Abstract
Objective
Research on the role of environmental lead exposure in the complex etiology of premature birth has yielded inconsistent results. We assessed the trimester-specific effect of prenatal lead exposure on gestational age and risk of premature delivery.
Methods
We used linear and logistic regression to identify critical windows of susceptibility to lead exposure upon gestational length.
Results
In single-trimester models, decreases in gestational length were most strongly associated with first and second trimester blood lead. In adjusted logistic regression models a one-standard deviation increase in second trimester blood lead was associated with an odds ratio of prematurity of 1.75 (95%CI: 1.02, 3.02).
Conclusions
Maternal whole blood lead levels measured during first and second trimesters yielded the most prominent inverse association with length of gestation and increased the risk of prematurity. .
INTRODUCTION
Premature births worldwide constitute a significant public health problem. Not only is premature delivery a major contributor to an estimated four million neonatal deaths per year (1, 2), but if preterm infants survive, they are at greater risk for a host of health problems in later life when compared to term infants. These include, but are not limited to: neurodevelopmental disabilities (3, 4); growth & metabolic disorders (5, 6); and respiratory disorders such as asthma (7-10). The role of environmental toxicants, including lead, in the complex etiology of this adverse birth outcome is suspected, but research results have been inconsistent (11).
Although tremendous reductions in lead exposure to the general population have been achieved in most of North America and Europe, worldwide, adults and children continue to be exposed through a variety of environmental media and informal sector occupations (12). Lead can persist in bone for decades after exposure, which in turn serves as in internal source of exposure (13, 14). Endogenous lead exposure is an important independent predictor of adverse health outcomes, such as: cognitive decline (15, 16), cardiovascular disease (13, 17), and decreased fetal growth (18-20). During pregnancy, these internal stores of lead mobilize to a marked degree, partitioning into red blood cells (~99%) and plasma (~1%). The pattern of association between plasma and whole blood lead remains largely unclear, but recent studies indicate that the relationship follows an exponential pattern and whole blood lead levels are not an accurate reflection of plasma lead levels (21, 22). This is of particular note since the lead present in plasma represents the circulating fraction of lead capable of crossing membranes, such as the placenta, and may serve as an important biomarker of fetal lead exposure.
A 2007 Institute of Medicine report on preterm births concluded the available epidemiologic data to date supports an adverse relationship between lead exposure and preterm delivery (11). However, given that a common hallmark of most of the reviewed studies was that lead exposure was assessed at delivery, either by measuring lead levels in cord blood or maternal whole blood, many were unable to address the issue of timing, i.e. what stage of pregnancy is the most vulnerable to lead’s impact on risk of prematurity. Of the studies which assessed lead at several time points, one found a significant inverse relationship with second trimester blood lead levels over 10 μg/dl (23) but several others had null findings (24, 25).
In the present study, the authors assessed the effect of prenatal lead exposure on length of gestational age and risk of premature delivery using trimester-specific maternal blood and plasma lead levels as biomarkers of fetal exposure to lead.
MATERIALS AND METHODS
Study Population
This analysis was based on data from a birth cohort study of fetal lead exposure and children’s cognitive development (26). Study subjects were recruited between May 1997 and July 1999 during prenatal visits at one of three clinics of the Mexican Institute of Social Security in Mexico City. Women were eligible if they lived in Mexico City and were willing to participate within the three year study period. Of the 2,273 women approached, 1,502 (66%) declined to be enrolled. We applied the following exclusion criteria to the 771 (34%) women who were willing to participate: if the mother was planning to leave the area within the study time period; daily consumption of alcoholic beverages; addiction to illegal drugs; continuous use of prescription drugs; diagnosis of multiple pregnancy, preeclampsia, renal or heart disease, gestational diabetes, or seizures that require medical treatment. A total of 280 eligible pregnant women were recruited and 182 eligible women with a negative pregnancy test who declared an intention to become pregnant in the near future were also recruited. Of the 182 women who declared an intention to become pregnant, 47 (26%) became pregnant and agreed to participate giving a total study population of 327.
