-V5 was in the complex associated with the antibody against GFP when GFP-CaMKIIβ was co-transfected (Fig. 6C, left panel). Likewise, GFP-CaMKIIβ was detected in the complex that was precipitated by the V5 antibody when V5-GSK-3α was co-transfected (Fig. 6C, right panel). Similarly, we found an association between CaMKIIβ and GSK-3β after co-expression of GFP-CaMKIIβ and V5-tagged GSK-3β (V5-GSK-3β) in HEK293A cells (Fig. 6D). These findings validate the idea that CaMKIIβ can function upstream of GSK-3 through direct interaction with and phosphorylation of GSK-3α and GSK-3β.
GSK-3 Phosphorylation by CaMKII Mediates Depolarization-dependent Neuron Survival
Several studies have reported that CaMKII mediates depolarization-dependent CGN survival (14, 67, 68). However, most of these observations arise from the use of KN62 or KN-93, which suppress all the CaMKs, including CaMKI, CaMKII, and CaMKIV. By contrast, See et al. (60) showed that constitutively active CaMKII exerted no discernible anti-apoptotic effect in CGNs. Therefore, the role of CaMKII in neuronal survival supported by depolarization remains ambiguous. To verify specifically the role of CaMKII in survival, we used myr-CaMKIINtide because of its previously validated selectivity and potency (58). As shown in Fig. 7A, both myr-CaMKIINtide and KN62 markedly reduced neuronal survival rates under depolarizing conditions. In support of this, the blockage of CaMKII activity by CaMKIIN transfection promoted apoptosis in depolarized neurons (Fig. 7B), indicating a requirement for CaMKII in the pro-survival pathway sustained by depolarization. In addition, a constitutively active mutant (CaMKIIβ T287D) protected CGNs under conditions that promoted apoptosis (Fig. 7B), demonstrating that activation of CaMKIIβ was sufficient to impair apoptosis that was induced by the removal of depolarizing stimulation. In summary, CaMKII is required for neuronal survival sustained by depolarization.
FIGURE 7.
GSK-3 phosphorylation by CaMKII mediates depolarization-dependent neuron survival. A, pharmacological inhibition of CaMKII promotes apoptosis. DIV7 CGNs in the 25 K condition were treated with DMSO, 10 μm KN62, or 15 μm myr-CaMKIINtide (CaMKIINtide) for 12 h, and apoptosis was quantified. B, manipulation of CaMKII activity modulates apoptosis. DIV5 CGNs were transfected with CaMKIIN or T287D for 36 h and switched to 25 or 5 K medium for 12 h. The percentages of apoptotic neurons to total transfected cells were scored. C, CaMKII promotes survival by phosphorylating GSK-3. DIV7 CGNs maintained in 25 K medium in the presence of DMSO, 10 μm KN62, or 15 μm myr-CaMKIINtide (CaMKIINtide) were treated with 20 μm myr-Ser(P)-9-tide or myr-Ser-9-tide for 12 h (there was an untreated control as well), and apoptosis was quantified. D, DIV5 CGNs transfected simultaneously with T287D and vector or S9A for 36 h were switched to 5 K medium for 12 h. The apoptotic rate was calculated. E, knockdown of GSK-3 attenuates CaMKII inhibition-induced apoptosis. CGNs were transfected simultaneously with CaMKIIN and negative control siRNA (NC), siGSK-3α-a plus siGSK-3β-a, or siGSK-3α-b plus siGSK-3β-b for 48 h. Then CGNs were switched to 25 K medium for 12 h, and quantification of apoptosis was performed. The values in A–E represent the mean ± S.E. of at least five independent experiments. * denotes p < 0.05; ** denotes p < 0.01; *** denotes p < 0.001.
