Abstract
Nuclear spin hyperpolarization can increase dramatically the sensitivity of the 13C magnetic resonance experiment, allowing dynamic measurements of the metabolism of hyperpolarized 13C-labeled substrates in vivo. Here, we report a preclinical study of the response of lymphoma tumors to the vascular disrupting agent, combretastatin-A4-phosphate, as detected by measuring changes in tumor metabolism of hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate. These measurements were compared with dynamic contrast agent enhanced magnetic resonance imaging (DCE-MRI) measurements of tumor vascular function and diffusion-weighted MRI (DW-MRI) measurements of the tumor cell necrosis that resulted from subsequent loss of tumor perfusion. The rate constant describing flux of hyperpolarized 13C label between [1-13C]pyruvate and lactate was decreased by 34% within 6 h of combretastatin-A4-phosphate treatment and remained at this lower level at 24 h. The rate constant describing production of labeled malate from hyperpolarized [1,4-13C2]fumarate increased 1.6-fold and 2.5-fold at 6 and 24 h after treatment respectively and correlated with the degree of necrosis detected in histological sections. While DCE-MRI measurements showed a substantial reduction in perfusion at 6 h after treatment, which had recovered by 24 h, diffusion-weighted imaging showed no change in the apparent diffusion coefficient of tumor water at 6 h after treatment, although there was a 32% increase at 24 h (p<0.02), when regions of extensive necrosis were observed by histology. Measurements of hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate metabolism may provide therefore a more sustained and sensitive indicator of response to a vascular disrupting agent than DCE-or DW-MRI respectively.
Keywords: Tumor microcirculation and microenvironment, Animal models of cancer, Noninvasive imaging in animal models, Imaging of animals, Magnetic resonance imaging (MRI), Novel assay technology, Tubulin-targeted agents
Introduction
Targeting the tumor vasculature is an attractive treatment option due to the ease of drug delivery and the distinctive phenotype shown by tumor blood vessels, which are frequently angiogenic, lacking in smooth muscle and with a rapidly proliferating endothelial cell population (1, 2). Vascular disrupting agents (VDAs) selectively target these endothelial cells, causing the vessels to collapse, which in turn eliminates the supply of oxygen and nutrients and leads ultimately to tumor cell necrosis (2).
Combretastatin A4 phosphate (CA4P), the water soluble pro-drug of CA4, is a tubulin-binding agent that induces a rapid shape change in proliferating endothelial cells by disrupting microtubule assembly, leading eventually to apoptotic endothelial cell death (3). CA4 at 100 mg/kg has been shown to yield rapid and prolonged interruption of blood flow in both human xenograft (MDA-MB-231 breast carcinoma) and murine (CaNT adenocarcinoma) tumor models, resulting in extensive tumor cell necrosis within 24 h of drug treatment (4). Following successful Phase I clinical trials (5), Phase II/III trials have been initiated with CA4P as both a single agent in anaplastic thyroid carcinoma (6) and combined with carboplatin and paclitaxel for ovarian cancer (7) and chemotherapy-naïve non-small cell lung carcinoma (8).
Since VDAs rarely cause tumor shrinkage in the early stages of treatment, clinical trials of these agents have used functional biomarkers of response, including reductions in tumor perfusion, changes in tumor cell metabolism, and ultimately cell death. Measurements of vascular function using dynamic contrast agent-enhanced (DCE) magnetic resonance imaging (MRI) and H2 15O Positron Emission Tomography (PET) have been most commonly used in CA4P clinical trials (5, 9, 10). Although DCE-MRI provides high sensitivity, repeatability between centers is limited by the wide variety of acquisition parameters and kinetic models (11). The need for an on site cyclotron to produce the short half life isotope, 15O, more significantly limits PET studies of the vasculature (12).
Diffusion weighted MRI (DW-MRI) has been used to image the tumor cell necrosis that results from a positive response to CA4P treatment, with a range of preclinical studies performed in a rat rhabdomyosarcoma (13, 14). In these studies, changes in ADC were highly dependent on the timing of the DW-MRI measurements relative to the administration of the treatment. At present, the use of DW-MRI as a clinical biomarker of vascular disrupting agents has been limited by a lack of uniform standards for data acquisition and processing (15). A recent Phase I trial combining CA4P with bevacizumab found good reproducibility between two centers, although no significant change in ADC was seen until after two doses of CA4P, again highlighting the importance of experiment timing (16).
Tumor response to VDAs can also be detected through changes in tumor cell metabolism. Preclinical models have shown significant (up to 80%) and rapid (within 2 h) reductions in uptake of the positron emitting glucose analogue, 18Fluoro-deoxyglucose (FDG), after treatment with both CA4P (17) and AVE8062, a combretastatin derivative (18); however, measurements of FDG uptake can be confounded by the presence of inflammation and hypoxia, both of which may arise following CA4P treatment (19).
We have shown previously that a reduction in tumor cell energy status, measured using 31P magnetic resonance spectroscopy (MRS), is a sensitive indicator of tumor response to treatment with a CA4P (10). Clinically, MRS of nuclei other than protons has thus far been limited by an inherent lack of sensitivity. Dynamic nuclear polarization (DNP) of 13C-labeled cell metabolites in vitro and subsequent intravenous injection dramatically enhances their sensitivity to detection in vivo (>10,000-fold) (20). Using this technique both the location of the molecule and, more importantly, the dynamics of its subsequent conversion into other cell metabolites can be imaged in vivo. The rate of conversion of hyperpolarized [1-13C]pyruvate into [1-13C]lactate in a murine model of lymphoma was shown to fall significantly as early as 24 h after cytotoxic therapy (21, 22), whereas the conversion of hyperpolarized [1,4-13C2]fumarate, a tricarboxylic acid cycle intermediate, into [1,4-13C2]malate was increased and shown to correlate in vitro with levels of tumor cell necrosis (23).
We show here that the exchange of hyperpolarized 13C label between pyruvate and lactate is reduced in murine lymphoma tumors within 24 h of treatment with CA4P and that the production of [1,4-13C2]malate from hyperpolarized [1,4-13C2]fumarate is increased over the same period. These measurements have been compared directly with DCE-MRI measurements of tumor perfusion and DW-MRI measurements of tumor cell necrosis. Despite the limited lifetime of the hyperpolarization and the need for specialist DNP equipment, the first clinical trials of hyperpolarized [1-13C]pyruvate are expected this year (24) so there is a reasonable expectation that these methods could translate to the clinic in the near future, where they could provide a sensitive adjunct to multi-parametric MRI measurements of tumor responses to VDAs.