Of the 327 pregnant women, 281 (86%) had complete information on gestational age, and 235 met the following additional inclusion criteria for this analysis: at least one measurement of plasma or blood lead during any of the three prenatal visits and complete information on maternal age, education, adverse birth outcome history, prior pregnancy, smoking during pregnancy and infant sex.
All mothers were given detailed information about the study procedures and ways to minimize lead exposure, and signed a written letter of informed consent prior to participation. The research protocol was approved by the Ethics and Research Committees of the National Institute of Public Health of Mexico, the Harvard School of Public Health, the Brigham and Women’s Hospital, the University of California, the University of Michigan School of Public Health, and the participating hospitals.
Maternal Blood and Plasma Lead Measurements
Maternal whole blood and plasma samples were collected at each prenatal visit at the Center for Environmental Health Research of the American British Cowdray Hospital. All blood collections, plasma and whole blood processing, and sample analyses were conducted under high-efficiency particulate air filtered conditions using trace metal clean techniques using methods previously described (27). Lead levels were analyzed using a Finnigan element inductively coupled plasma high-resolution mass spectrometer (Thermo Finnigan, Bermen, Germany) at the University of California Santa Cruz. Plasma hemoglobin and ferritin levels were also measured in order to evaluate the potential contribution of hemolysis to plasma lead levels (22).
Measurement of Gestational Length and Potential Confounders
Gestational length was estimated by date of last maternally-recalled menstrual period. Premature delivery was defined as the occurrence of birth prior to 37 weeks (259 days) gestation. Information on demographic, socioeconomic, and other factors that could confound the relationship between lead and gestational length was collected through questionnaire.
Statistical Analysis
In the initial study design, individuals were seen three times during pregnancy which corresponded to the first visit being < 20 weeks, the second visit between 20-28 weeks, and the last visit after 28 weeks. Due to this specified schedule, and to postponed visits for some individuals, the three study visits did not always correspond to clinical trimesters. Thus, we recoded the study visits to correspond to the first (<13th week), second (between the 13th-27th week) and third (>27th week) trimester classification.
All descriptive statistics and transformations were performed prior to bivariate analysis. Potential outliers were detected using the Extreme Studentized Deviate Many-Outlier procedure (28). Characteristics of the final study population were compared with excluded participants using two sample tests (t-test or chi-squared) for continuous versus categorical variables. All maternal blood and plasma lead measures were loge-transformed prior to statistical analysis. Plasma lead samples that had potential contamination due to hemolysis, as assessed by plasma hemoglobin and ferritin levels, were excluded (N=5). Spearman correlation coefficients were calculated on cord blood lead and all trimester-specific plasma and blood lead measures.
Initially, “single-trimester” multiple linear regression models were fitted to describe the relationships between gestational age and trimester-specific measures of blood, plasma lead, plasma-to-blood ratio, and cord blood lead adjusted for covariates of interest. Models were also estimated using the average blood and plasma of those with all three trimester-specific measures. Potential confounding variables were chosen based on biologic plausibility (regardless of statistical significance) and those significantly associated (p < 0.1) with gestational age in bivariate analysis. Covariates of interest included in multiple linear regression models were: maternal age, years of maternal education, history of adverse birth outcome, cigarette smoking during pregnancy, history of previous pregnancy, and infant sex. A similar model building strategy was used for logistic regression models. In order to compare the regression coefficients between the different lead biomarkers in our models, we centered and standardized the effect estimates for a one standard deviation (SD) change in each exposure metric. We then generated “multi-trimester” models, incorporating, in each model, the data from either plasma or whole blood lead concentrations from early (trimester 1) and late (trimester 3) pregnancy.
Regression plots of participants with all three trimester-specific lead biomarkers were constructed to explore the differences in the shape of the association between trimester-specific blood lead and gestational length. To construct these plots, residuals of the regression of gestational length adjusted for maternal age, years of maternal education, history of adverse birth outcome, cigarette smoking during pregnancy, history of previous pregnancy, and infant sex was the dependent variable and residuals of the regression of a 1- SD change in lead biomarker, adjusted for the same covariates, was the independent variable.