To determine whether inhibitory phosphorylation of GSK-3 at Ser-21/9 mediates the pro-survival effects of CaMKII, the aforementioned myr-Ser(P)-9-tide (which mimics phosphorylation of GSK-3) was used along with either a pan-CaMKII inhibitor (KN62) or a specific CaMKII inhibitor (myr-CaMKIINtide). As expected, treatment with myr-Ser(P)-9-tide dramatically prevented the neuronal apoptosis that was induced by KN62 or myr-CaMKIINtide (Fig. 7C), whereas myr-Ser-9-tide by itself had no influence on neuronal viability. Protection from neuronal apoptosis resulting from CaMKIIβ T287D transfection was partially reversed by GSK-3β S9A co-transfection (Fig. 7D). These results further establish that the pro-survival effect of CaMKII depends on GSK-3 phosphorylation. To control for possible nonspecific effects of the compounds or peptides, siRNA-mediated knockdown of GSK-3α/β was employed. Depletion of GSK-3α/β by siRNAs significantly attenuated apoptosis caused by CaMKIIN (Fig. 7E). Taken together, these findings further support the notion that Ser-21/9 phosphorylation/inhibition of GSK-3 by CaMKII mediates neuronal survival.
DISCUSSION
To our knowledge, this is the first study to identify CaMKII as a direct upstream kinase of GSK-3. We have provided evidence that both GSK-3α and GSK-3β play a crucial role in neuronal apoptosis and that Ser-21/9 phosphorylation of GSK-3α/β is critical for depolarization maintenance of CGN survival. Furthermore, our data show that CaMKIIβ can physically associate with GSK-3α/β and directly phosphorylate GSK-3α/β at Ser-21/9. We demonstrate that direct phosphorylation of GSK-3 by CaMKII mediates depolarization-dependent neuronal survival.
Neuronal viability depends on survival signals that are produced by membrane electrical activity and by trophic molecules, including neurotrophins and agents that elevate the intracellular cAMP level (2, 4, 69, 70). Neurotrophins (such as NGF, BDNF, and IGF-1) lead to activation of Akt, which in turn suppresses GSK-3 by direct phosphorylation of Ser-21/9 (15, 20, 30). We and another group have discovered that PKA phosphorylates and inactivates GSK-3β to promote neuronal survival in response to increased intracellular cAMP (21, 22). With regard to depolarization-dependent neuronal survival, the signaling pathways upstream of GSK-3 are still unclear. We demonstrate here that CaMKII, but not Akt/PKB, p90RSK, or PKA, phosphorylates and inhibits GSK-3 to support survival following depolarization. Hence, in response to distinct stimuli or in different contexts, multiple kinases may converge on Ser-21/9 of GSK-3 to regulate neuronal viability. Although CaMKII-mediated phosphorylation of GSK-3 is evident in our study, identifying the phosphatase(s) involved in regulating the phosphorylation level of GSK-3 following depolarization will also be important and will require further investigation.
The role of PI3K-Akt signaling in depolarization-mediated survival has been controversial. The increased Akt phosphorylation at either Thr-308 (71) or Ser-473 (72) induced by depolarization has been observed previously. However, we found that the phosphorylation of Akt (both Ser-473 and Thr-308) and its activity were barely detectable and exhibited no significant difference between 25 K- and 5 K-treated CGNs (Fig. 4A). This finding is supported by data from other groups (71, 73), demonstrating that the PI3K-Akt pathway is not activated by depolarization. Although wortmannin and LY294002 have also been used to suggest the involvement of the PI3K-Akt signaling in survival maintained by depolarization (74–76), other studies and our results (data not shown) indicated that wortmannin and LY294002 did not affect survival in 25 K (69, 72). We used wortmannin or LY294002 to effectively inhibit the PI3K-Akt pathway and showed that GSK-3 phosphorylation was independent of PI3K-Akt signaling (Fig. 4A). Therefore, the PI3K-Akt pathway was not activated by depolarization and was not involved in the depolarization-induced increase of GSK-3 phosphorylation. PI3K-Akt signaling may represent a survival-promoting pathway mediated by neurotrophic factors but not by depolarization.