Materials and Methods
Tumor implantation, treatment and histology
Tumors were established, as described previously, by subcutaneous inoculation of 5×106 EL4 murine lymphoma cells (no authentication was performed by the authors) in female C57BL/6 mice and allowed to grow for 10 days. Drug-treated animals received an intra peritoneal injection of 100 mg/kg CA4P (structure shown in Figure 1; provided by Oxigene, San Francisco, USA). Procedures were conducted in accordance with project and personal licenses issued under the United Kingdom Animals (Scientific Procedures) Act 1986. Tumor cell death was assessed histologically by haematoxylin and eosin staining of tissue sections obtained post mortem. Slides were scanned using an Aperio XT (Aperio Technologies, California, US) and the area fraction containing the fragmented nuclei of dead cells, relative to the total area of two fields of view, was evaluated in two sections per excised tumor using ImageJ (National Institutes of Health, Bethesda, U.S.A.).
Figure 1.

Structure of Combretastatin-A4, the active form of the prodrug Combretastatin-A4-Phosphate
Hyperpolarization of [1-13C]pyruvate and [1,4-13C2]fumarate
[1-13C] pyruvic acid samples (44 mg, 14M; 91% 13C) contained 15 mM trityl radical ((Tris(8-carboxy-2,2,6,6-(tetra-(hydroxyethyl)-benzo-[1,2-4,5′]-bis-(1,3)-dithiole-4-yl)-methyl sodium salt) (OX063; GE Healthcare, Amersham, U.K.) and 1.4 mM of an aqueous solution of a gadolinium chelate (ProHance; Bracco International B.V., The Netherlands). [1,4-13C2]fumaric acid (381.2mg, 3.4 M; 99% 13C) (Cambridge Isotope Laboratories, U.S.A.) was dissolved in 4.5 g dimethyl sulfoxide (DMSO; Sigma Aldrich, U.K.), containing 18.5 mM trityl radical ((Tris(8-carboxy-2,2,6,6(tetra(methoxyethyl) benzo-[1,2-4,5′]bis-(1,3)dithiole-4-yl)methyl sodium salt) (AH111501; GE Healthcare, Amersham, U.K.) and 0.8 mM of anhydrous gadolinium chelate ((1,3,5-tris-(N-(DO3A-acetamido)-N-methyl-4-amino-2-methylphenyl)-1,3,5]tria-zinane-2,4,6-trione) (Gd-3, GE Healthcare, Amersham, U.K.). A 40 mg aliquot of this fumarate solution was used for each experiment.
Pyruvate was hyperpolarized as described previously (20). Briefly, the liquid sample was lowered into a GE Healthcare DNP prototype Hyperpolarizer and cooled rapidly to ~1.2 K using liquid helium at a pressure of ~1 mbar. Polarization of the electron spins on the trityl radical was transferred to the 13C label using microwave irradiation at 93.975 GHz and 100 mW for 45 minutes. The sample was then rapidly dissolved in 6 ml of HEPES buffer (40 mM HEPES, 94 mM NaOH, 30 mM NaCl and 100 mg/l EDTA) pressurized to 10 bar and at 180°C; the eluted sample was cooled to ~37°C before injection. The level of polarization ranged from 20-40% and the final concentration of [1-13C]pyruvate was 75 mM.
For [1,4-13C2]fumarate, 10 μl aliquots were dropped into liquid nitrogen to form pellets, which were transferred to a pre-cooled dissolution cup (25) and lowered into the Hyperpolarizer. The sample was dissolved using 6 ml phosphate buffer (40 mM phosphate, 50 mM NaCl, 40 mM NaOH, 100 mg/l EDTA), yielding a final fumarate concentration of 20 mM. The polarization ranged from 15-25%.
Magnetic Resonance Spectroscopy (MRS) and Imaging (MRI)
Animals were anaesthetized by intraperitoneal injection of 10 ml/kg 5:4:31 mixture of Hypnorm (VetaPharma Ltd., Leeds, U.K.), Hypnovel (Roche, Basel, Switzerland) and saline. A catheter was inserted into the tail vein and the animal was placed inside a cradle with a 24 mm diameter surface coil tuned to 13C (100MHz) positioned over the tumor. The assembly was placed in a quadrature 1H-tuned volume coil (Varian, Palo Alto, CA), in a 9.4-T vertical wide-bore magnet (Oxford Instruments, UK). Transverse 1H images were acquired using a gradient-echo pulse sequence (30° pulse; repetition time (TR), 300 ms; echo time (TE), 2.2 ms; field-of-view (FOV) 35 × 35 mm in a data matrix of 256 × 256 with 2 averages per increment; slice thickness 2 mm and 21 transverse slices). The tumor slice for hyperpolarized experiments was selected from these images and a slice selective excitation pulse and acquire sequence was used for 13C spectroscopy.
After injection of 0.2 ml hyperpolarized pyruvate, 160 13C spectra were acquired from a 6 mm thick tumor slice at 1s intervals with a nominal flip angle of 10°. Approximately 45 min after the pyruvate experiment, which was the time taken to hyperpolarize the fumarate sample, 0.2 ml of hyperpolarized fumarate was injected into the same animal and 80 13C spectra were acquired at 2 s intervals with a nominal flip angle of 20°. In both experiments, every 16th spectrum was acquired from the entire sensitive volume of the surface coil. The body temperature of the animals was maintained using a flow of warm air. It was not possible to co-inject pyruvate and fumarate as the resonances of the resulting hyperpolarized lactate and malate were insufficiently resolved in the spectra obtained in vivo.