Regression diagnostics were performed on all models to evaluate multicollinearity and assess violations of the linear regression model assumptions. Potential non-linearity between continuous predictor variables and gestational age were explored by plotting generalized additive models that included smoothing parameters for continuous variables. When influential data points were detected, new models were fit excluding the observations. Data were analyzed using SAS 9.1, SAS Institute Inc. Cary, NC, 2002-2003 and R 2.9.1, The R Foundation for Statistical Computing, Boston MA 2007.
RESULTS
Our final study population included 235 pregnant women who had complete birth and covariate information with a total of 22 (9.4%) premature deliveries. There were no significant differences in population characteristics when compared with the 46 mother-infant pairs who were excluded from the analysis (Table 1). In the 1st, 2nd, and 3rd trimesters, mean plasma and whole blood lead levels, respectively, were: 0.17, 0.13, 0.16 μg/dL and 7.2, 6.3, 6.8 μg/dL. As expected, maternal blood and plasma lead were moderate to highly correlated (Spearman’s Rho: 0.45 – 0.78, all p < 0.05). These correlation coefficients were highest between adjacent trimesters (i.e. trimesters 1 & 2 or trimesters 2 & 3) and lowest between trimesters 1 & 3. Trimester 3 whole blood lead and umbilical cord blood lead at delivery were also correlated (Spearman’s Rho: 0.57, P < 0.001.)
Table 1.
Characteristics of Final Study Population Compared to Eligible Participants With Missing Data, Mexico City, Mexico, 1997-1999
| Included | Not Included | ||||
|---|---|---|---|---|---|
| Maternal Characteristics | N | Mean (SD) | N | Mean (SD) | P-Value |
| Age (years) | 235 | 27.1 (5.4) | 43 | 25.4 (4.3) | 0.07 |
| Education (years) | 235 | 10.7 (3.1) | 43 | 10.6 (3.5) | 0.86 |
| Smoking during pregnancy (%) | 235 | 4.3 | 40 | 2.5 | 0.60 |
| Number of prior pregnancies | 235 | 1.8 (1.1) | 46 | 1.7 (0.92) | 0.72 |
| Primiparity (%) | 235 | 58.7 | 46 | 60.0 | 0.87 |
| History of adverse pregnancy (%) | 235 | 7.7 | 44 | 11.4 | 0.41 |
| Married (%) | 235 | 73.6 | 43 | 74.4 | 0.91 |
| Weight gain during pregnancy (kg) | 176 | 8.6 (3.4) | 7 | 7.8 (4.6) | 0.75 |
| Blood lead (ug/dL) | |||||
| Trimester 1 | 100 | 7.2 (5.2) | 2 | 7.9 (5.7) | 0.84 |
| Trimester 2 | 187 | 6.3 (4.3) | 3 | 5.7 (4.8) | 0.51 |
| Trimester 3 | 200 | 6.8 (4.5) | 2 | 8.6 (6.5) | 0.66 |
| Plasma Lead (ug/dL) | |||||
| Trimester 1 | 89 | 0.17 (0.16) | 2 | 0.15 (0.07) | 0.70 |
| Trimester 2 | 190 | 0.13 (0.10) | 3 | 0.15 (0.16) | 0.71 |
| Trimester 3 | 198 | 0.16 (0.26) | 8 | 0.27 (0.50) | 0.48 |
| Infant Characteristics | |||||
|
| |||||
| Gestational Age (days) | 235 | 270.9 (11.2) | 46 | 269.7 (12.4) | 0.56 |
| Birthweight (grams) | 235 | 3337.3 (692.1) | 46 | 3126.2 (660.4) | 0.09 |
| Sex (% Male) | 235 | 52.3 | 46 | 52.2 | 0.98 |
| Umbilical Cord Blood Lead (ug/dL) | 119 | 5.9 (3.8) | 18 | 6.0 (4.5) | 0.66 |
There was a general negative relationship between biomarkers of lead during each trimester of pregnancy and gestational age after adjusting for maternal age, maternal education, history of adverse birth outcome, cigarette smoking during pregnancy, history of previous pregnancy, and infant sex with the strongest negative effect estimates found in the first trimester for both plasma and blood lead levels (Table 2). In these trimester-specific models, decreased gestational age was significantly associated with blood lead in the 1st trimester (standardized coefficient, −2.76 days; 95%CI: −5.21, −0.31) and during the 2nd trimester (standardized coefficient, −1.77 days; 95%CI: −3.39, −0.15). Plasma lead showed a marginally significant association with gestational age during the 1st trimester (standardized coefficient, −2.38 days; 95%CI: −4.97, 0.21) and 3rd trimester (standardized coefficient, −1.28 days; 95%CI: −2.63, 0.06). When looking at the ratio between plasma and blood during each trimester, a 1-SD increase in the first trimester ratio of plasma-to-blood lead resulted in a 3.23-day decrease (95% CI: −6.01, −0.44) in gestational age after controlling for covariates of interest. Cord blood lead predicted a non-significant decrease of 0.68 days (95%CI: −2.37, 1.00) of gestation per 1-SD increase in exposure and this effect was similar to effects found for a 1-SD increase in third trimester blood lead.