Previous studies of GSK-3 in neuronal death have predominantly emphasized GSK-3β (rather than GSK-3α) with evidence that was primarily based on pharmacological inhibitors (21, 26, 29, 77). However, such pharmacological agents do not discriminate between GSK-3α and GSK-3β. A recent study found that GSK-3β, but not GSK-3α, was crucial in trophic deprivation-induced CGN apoptosis (48). In contrast, our work has revealed critical roles of not only GSK-3β but also GSK-3α in neuronal apoptosis. First, our results, which are consistent with the findings of Hongisto et al. (48), showed that both GSK-3α and GSK-3β contribute significantly to the total GSK-3 protein complement in CGNs, suggesting that the role of GSK-3α should not be ignored. Second, in addition to GSK-3β, GSK-3α is also subject to Ser-21 phosphorylation and inhibition in response to depolarization (Fig. 1A). Third, compared with a WT control, the dominant-negative GSK-3α (K148A) significantly represses apoptosis (Fig. 2B), and the constitutively active GSK-3α S21A enhances apoptosis (Fig. 3A). This result, along with the similar results concerning GSK-3β, indicated that manipulating either GSK-3α or GSK-3β activity independently can modulate neuronal apoptosis. Finally, isoform-specific knockdown of GSK-3α or GSK-3β by siRNAs significantly attenuates neuronal apoptosis without affecting the level of the nontargeted isoform. This finding is somewhat corroborated by a recent study showing that depletion of either GSK-3α or GSK-3β alone is sufficient to protect CGNs from glutamate insult (27). Together, the data suggest that no functional redundancy exists in the pathways of neuronal apoptosis with respect to GSK-3α and GSK-3β, while clearly demonstrating that both isoforms are vital for neuronal apoptosis.
In this study, we have extended understanding of the roles of Ser-21/9 phosphorylation of GSK-3α/β in neuronal survival. A recent study (48) suggested that dephosphorylation of Ser-21/9 alone was insufficient to induce neuronal death based on the evidence that 7-DIV cultured CGNs isolated from GSK-3α/β S21A/S9A knock-in mice survive normally in serum plus 25 mm KCl medium. However, in CGN culture medium, serum consists of multiple tropic factors and provides a survival-promoting effect of unknown origin (37), thereby complicating the results. Therefore, we assessed the pro-apoptotic ability of constitutively active GSK-3 mutants in 25 K or serum plus 25 mm KCl medium and found that expression of GSK-3α S21A or GSK-3β S9A, as compared with their WT controls, significantly enhanced apoptosis of CGNs in 25 K culture but not in serum plus 25 mm KCl culture (Fig. 3A). Furthermore, we generated a cell-permeable phosphopeptide (residues 4–14 of GSK-3β) that acts as a pseudosubstrate, like the Ser-9-phosphorylated N terminus of GSK-3β. The phosphorylated myr-Ser(P)-9-tide (but not the unphosphorylated version) potently repressed the activity of GSK-3β and significantly protected CGNs from apoptosis in 5 K medium (Fig. 3B), confirming that the Ser-9-phosphorylated GSK-3β is protective against the apoptosis that results from the deprivation of depolarization. Overall, these results confirm and expand upon previous studies (21, 48) and demonstrate that Ser-21/9 phosphorylation of GSK-3α/β specifically mediates the survival-favoring effect of depolarization but not of serum plus depolarization.
The precise substrates underlying the pro-apoptotic effects of GSK-3 in response to potassium deprivation remain to be defined. At present, the most widely reported association between GSK-3 and apoptosis involves the mitochondrion-dependent apoptotic pathway (26). GSK-3 has been proposed to target several key proteins that deliver the apoptotic signal to the mitochondria, including the pro-apoptotic members of the Bcl-2 family of proteins such as Bax and Bim (48, 78). In addition, GSK-3 accelerates the degradation of the anti-apoptotic Bcl-2 family member Mcl-1 (79) and phosphorylates the voltage-dependent anion channel (protein implicated in maintaining mitochondrial membrane potential) to facilitate apoptosis (80). This is similar to a well documented mitochondrion-mediated mechanism where CGNs undergo typical apoptosis upon potassium deprivation (9, 81). Thus, potential substrates of GSK-3 that mediate the pro-apoptotic effects of potassium withdrawal likely include key mitochondrial components of the apoptotic signaling pathway.