MRS Data Analysis
The integrated peak intensities of hyperpolarized [1-13C]pyruvate and [1-13C]lactate were fit to the modified Bloch equations for two-site exchange to obtain the rate constants kL and kP and the apparent spin lattice relaxation rates as described previously (21)
| (1) |
| (2) |
| (3) |
where Lz and Pz are the z magnetizations of the 13C nucleus in the lactate and pyruvate carboxyl carbons, ρL and ρP are the spin lattice relaxation rates (1/T1L,P), and L∞ and P∞ are the equilibrium magnetizations (i.e. at t = ∞). Due to the large number of parameters to be fitted, it was assumed that the spin-lattice relaxation rates of the metabolites were equivalent (ρP =ρL). The integrated intensities of hyperpolarized [1,4-13C2]fumarate and [1,4-13C2]malate were fit to the same equations, with M and F referring to malate and fumarate respectively, the apparent rate constants being kF and kM and by setting ρF=ρM. We have shown previously, and again confirmed in these experiments (data not shown), that the fitted forward rate constants (kP or kF) were not affected by the assumption of equal T1s over the range of T1 values encountered for these metabolites (21, 23). Results are expressed as mean ± standard deviation, unless stated otherwise. Statistical significance was tested using Prism (Graphpad, U.S.A.) with an unpaired one-tailed t-test at the 95% confidence level.
Measurements of enzyme activities and metabolite concentrations
Tumors were excised and freeze-clamped 30 s after injection of a 0.2 ml bolus of 75 mM pyruvate and 20 mM fumarate (non hyperpolarized). These freeze-clamped samples were used to determine lactate dehydrogenase (LDH) and fumarase activities, as well as metabolite concentrations at different time points after CA4P treatment. A small amount of each freeze-clamped tumor was homogenized and enzyme activities were determined spectrophotometrically (26). The remaining frozen tumor was extracted using 7% perchloric acid and neutralized using 2M K2CO3. The concentrations of pyruvate and lactate in these neutralized extracts were measured using NADH-linked enzymatic assays, assuming a millimolar extinction coefficient for NADH of 6.22 (27, 28). Fumarate and malate concentrations in the same extracts were measured fluorometrically using the fluorescence of acetylpyridine-adenine dinucleotide (APAD) (29) and concentrations were determined by comparison with known standards. Results are expressed as mean ± standard error and unpaired two-tailed t-tests at the 95% confidence level were performed using Excel (Microsoft, U.S.A.).
The remainder of each extract was then treated with 0.5 g Chelex-100 resin (Sigma Aldrich, U.K.) for 1 hour and then lyophilized for 1H NMR analysis. The dried sample was dissolved in 0.1M Na2HPO4 and 0.1M KH2PO4 (pH 8.10) in D2O containing 4.2 mM sodium trimethylsilyl-2,2,3,3-tetradeuteroproprionate (TSP) as a chemical shift and intensity standard (Sigma Aldrich, U.K.). 1H NMR spectra were acquired at 500 MHz in to 32,768 data points using a 90° pulse, a sweep width of 16 kHz, a repetition time of 6 s and were the sum of 256 transients. Peaks from metabolites of interest were integrated with reference to the TSP standard and compared with samples of known concentration.
Dynamic Contrast Enhanced Magnetic Resonance Imaging (DCE-MRI)
As the gadolinium contrast agent can increase the rate of polarization loss from the hyperpolarized substrates the DCE-MRI data were acquired from a separate cohort of animals, before and after CA4P treatment, using a T1-weighted spin echo pulse sequence (coronal tumor slice; FOV, 60 mm × 30 mm; data matrix 256 × 64; slice thickness, 1.5 mm, five slices with 0.25 mm gaps between slices; TR, 110 ms; TE, 9 ms). Additionally, spin-lattice relaxation rates (R1 (1/T1)) were measured using an inversion recovery fast low angle shot (FLASH) pulse sequence (TR, 5.5 ms; TE, 2.5 ms; 10 s delay between images, 11 inversion times between 50 ms and 10 s, 2 averages per inversion time). Four control images and one R1 map were collected before gadolinium diethylenetriaminepentaacetate (Gd-DTPA) (Magnevist, Schering), diluted in sterile saline (0.9% sodium chloride), was administered intravenously to give 200 μmole/kg body weight; imaging was then continued for ten minutes. Inversion recovery data were fitted, pixel-by-pixel, to the mono-exponential function:
| (4) |
where TI is the inversion time. This gave estimates for S0, spin density, R1,pre, the relaxation rate before contrast agent administration, and k, a factor accounting for imperfections in flip angles. Spin-echo images were converted to relaxation rate maps using:
| (5) |
S0,pre was estimated from control images using the measured R1,pre values, and was assumed not to change during the experiment. The effect of Gd-DTPA on transverse relaxation was assumed to be negligible at the short TE used. The area under ΔR1 curve (AUC) was calculated from a region of interest covering the whole tumor (30, 31). Statistical significance was tested using Prism (Graphpad, U.S.A.), with one-way analysis of variance (ANOVA) on the Gd-DTPA uptake curves and a paired two-tailed t-test at the 95% confidence level for the AUC.
Diffusion Weighted Magnetic Resonance Imaging (DW-MRI)
The apparent diffusion coefficient (ADC) was measured before and after treatment in all animal cohorts; 8 to 10 slices were acquired covering the whole tumor using a navigated dual-echo spin echo pulse sequence (FOV, 35 × 35 mm2; data matrix, 128 × 64; slice thickness 2 mm; TR, 1.0 s; TE, 35 ms and 45 ms, the second echo being used as the navigator echo; two signal averages per b-value) (32), with diffusion-sensitizing gradients applied along the slice axis (gradient length (∂), 7.5 ms; delay between gradients (Δ), 13 ms; and five b-values (0, 68, 271, 609 and 1082 s/mm2).
ADC maps were generated for each slice on a pixel-by-pixel basis by fitting the signal intensity of the five diffusion weighted images and the corresponding b-values to the monoexponential function:
| (6) |
where S0 is the signal amplitude without diffusion gradients, b is the b-value, given by the amplitude, length and separation of the diffusion gradients, and D is the apparent diffusion coefficient in the direction of the diffusion gradients. A region of interest (ROI) just inside the boundary of the tumor was selected in each slice using a custom routine written in Matlab (Mathworks, Natick). ADC histograms were generated for each ROI, as well as for the whole tumor. No statistically significant differences were found between the ADC histogram distributions or median pixel values of each tumor slice or the whole tumor compared to the central tumor slice as assessed by a paired two-tailed t-test at the 95% confidence level. The mean ADC obtained from the multi-slice data was (0.45±0.04)×10−3mm2s−1 for untreated animals and 0.51±0.03 at 24 h after treatment, while for the single slice data the mean ADC was 0.44±0.04 and 0.55±0.09 respectively (mean ± s.d., n=6 animals). The ADC maps and median ADC values of the central tumor slice were therefore considered representative and used to assess treatment response using a paired two-tailed t-test at the 95% confidence level.
Results
Intravenous injection of hyperpolarized [1-13C]pyruvate or [1,4-13C2]fumarate resulted in readily detectable signals from these metabolites and in the production of [1-13C]lactate and [1,4-13C2]malate respectively in the lymphoma tumors (Figure 2). The flux of 13C label from hyperpolarized [1-13C]pyruvate at 173p.p.m. to [1-13C]lactate at 185p.p.m. was clearly decreased 24 h after treatment with CA4P (Figure 2A, B), whereas the fumarase catalyzed hydration of hyperpolarized [1,4-13C2]fumarate (177p.p.m.) to [1,4-13C2]malate (182, 184p.p.m.), which was barely detectable before treatment (Figure 2C), showed a large increase at 24 h after treatment (Figure 2D).
Figure 2.
13C MR spectra acquired from tumors following intravenous injection of hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate. Sequential spectra collected from a 6 mm tumor slice over a period of 160 s (starting ~10 s post injection), showing flux of hyperpolarized 13C label between [1-13C]pyruvate (172.9p.p.m.) and [1-13C]lactate (185.1p.p.m.) in an untreated tumor (A) and 24 h after CA4P treatment (B) and between [1,4-13C2]fumarate (177.2p.p.m.) and [1,4-13C2]malate (182.2, 183.6p.p.m.) in an untreated tumor (C) and 24 hours after CA4P treatment (D). Only every 4th spectrum is shown for clarity.
The time courses of the pyruvate, lactate, fumarate and malate signal intensities are shown in Figure 3. These data were fit to the modified Bloch equations for two-site exchange. The rate constant describing label flux between pyruvate and lactate (kP) was reduced by 34% within 6 h of treatment, from 0.10±0.03s−1 to 0.07±0.02s−1 (n=10 animals, p<0.01) and was at the same level at 24 h (0.07±0.03s−1; n=7 animals, p<0.05), whereas the rate constant describing the production of labeled malate (kF) was increased 1.6-fold at 6 h after treatment (0.010±0.004s−1 to 0.016±0.012s−1; n=7 animals, p<0.1) and 2.5-fold at 24 h (0.025±0.018s−1; n=7 animals, p<0.05). The peak of the malate signal appeared later in untreated tumors (19.7 s) compared with both treatment time points (13 s at 6 and 24 h respectively), as was observed previously in etoposide-treated lymphoma tumors (23).
Figure 3.
Time dependent changes in the concentrations of hyperpolarized 13C labeled metabolites in treated and untreated tumors. Data were normalized to the initial signal intensity from the injected metabolite and were averaged over all animals within each cohort (data shown as mean ± S.E.M.). (A) [1-13C]pyruvate (B) [1-13C]lactate (C) [1,4-13C2]fumarate (D) [1,4-13C2]malate.
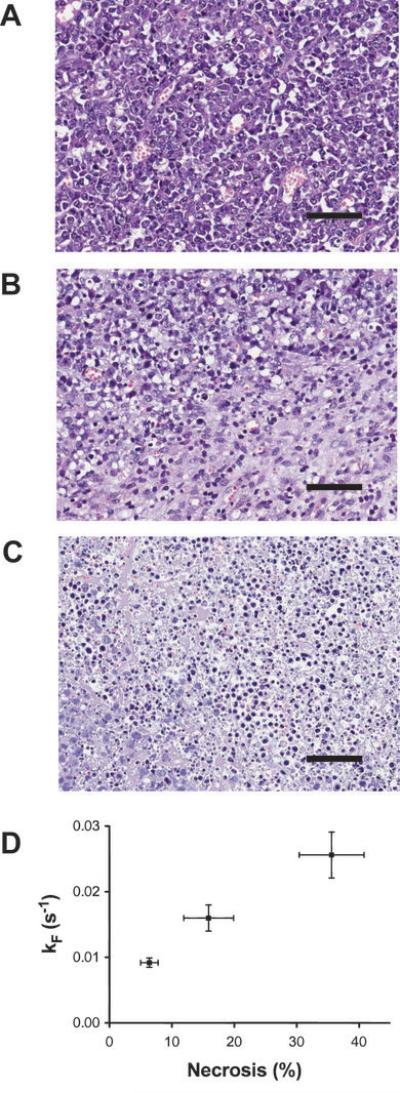
The maximum amplitude of the [1,4-13C2]malate signals relative to the signal from [1,4-13C2]fumarate increased from 8±1% in untreated tumors, to 13±3% at 6 h and 20±2% at 24 h after CA4P treatment (mean ± S.E.; n=7 animals per group). Histological assessment of tumor sections obtained post mortem showed the presence of necrosis in the drug-treated tumors. This rose from a background level of 6.4±1.4 % (n=7 animals) in untreated tumors, to 15.9±4 % (2.5-fold higher; n=3 animals, p<0.05) at 6 h after treatment and 35.6±5.2% (6-fold higher; n=7 animals, p<0.01) at 24 h after treatment. Tumors at 6 h after treatment (Figure 4B) showed small and diffuse areas of necrosis compared to the untreated tumors (Figure 4A), while at 24 h widespread necrosis was observed (Figure 4C). We have shown previously that malate production from fumarate is a measure of tumor cell necrosis in vitro. The data presented here suggest that this is also the case in vivo, since there was a good correlation between the rate of malate production (y) (Figure 4D) and the percentage necrosis (x) observed in histological sections.
Figure 4.
Sections of tumors stained with hemotoxylin and eosin; scale bar 280 μm. Untreated tumors (A); tumors 6 h after treatment with CA4P (B) and 24 h after treatment (C). The tumors at 6 h after treatment show small areas of necrosis, whereas there is widespread necrosis at 24 h. The relationship between the apparent rate constant describing malate production and the area of necrosis (%), determined from analysis of histological sections, is shown in (D). Error bars for both axes represent standard error on the mean, taken over all animals in each cohort.