Our data establish for the first time that GSK-3 is an in vivo substrate of CaMKII. GSK-3 is therefore a reasonable candidate to mediate other biological effects of CaMKII. Of particular interest are some common activity-dependent cellular functions, such as neurite outgrowth (24, 54) and synaptic plasticity (54, 82); activation of CaMKII and inhibition of GSK-3 are both observed in these functions, although a direct interaction has not been described previously. Therefore, our study not only reveals that inhibitory phosphorylation of GSK-3 mediated by CaMKII is required for neuronal survival but also uncovers a novel Ca2+/CaM/CaMKII/GSK-3 pathway that may play a fundamental role in other activity-dependent cellular processes.
Acknowledgments
We thank Dr. Thilo Hagen, Dr. Thomas R. Soderling, and Dr. Yasunori Hayashi for providing constructs. We are grateful to Dr. Philip Cohen for helpful discussions and comments on the synthesis of myr-Ser(P)-9-tide and myr-Ser-9-tide.
This work was supported by National Natural Science Foundation of China Grants 30870786, U0632006 and 81030024, the Ministry of Science and Technology of China Grant 2009ZX09103-043, the Natural Science Foundation of Guangdong Province China Grant 9351008901000003, National Basic Research Program of China (973 Program) 2011CB504105, and the Canadian Institutes of Health Research Grant FRN 12858.
- CGN
- cerebellar granule neuron
- GSK-3
- glycogen synthase kinase-3
- CaMKII
- Ca2+/calmodulin-dependent protein kinase II
- CaMKIINtide
- calmodulin kinase IINtide
- myr-CaMKIINtide
- myristoylated calmodulin kinase IINtide
- DIV
- days in vitro
- NC
- negative control.
REFERENCES
- 1.Oppenheim R. W. (1991) Annu. Rev. Neurosci. 14, 453–501 [DOI] [PubMed] [Google Scholar]
- 2.Buss R. R., Sun W., Oppenheim R. W. (2006) Annu. Rev. Neurosci. 29, 1–35 [DOI] [PubMed] [Google Scholar]
- 3.Franklin J. L., Johnson E. M., Jr. (1992) Trends Neurosci. 15, 501–508 [DOI] [PubMed] [Google Scholar]
- 4.Mennerick S., Zorumski C. F. (2000) Mol. Neurobiol. 22, 41–54 [DOI] [PubMed] [Google Scholar]
- 5.Galli-Resta L., Ensini M., Fusco E., Gravina A., Margheritti B. (1993) J. Neurosci. 13, 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., Tenkova T. I., Stefovska V., Turski L., Olney J. W. (1999) Science 283, 70–74 [DOI] [PubMed] [Google Scholar]
- 7.Borsello T., Di Luzio A., Ciotti M. T., Calissano P., Galli C. (2000) Neuroscience 95, 163–171 [DOI] [PubMed] [Google Scholar]
- 8.Gallo V., Kingsbury A., Balázs R., Jørgensen O. S. (1987) J. Neurosci. 7, 2203–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contestabile A. (2002) Cerebellum 1, 41–55 [DOI] [PubMed] [Google Scholar]
- 10.D'Mello S. R., Galli C., Ciotti T., Calissano P. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 10989–10993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood K. A., Dipasquale B., Youle R. J. (1993) Neuron 11, 621–632 [DOI] [PubMed] [Google Scholar]
- 12.Ghosh A., Greenberg M. E. (1995) Science 268, 239–247 [DOI] [PubMed] [Google Scholar]
- 13.West A. E., Griffith E. C., Greenberg M. E. (2002) Nat. Rev. Neurosci. 3, 921–931 [DOI] [PubMed] [Google Scholar]