The hyperpolarized signals from all metabolites were observed to decay more rapidly following drug treatment (Figure 3) and there was a significant decrease in the fitted relaxation time constants for pyruvate (25.4 and 28.1s at 6 and 24 hours after treatment compared to 30.9s before; n=7 animals, p<0.05) and fumarate (17.7s and 18.2s compared to 26.4s before; n=7 animals, p<0.05). The relaxation time for tissue water protons was also found to decrease, from 2.25±0.05 s before treatment (n=8 animals) to 2.11±0.06 s (n=4 animals, p=0.019) and 2.09±0.04 s (n=5 animals, p=0.023) at 6 h and 24 h after treatment with CA4P respectively. These decreases in T1 relaxation times may be explained by dipole-dipole interactions between the 13C and 1H nuclei and paramagnetic deoxyhemoglobin and methemoglobin, which are known to accumulate in the regions of hemorrhage (33) induced by CA4P treatment (4).
Measurements on tumor extracts (Table 1) showed an increase in LDH activity at 6 h after treatment but no significant change in fumarase activity over the treatment time course. The lactate concentration in tumor extracts appeared to decrease by ~10% following treatment, confirmed also by 1H NMR, but due to the large spread in the untreated data, this was not significant. The pyruvate concentration fell by 45% at 6 h after treatment but by 24 h was comparable to the pretreatment level; pyruvate could not be detected reliably in the 1H NMR spectra.
Table 1.
Enzyme activities (A) and metabolite concentrations, determined by enzymatic assay, (B) in tumor extracts before and after treatment with Combretastatin-A4-Phosphate at 100mg/kg
| Lactate Dehydrogenase | Fumarase | |||||
|---|---|---|---|---|---|---|
| n | U/mg protein | U/gwet wt | n | U/mg protein | U/g wet wt. | |
| Untreated | 3 | 1.40±0.13 | 79±24 | 7 | 0.17±0.02 | 9.0±0.6 |
| 6 hrs | 4 | 2.10±0.20* | 105±16 | 5 | 0.20±0.02 | 10.3±0.4 |
| 24 hrs | 4 | 1.76±0.17 | 91±18 | 5 | 0.24±0.03 | 10.1±0.8 |
| Lactate | Pyruvate | Fumarate + Malate | |||
|---|---|---|---|---|---|
| n | μmol/g wet wt. | μmol/g wet wt. | n | μmol/g wet wt | |
| Untreated | 6 | 11.2±1.3 | 0.60±0.06 | 4 | 0.75±0.08 |
| 6 hrs | 5 | 10.3±0.7 | 0.33±0.07 * | 3 | 0.62±0.12 |
| 24 hrs | 5 | 10.5±0.3 | 0.55±0.02 | 3 | 0.84±0.07 |
(p<0.05 by unpaired t-test)
Fumarate could not be measured separately from malate in the fluorometric assay of tumor extracts, presumably because of contaminating fumarase activity that remained following the extraction process. The results from the assay thus provide a measure of the total concentrations of both fumarate and malate (Table 1). Instead, the ratio of the fumarate and malate signals observed in the 1H NMR measurements was used to probe the relative concentrations of these metabolites, increasing by 2.4-fold (p<0.1, n=3 animals) and 3.7-fold (n=3 animals, p<0.05) at 6 and 24 h after treatment respectively.
Treatment response was also assessed using DCE-MRI measurements of tumor perfusion and DW-MRI measurements of tumor cell necrosis. The pattern of changes in the DCE- and DW-MRI parameters were similar to what has been observed previously in other tumor types with this drug (4, 34-36). One-way analysis of variance between the curves of Gd-DTPA uptake measured in the DCE-MRI experiments (Figure 5A) showed significant (one-way ANOVA; n=3 animals, p<0.001) suppression of uptake at 6 h after treatment, returning to above pretreatment values (n=5 animals, p<0.05) at 24 h. Since Gd-DTPA is neither freely diffusible, nor a pure blood pool agent, uptake is affected by changes in both blood flow and vessel permeability, therefore as CA4P is expected to increase the permeability of surviving vessels (37), a combination of restored perfusion and increased permeability may explain the increase in uptake beyond pretreatment levels at 24 h. The corresponding Area-Under-the-Curve (AUC, Figure 5B) up to 10 min after injection of the contrast agent fell from 9.67±0.43 mM to 6.85±0.95 mM (n=3 animals, p=0.030) at 6 h after treatment and rose to 12.8±0.9mM (n=5 animals, p=0.005) at 24 h (mean±S.E.).
Figure 5.

Dynamic contrast agent enhanced magnetic resonance imaging measurements of tumor perfusion. (A) Estimated contrast agent concentration, following i.v. injection at t=0, in untreated tumors and in tumors at 6 h and 24 h after CA4P treatment. The error bars indicate S.D; significant difference between all curves was tested by one-way ANOVA (p<0.001). (B) The area under the uptake curve (AUC) showed a slight reduction in contrast agent uptake at 6 h (p=0.030, paired two-tailed t-test) after CA4P treatment, while at 24 h there was a slight but significant increase in uptake compared to pre-treatment levels (p=0.002, paired two-tailed t-test). Horizontal bars indicate the median value.
The median apparent diffusion coefficient (ADC) of tumor water, measured using DW-MRI, was unchanged at 0.39±0.05 × 10−3mm2/s at 6 h after treatment (n=6 animals, p=0.53) compared to untreated levels 0.42±0.04 × 10−3mm2/s (Figure 6B), despite the onset of diffuse cellular necrosis observed in tumor sections obtained post mortem (Figure 4). The tumor area in the ADC maps also remained largely homogeneous, with both ADC histograms (Figure 6A) and visual inspection (Figure 6C, D) revealing no significant change from untreated tumors. At 24 h after treatment, the median ADC showed a 32% increase to 0.55±0.09 × 10−3mm2/s (n=7 animals, p=0.018), much broader histograms were observed (Figure 6A) and large necrotic regions with increased ADC could be distinguished within the tumor on the ADC maps (Figure 6D).
Figure 6.
Diffusion-weighted imaging shows an increase in the apparent diffusion coefficient of water (ADC) at 24 h after treatment with CA4P but not at 6 h. Average histograms of ADC values over all animals imaged are shown in (A), reflecting increased heterogeneity in the tumors at 24 h after treatment. (B) The ADC measured along the slice-selective direction shows a 32% increase (p<0.05) at 24 h after CA4P treatment but is not sensitive to changes occurring at 6 h after treatment (p=0.53). Each data point is the median pixel value in a region of interest drawn within the tumor boundary on the motion-corrected diffusion weighted image. Representative images before (C), at 6 h (D) and at 24 h (E) after treatment. The tumors are outlined in white.
Discussion
Tumor responses to treatment are usually assessed from MRI and CT measurements of reductions in tumor size. The Response Evaluation Criteria in Solid Tumors (RECIST) define complete response as total tumor regression while partial response is defined as a 30% reduction in the sum of the longest tumor axes (38). However, since vascular disrupting agents such as Combretastatin often elicit a cytostatic response at early treatment time points, the RECIST criteria are of limited value (11, 38) and interest has focused instead on measuring functional biomarkers of response, including reductions in tumor perfusion, changes in tumor cell metabolism, and ultimately cell death.
We have shown here that hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate are sensitive markers of response to CA4P treatment. The apparent rate constant describing label exchange between [1-13C]pyruvate and [1-13C]lactate, kP, is influenced by a number of factors including tumor cell lactate dehydrogenase (LDH) activity, the concentration of the coenzyme NAD(H), tumor lactate concentration, membrane transport of pyruvate and lactate and pyruvate delivery to the tumor (21). Treatment with CA4P resulted in a 34% decrease in kP at 6 h after treatment and it remained at this lowered level at 24 h after treatment. The decrease in kP at 6 h is likely explained by a decrease in tumor perfusion. DCE-MRI measurements showed a decrease in the AUC of Gd-DTPA uptake of 29% at 6 h, while independent ex vivo measurements of the tumor pyruvate concentration following injection of unlabelled pyruvate showed a decrease of 45%. The absence of a significant change in lactate concentration between 6 and 24 h after CA4P treatment is consistent with the notion that the hyperpolarized [1-13C]pyruvate is labeling, by passive exchange, a pre-existing lactate pool, rather than there being significant net conversion of pyruvate to lactate. The evidence that we are measuring predominantly exchange, rather then net conversion of pyruvate into lactate, has been published previously (21, 39). The decrease in kP at 6 h after CA4P treatment occurred despite a 50% increase in LDH activity, which by 24 h had declined towards pretreatment levels. Such up-regulation of LDH activity is likely to be a consequence of the disruption to blood flow resulting in increased tumor hypoxia, which can last for up to at least 6 h after CA4P treatment (35); hypoxia will induce expression of HIF-1α, which mediates up-regulation of the glycolytic enzymes, including LDH (40).
Restoration of tumor perfusion at 24 h after CA4P treatment, which was evident from the DCE-MRI measurements, was not accompanied by an increase in kP, indicating that factors other than pyruvate delivery are determining the rate constant at this time point. Since there was no significant difference in LDH activity and only a small decline in lactate concentration relative to control, a loss of coenzyme NAD(H) or decreased membrane transport may be responsible. These factors could also have some influence in the decline at 6 h. A further explanation may be that although there was restoration of perfusion, this was heterogeneous, with those regions of increased perfusion having lower levels of lactate and so showing lower levels of detectable label exchange.
From the perspective of response monitoring, however, the important point is that the hyperpolarized [1-13C]pyruvate experiment detects both the initial and persistent damage to the tumor by probing first its perfusion then metabolic state, at a time when DCE-MRI measurements indicated recovery of perfusion. The hyperpolarized pyruvate experiment should, therefore, be less sensitive than DCE-MRI to the timing of the measurement after CA4P treatment, which could be advantageous in the clinic. The advantage of DCE-MRI, however, is that the technique has better spatial resolution; the projected resolution for hyperpolarized 13C imaging in the clinic is only of the order of 2.5 mm and therefore DCE-MRI may be better able to detect response if this is very heterogeneous.
In a previous study we presented evidence in vitro that the production of [1,4-13C2]malate from hyperpolarized [1,4-13C2]fumarate is related to tumor cell necrosis [23]. Since fumarate shows only relatively slow transport across the plasma membrane, the onset of necrosis results in an increased rate of access of the labeled fumarate to intracellular fumarase, and hence in formation of labeled malate within the relatively short lifetime of the hyperpolarized 13C label. The results presented here, in which we have shown that the rate of production of [1,4-13C2]malate from [1,4-13C2]fumarate correlates with the levels of necrosis detected in histological sections of tumors, demonstrate that this is also the case in vivo. This correlation between necrosis and the rate of malate production from fumarate has also been demonstrated recently in a human breast cancer cell line (41).
The production of malate was detectable, although small, in untreated tumors and this increased within 6 h of CA4P treatment, with a 1.6-fold increase in the rate constant describing malate production (kF), which occurred despite the decrease in tumor perfusion. The significant increase in the malate/fumarate 13C signal intensity ratio at this time point was paralleled by an increase in the malate/fumarate concentration ratio measured in tumor extracts using 1H spectroscopy. Thus unlike the hyperpolarized [1-13C]pyruvate experiment, where we appear to be measuring primarily exchange of label between the injected pyruvate and a pre-existing lactate pool, in the hyperpolarized [1,4-13C]fumarate experiment we seem to be measuring principally the net production of malate from fumarate. The DW-MRI experiment, however, showed no change in the median ADC of tumor water over this period and the tumor area on diffusion images remained homogeneous. This is consistent with previous studies, which have shown that in xenograft tumors with small and diffuse regions of necrosis, there can be no change in ADC even with necrotic fractions of up to 40% (42). At 24 h after CA4P treatment the necrosis observed in tumor sections obtained post mortem had increased by a factor of 6 compared to untreated tumors, from 6.4% to 35.6% and there was a 2.5-fold increase in the rate constant kF. The ADC maps at this time point showed regions of extensive necrosis and there was a 32% increase in the median ADC. The hyperpolarized fumarate experiment, therefore, detects diffuse tumor necrosis and so may be a more sensitive and enduring measure of necrosis than the DW-MRI experiment. However, the hyperpolarized fumarate experiment requires intravenous injection and again does not have the spatial resolution that can be obtained with DW-MRI.