- 14.Bok J., Wang Q., Huang J., Green S. H. (2007) Mol. Cell. Neurosci. 36, 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaillant A. R., Mazzoni I., Tudan C., Boudreau M., Kaplan D. R., Miller F. D. (1999) J. Cell Biol. 146, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linseman D. A., Bartley C. M., Le S. S., Laessig T. A., Bouchard R. J., Meintzer M. K., Li M., Heidenreich K. A. (2003) J. Biol. Chem. 278, 41472–41481 [DOI] [PubMed] [Google Scholar]
- 17.Midorikawa R., Takei Y., Hirokawa N. (2006) Cell 125, 371–383 [DOI] [PubMed] [Google Scholar]
- 18.Woodgett J. R. (1990) EMBO J. 9, 2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen P., Frame S. (2001) Nat. Rev. Mol. Cell Biol. 2, 769–776 [DOI] [PubMed] [Google Scholar]
- 20.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 21.Li M., Wang X., Meintzer M. K., Laessig T., Birnbaum M. J., Heidenreich K. A. (2000) Mol. Cell. Biol. 20, 9356–9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang X., Yu S. X., Lu Y., Bast R. C., Jr., Woodgett J. R., Mills G. B. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11960–11965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z., Wang Z., Gu Y., Feil R., Hofmann F., Ma L. (2009) J. Neurosci. 29, 1350–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naska S., Park K. J., Hannigan G. E., Dedhar S., Miller F. D., Kaplan D. R. (2006) J. Neurosci. 26, 13344–13356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eldar-Finkelman H., Seger R., Vandenheede J. R., Krebs E. G. (1995) J. Biol. Chem. 270, 987–990 [DOI] [PubMed] [Google Scholar]
- 26.Beurel E., Jope R. S. (2006) Prog. Neurobiol. 79, 173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang M. H., Chuang D. M. (2007) J. Biol. Chem. 282, 3904–3917 [DOI] [PubMed] [Google Scholar]
- 28.Hetman M., Cavanaugh J. E., Kimelman D., Xia Z. (2000) J. Neurosci. 20, 2567–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin P. C., Majdzadeh N., D'Mello S. R. (2005) Brain. Res. Mol. Brain. Res. 137, 193–201 [DOI] [PubMed] [Google Scholar]
- 30.Crowder R. J., Freeman R. S. (2000) J. Biol. Chem. 275, 34266–34271 [DOI] [PubMed] [Google Scholar]
- 31.Chen G., Bower K. A., Ma C., Fang S., Thiele C. J., Luo J. (2004) FASEB J. 18, 1162–1164 [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Yang Y., Ying C., Li W., Ruan H., Zhu X., You Y., Han Y., Chen R., Wang Y., Li M. (2007) Neuropharmacology 52, 1678–1684 [DOI] [PubMed] [Google Scholar]
- 33.Jope R. S., Johnson G. V. (2004) Trends Biochem. Sci. 29, 95–102 [DOI] [PubMed] [Google Scholar]
- 34.Ma C., Ying C., Yuan Z., Song B., Li D., Liu Y., Lai B., Li W., Chen R., Ching Y. P., Li M. (2007) J. Biol. Chem. 282, 30901–30909 [DOI] [PubMed] [Google Scholar]
- 35.Yuan Z., Gong S., Luo J., Zheng Z., Song B., Ma S., Guo J., Hu C., Thiel G., Vinson C., Hu C. D., Wang Y., Li M. (2009) Mol. Cell. Biol. 29, 2431–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M., Linseman D. A., Allen M. P., Meintzer M. K., Wang X., Laessig T., Wierman M. E., Heidenreich K. A. (2001) J. Neurosci. 21, 6544–6552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller T. M., Johnson E. M., Jr. (1996) J. Neurosci. 16, 7487–7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doble B. W., Patel S., Wood G. A., Kockeritz L. K., Woodgett J. R. (2007) Dev. Cell 12, 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. (1993) EMBO J. 12, 803–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frame S., Cohen P., Biondi R. M. (2001) Mol. Cell 7, 1321–1327 [DOI] [PubMed] [Google Scholar]
- 41.Dajani R., Fraser E., Roe S. M., Young N., Good V., Dale T. C., Pearl L. H. (2001) Cell 105, 721–732 [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. (2005) Cell 120, 137–149 [DOI] [PubMed] [Google Scholar]
- 43.Cole A. R., Knebel A., Morrice N. A., Robertson L. A., Irving A. J., Connolly C. N., Sutherland C. (2004) J. Biol. Chem. 279, 50176–50180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coghlan M. P., Culbert A. A., Cross D. A., Corcoran S. L., Yates J. W., Pearce N. J., Rausch O. L., Murphy G. J., Carter P. S., Roxbee Cox L., Mills D., Brown M. J., Haigh D., Ward R. W., Smith D. G., Murray K. J., Reith A. D., Holder J. C. (2000) Chem. Biol. 7, 793–803 [DOI] [PubMed] [Google Scholar]
- 45.Bhat R., Xue Y., Berg S., Hellberg S., Ormö M., Nilsson Y., Radesäter A. C., Jerning E., Markgren P. O., Borgegård T., Nylöf M., Giménez-Cassina A., Hernández F., Lucas J. J., Díaz-Nido J., Avila J. (2003) J. Biol. Chem. 278, 45937–45945 [DOI] [PubMed] [Google Scholar]
- 46.Bain J., Plater L., Elliott M., Shpiro N., Hastie C. J., McLauchlan H., Klevernic I., Arthur J. S., Alessi D. R., Cohen P. (2007) Biochem. J. 408, 297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagen T., Di Daniel E., Culbert A. A., Reith A. D. (2002) J. Biol. Chem. 277, 23330–23335 [DOI] [PubMed] [Google Scholar]
- 48.Hongisto V., Vainio J. C., Thompson R., Courtney M. J., Coffey E. T. (2008) Mol. Cell. Biol. 28, 1515–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ter Haar E., Coll J. T., Austen D. A., Hsiao H. M., Swenson L., Jain J. (2001) Nat. Struct. Biol. 8, 593–596 [DOI] [PubMed] [Google Scholar]
- 50.Dudek H., Datta S. R., Franke T. F., Birnbaum M. J., Yao R., Cooper G. M., Segal R. A., Kaplan D. R., Greenberg M. E. (1997) Science 275, 661–665 [DOI] [PubMed] [Google Scholar]
- 51.Dudley D. T., Pang L., Decker S. J., Bridges A. J., Saltiel A. R. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 7686–7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favata M. F., Horiuchi K. Y., Manos E. J., Daulerio A. J., Stradley D. A., Feeser W. S., Van Dyk D. E., Pitts W. J., Earl R. A., Hobbs F., Copeland R. A., Magolda R. L., Scherle P. A., Trzaskos J. M. (1998) J. Biol. Chem. 273, 18623–18632 [DOI] [PubMed] [Google Scholar]
- 53.White R. R., Kwon Y. G., Taing M., Lawrence D. S., Edelman A. M. (1998) J. Biol. Chem. 273, 3166–3172 [DOI] [PubMed] [Google Scholar]
- 54.Wayman G. A., Lee Y. S., Tokumitsu H., Silva A. J., Silva A., Soderling T. R. (2008) Neuron 59, 914–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. (1990) J. Biol. Chem. 265, 4315–4320 [PubMed] [Google Scholar]
- 56.Tokumitsu H., Inuzuka H., Ishikawa Y., Ikeda M., Saji I., Kobayashi R. (2002) J. Biol. Chem. 277, 15813–15818 [DOI] [PubMed] [Google Scholar]
- 57.Tokumitsu H., Enslen H., Soderling T. R. (1995) J. Biol. Chem. 270, 19320–19324 [DOI] [PubMed] [Google Scholar]