In conclusion, we have shown that the reduced rate of label exchange between [1-13C]pyruvate and [1-13C]lactate at 6 h after treatment reflects reduced tumor perfusion, while at 24 h it is sensitive to persistent damage to the tumor, at a time when DCE-MRI measurements showed perfusion had recovered. Over the same time course, the increased hydration of [1,4-13C2]fumarate to [1,4-13C2]malate parallels the progressive increase in cellular necrosis observed in histological sections and is sensitive to diffuse areas of necrosis that develop as early as 6 h after drug administration. As the production of malate from fumarate is a positive contrast experiment, it is less susceptible to changes in substrate delivery than the pyruvate experiment. Diffusion Weighted Imaging by comparison, only detected response once the necrosis was widespread at 24 hours. The combination of hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate probes both the energy status of viable tumor cells and the onset of cell death following treatment. Hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate, when combined with 13C spectroscopic imaging (21, 23) may be useful therefore in the clinic as sensitive imaging biomarkers of response to vascular targeted therapy.
Acknowledgements
The work was supported by a Cancer Research UK Programme grant to K.M.B. (C197/A3514) and by a Translational Research Program Award from The Leukemia & Lymphoma Society. The polarizer and related materials were provided by GE-Healthcare. F.A.G was in receipt of a Cancer Research UK & Royal College of Radiologists (UK) clinical research training fellowship, T.H.W a GE Healthcare-BBSRC CASE studentship and B.W.C.K. a Cancer Research UK studentship. We thank Dai Chaplin (Oxigene) for the generous gift of CA4P. We also thank Wm. Brad O’Dell for assistance with metabolite concentration and enzyme activity assays.
Notes: The work was supported by a Cancer Research UK Programme grant to K.M.B. (C197/A3514) and by a Translational Research Program Award from The Leukemia & Lymphoma Society.
Footnotes
Conflict of interest: The polarizer and related materials in this work were provided by GE-Healthcare. K.M.B. and M.I.K. hold patents with GE-Healthcare.
References
- 1.Hinnen P, Eskens FALM. Vascular disrupting agents in clinical development. Br J Cancer. 2007;96:1159–65. doi: 10.1038/sj.bjc.6603694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–35. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 3.Iyer S, Chaplin DJ, Rosenthal DS, Boulares AH, Li L, Smulson ME. Induction of Apoptosis in Proliferating Human Endothelial Cells by the Tumor-specific Antiangiogenesis Agent Combretastatin A-4. Cancer Res. 1998;58:4510–4. [PubMed] [Google Scholar]
- 4.Dark GG, Hill SA, Prise VE, Tozer GM, Pettit GR, Chaplin DJ. Combretastatin A-4, an agent that displays potent and selective toxicity toward tumor vasculature. Cancer Res. 1997;57:1829–34. [PubMed] [Google Scholar]
- 5.Galbraith SM, Maxwell RJ, Lodge MA, et al. Combretastatin A4 phosphate has tumor antivascular activity in rat and man as demonstrated by dynamic magnetic resonance imaging. J Clin Oncol. 2003;21:2831–42. doi: 10.1200/JCO.2003.05.187. [DOI] [PubMed] [Google Scholar]
- 6.Cooney MM, Savvides P, Agarwala S, et al. Phase II study of combretastatin A4 phosphate (CA4P) in patients with advanced anaplastic thyroid carcinoma (ATC) ASCO Meeting Abstracts. 2006;24:5580. [Google Scholar]
- 7.Zweifel M, Jayson G, Reed N, et al. Combretastatin A-4 phosphate (CA4P) carboplatin an paclitaxel in patients with platinum-resistant ovarian cancer. ASCO Meeting Abstracts. 2009;27 doi: 10.1093/annonc/mdq708. [DOI] [PubMed] [Google Scholar]
- 8.Gridelli C, Rossi A, Maione P, et al. Vasular Disrupting Agents: A Novel Mechanism of Action in the Battle Against Non-Small Cell Lung Cancer. The Oncologist. 2009;14:612–20. doi: 10.1634/theoncologist.2008-0287. [DOI] [PubMed] [Google Scholar]
- 9.Anderson HL, Yap JT, Miller MP, Robbins A, Jones T, Price PM. Assessment of Pharmacodynamic Vascular Response in a Phase I trial of Combretastatin A4 Phosphate. J Clin Oncol. 2003;21:2823–30. doi: 10.1200/JCO.2003.05.186. [DOI] [PubMed] [Google Scholar]
- 10.Beauregard DA, Hill SA, Chaplin DJ, Brindle KM. The susceptibility of tumors to the antivascular dug combretastatin A4 phosphate correlates with vascular permeability. Cancer Res. 2001;61 [PubMed] [Google Scholar]
- 11.O’Connor JPB, Jackson A, Asselin M, Buckley DL, Parker GJM, Jackson GC. Quantitative imaging biomarkers in the clinical development of targeted therapeutics: current and future perspectives. Lancet Oncol. 2008;9:766–76. doi: 10.1016/S1470-2045(08)70196-7. [DOI] [PubMed] [Google Scholar]
- 12.Weber WA. Positron Emission Tomography As an Imaging Biomarker. J Clin Oncol. 2006;24:3282–92. doi: 10.1200/JCO.2006.06.6068. [DOI] [PubMed] [Google Scholar]
- 13.Thoeny HC, De Keyzer F, Chen F, et al. Diffusion-Weighted Magnetic Resonance Imaging Allows Noninvasive In Vivo Monitoring of the Effects of Combretastatin A-4 Phosphate after Repeated Administration. Neoplasia. 2005;7:779–87. doi: 10.1593/neo.04748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thoeny HC, Keyzer FD, Vandecaveye V, et al. Effect of vascular targeting agent in rat tumor model: Dynamic-contrast enhanced versus diffusion-weighted MR imaging. Radiology. 2005;237:492–9. doi: 10.1148/radiol.2372041638. [DOI] [PubMed] [Google Scholar]
- 15.Hamstra DA, Rehemtulla A, Ross BD. Diffusion Magnetic Resonance Imaging: A biomarker for treatment response in oncology. J Clin Oncol. 2007;25:4104–9. doi: 10.1200/JCO.2007.11.9610. [DOI] [PubMed] [Google Scholar]