- 58.Chang B. H., Mukherji S., Soderling T. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10890–10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yano S., Tokumitsu H., Soderling T. R. (1998) Nature 396, 584–587 [DOI] [PubMed] [Google Scholar]
- 60.Sée V., Boutillier A. L., Bito H., Loeffler J. P. (2001) FASEB J. 15, 134–144 [DOI] [PubMed] [Google Scholar]
- 61.Marshall J., Dolan B. M., Garcia E. P., Sathe S., Tang X., Mao Z., Blair L. A. (2003) Neuron 39, 625–639 [DOI] [PubMed] [Google Scholar]
- 62.Kennedy M. B. (2008) Neuron 60, 401–402 [DOI] [PubMed] [Google Scholar]
- 63.Griffith L. C. (2004) J. Neurosci. 24, 8391–8393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. (1990) J. Neurosci. 10, 1788–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Griffith L. C. (2004) J. Neurosci. 24, 8394–8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erxleben C., Liao Y., Gentile S., Chin D., Gomez-Alegria C., Mori Y., Birnbaumer L., Armstrong D. L. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3932–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hack N., Hidaka H., Wakefield M. J., Balázs R. (1993) Neuroscience 57, 9–20 [DOI] [PubMed] [Google Scholar]
- 68.Borodinsky L. N., Coso O. A., Fiszman M. L. (2002) J. Neurochem. 80, 1062–1070 [DOI] [PubMed] [Google Scholar]
- 69.D'Mello S. R., Borodezt K., Soltoff S. P. (1997) J. Neurosci. 17, 1548–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rydel R. E., Greene L. A. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 1257–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumari S., Liu X., Nguyen T., Zhang X., D'Mello S. R. (2001) Brain Res. Mol. Brain. Res. 96, 157–162 [DOI] [PubMed] [Google Scholar]
- 72.Lafon-Cazal M., Perez V., Bockaert J., Marin P. (2002) Eur. J. Neurosci. 16, 575–583 [DOI] [PubMed] [Google Scholar]
- 73.Zhong J., Deng J., Huang S., Yang X., Lee W. H. (2004) J. Neurosci. Res. 75, 794–806 [DOI] [PubMed] [Google Scholar]
- 74.Shimoke K., Yamagishi S., Yamada M., Ikeuchi T., Hatanaka H. (1999) Brain Res. Dev. Brain. Res. 112, 245–253 [DOI] [PubMed] [Google Scholar]
- 75.Miller T. M., Tansey M. G., Johnson E. M., Jr., Creedon D. J. (1997) J. Biol. Chem. 272, 9847–9853 [DOI] [PubMed] [Google Scholar]
- 76.Wiedmann M., Wang X., Tang X., Han M., Li M., Mao Z. (2005) J. Neurosci. Res. 81, 226–234 [DOI] [PubMed] [Google Scholar]
- 77.Hetman M., Hsuan S. L., Habas A., Higgins M. J., Xia Z. (2002) J. Biol. Chem. 277, 49577–49584 [DOI] [PubMed] [Google Scholar]
- 78.Linseman D. A., Butts B. D., Precht T. A., Phelps R. A., Le S. S., Laessig T. A., Bouchard R. J., Florez-McClure M. L., Heidenreich K. A. (2004) J. Neurosci. 24, 9993–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maurer U., Charvet C., Wagman A. S., Dejardin E., Green D. R. (2006) Mol. Cell 21, 749–760 [DOI] [PubMed] [Google Scholar]
- 80.Pastorino J. G., Hoek J. B., Shulga N. (2005) Cancer Res. 65, 10545–10554 [DOI] [PubMed] [Google Scholar]
- 81.Putcha G. V., Harris C. A., Moulder K. L., Easton R. M., Thompson C. B., Johnson E. M., Jr. (2002) J. Cell Biol. 157, 441–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu L. Q., Wang S. H., Liu D., Yin Y. Y., Tian Q., Wang X. C., Wang Q., Chen J. G., Wang J. Z. (2007) J. Neurosci. 27, 12211–12220 [DOI] [PMC free article] [PubMed] [Google Scholar]