- 16.Koh DM, Blackledge M, Collins DJ, et al. Reproducibility and changes in the apparent diffusion coefficients of solid tumours treated with combretastatin A4 phosphate and bevacizumab in a two-centre phase I clinical trial. Eur Radiol. 2009;19:2728–38. doi: 10.1007/s00330-009-1469-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S, Moore JV, Waller ML, et al. Positron emission tomography of murine liver metastases and the effects of treatment by combretastatin A-4. Eur J Nucl Med. 1999;26:231–8. doi: 10.1007/s002590050382. [DOI] [PubMed] [Google Scholar]
- 18.Kim TJ, Ravoori M, Landen CN, et al. Antitumor and Antivascular Effects of AVE8062 in Ovarian Carcinoma. Cancer Res. 2007;67:9337–45. doi: 10.1158/0008-5472.CAN-06-4018. [DOI] [PubMed] [Google Scholar]
- 19.Jadvar H, Parker JA. Infection and Inflammation. Springer; London: 2005. pp. 235–42. [Google Scholar]
- 20.Ardenkjaer-Larsen JH, Fridlund B, Gram A, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–63. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day SE, Kettunen MI, Gallagher FA, et al. Detecting tumor response to treatment using hyperpolarized 13C magnetic resonance imaging and spectroscopy. Nat Med. 2007;13:1382–7. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- 22.Witney TH, Kettunen MI, Day SE, et al. A comparison between radiolabeled fluorodeoxyglucose uptake and hyperpolarized 13C-labeled pyruvate utilization as methods for detecting tumor response to treatment. Neoplasia. 2009;11:574–82. doi: 10.1593/neo.09254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher FA, Kettunen MI, Hu D, et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0911447106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigneron D, Kurhanewicz J, Nelson S, et al. Towards Clinical Studies of Hyperpolarized Carbon-13 Metabolic Imaging. Proceedings of the 17th annual scientific meeting of the ISMRM; Hawai’i. 2009. [Google Scholar]
- 25.Gallagher FA, Kettunen MI, Day SE, et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature. 2008;453:940–4. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 26.Vassault A. Lactate Dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie GmbH; 1983. pp. 118–26. [Google Scholar]
- 27.Czok R, Lamprecht W. Pyruvate, Phosphoenolpyruvate and D-Glycerate-2-phosphate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie GmbH; 1974. pp. 1446–51. [Google Scholar]
- 28.Gutmann I, Wahlefeld AW. L-(+)-Lactate: Determination with Lactate Dehydrogenase and NAD. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie GmbH; 1974. pp. 1464–8. [Google Scholar]
- 29.Goldberg ND, Passonneau JV. L-Malate and Fumarate. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Verlag Chemie GmbH; 1974. pp. 1600–3. [Google Scholar]
- 30.Minowa T, Kawano K, Kuribayashi H, et al. Increase in tumour permeability following TGF-β type I receptor-inhibitor treatment observed by dynamic contrast-enhanced MRI. Br J Cancer. 2009;101:1884–90. doi: 10.1038/sj.bjc.6605367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muruganandham M, Lupu M, Dyke J, et al. Preclinical evaluation of tumor microvascular response to a novel antiangiogenic/antitumor agent RO0281501 by dynamic contrast-enhanced MRI at 1.5T. Mol Cancer Ther. 2006;5:1950–7. doi: 10.1158/1535-7163.MCT-06-0010. [DOI] [PubMed] [Google Scholar]
- 32.de Crespigny AJ, Marks MP, Enzmann DR, Moseley ME. Navigated diffusion imaging of normal and ischemic human brain. Magn Reson Med. 1995;33:720–8. doi: 10.1002/mrm.1910330518. [DOI] [PubMed] [Google Scholar]
- 33.Allkemper T. Haemorrhage. In: Reimer P, Parizel PM, Stichnoth FA, editors. Clinical MR Imaging: A practical approach. 2nd ed Springer; Berlin: 2006. pp. 64–76. [Google Scholar]
- 34.Chen G, Horsman MR, Pedersen M, Pang Q, Stodkilde-Jorgensen H. The effect of combretastatin A4 disodium phosphate and 5,6-dimethyxanthenone-4-acetic acid on water diffusion and blood perfusion in tumors. Acta Oncologica. 2008;47:1071–6. doi: 10.1080/02841860701769750. [DOI] [PubMed] [Google Scholar]
- 35.Horsman MR, Ehrnrooth E, Ladekari M, Overgaard J. The effect of combretastatin A-4 disodium phosphate in a C3H mouse mammary carcinoma and a variety of murine spontaneous tumors. Int J Radiat Oncol Biol Phys. 2000;42:895–8. doi: 10.1016/s0360-3016(98)00299-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhao D, Jiang L, Hahn EW, Mason RP. Tumor physiologic response to combretastatin A4 phosphate assessed by MRI. Int J Radiat Oncol Biol Phys. 2005;62:872–80. doi: 10.1016/j.ijrobp.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Griggs J, Metcalfe JC, Hesketh R. Targeting tumour vasculature: the development of combretastatin A4. Lancet Oncol. 2001;2:82–7. doi: 10.1016/S1470-2045(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Kettunen MI, Hu D, Witney TH, et al. Magnetization Transfer Measurement of Exchange between Hyperpolarized [1-13C]Pyruvate and [1-13C]Lactate in a Murine Lymphoma. Magn Reson Med. 2010;63:872–80. doi: 10.1002/mrm.22276. [DOI] [PubMed] [Google Scholar]
- 40.Simon MC. Coming up for air: HIF-1 and mitochondrial oxygen consumption. Cell Metabolism. 2006;3:150–1. doi: 10.1016/j.cmet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Witney TH, Kettunen MI, Hu D, Gallagher FA, Brindle KM. Detecting response to treatment in human breast adenocarcinoma using a co-administration of hyperpolarized [1-13C]pyruvate and [1,4-13C2]fumarate. Intl Soc Mag Reson Med; Stockholm: 2010. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lyng H, Haraldseth O, Rofstad EK. Measurement of Cell Density and Necrotic Fraction in Human Melanoma Xenografts by Diffusion Weighted Magnetic Resonance Imaging. Magn Reson Med. 2000;43:828–36. doi: 10.1002/1522-2594(200006)43:6<828::aid-mrm8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]