Table 2.
Separate Linear Regression Models for Gestational Age and Biomarkers of Lead Exposure at Different Trimesters, Mexico City, Mexico, 1997-1999
| Variable | N | β | P-Value | 95% CI |
|---|---|---|---|---|
| Blood Lead (ug/dL) | ||||
| Trimester 1 | 99 | −2.76 | 0.03 | −5.21, −0.31 |
| Trimester 2 | 187 | −1.77 | 0.03 | −3.39, −0.15 |
| Trimester 3 | 200 | −0.47 | 0.48 | −1.78, 0.84 |
| Average | 85 | −1.49 | 0.17 | −3.63, 0.64 |
| Plasma Lead (ug/dL) | ||||
| Trimester 1 | 89 | −2.38 | 0.07 | −4.97, 0.21 |
| Trimester 2 | 190 | −1.34 | 0.11 | −2.98, 0.29 |
| Trimester 3 | 195 | −1.28 | 0.06 | −2.63, 0.06 |
| Average | 70 | −0.28 | 0.82 | −2.81, 2.25 |
| Plasma to Blood Lead Ratio | ||||
| Trimester 1 | 81 | −3.23 | 0.02 | −6.01, −0.44 |
| Trimester 2 | 171 | −1.41 | 0.10 | −3.10, 0.29 |
| Trimester 3 | 189 | −1.30 | 0.06 | −2.67, 0.07 |
| Average | 68 | −1.27 | 0.34 | −3.89, 1.35 |
| Cord Blood Lead (ug/dL) | ||||
| 118 | −0.68 | 0.42 | −2.37, 1.00 |
Abbreviation: N, number; β, beta coefficient; CI, confidence interval.
Coefficients are mean change in gestational age (days) per increase of 1 SD in centered log lead concentration.
Each model is adjusted for sex, maternal age, maternal education, history of adverse birth outcome, cigarette smoking, and parity.
In order to further explore the relationship between early/late lead exposure and gestational length, we fit models controlling for trimester 1 and trimester 3 blood and plasma lead (Table 3). In the blood lead model, we find that even after controlling for trimester 3 blood lead, trimester 1 blood lead was significantly associated with a decrease in 2.77 days (95%CI: −5.53, −0.02) of gestational length. Trimester 1 plasma lead and the ratio of plasma-to-blood lead had larger negative associations with gestational length, after controlling for trimester 3 lead biomarkers, though in each model they failed to reach statistical significance at a p-value < 0.05.
Table 3.
Separate Linear Regression Models for Gestational Age Including Several Biomarkers of Trimester Specific Lead Exposure in the Same Model, Mexico City, Mexico, 1997-1999
| Variable | N | β | P-Value | 95% CI |
|---|---|---|---|---|
| Blood Lead (ug/dL) | ||||
| Trimester 1 | 88 | −2.77 | 0.05 | −5.53, −0.02 |
| Trimester 3 | 88 | 0.56 | 0.65 | −1.91, 3.04 |
| Plasma Lead (ug/dL) | ||||
| Trimester 1 | 72 | −1.65 | 0.29 | −4.74, 1.43 |
| Trimester 3 | 72 | −0.39 | 0.81 | −3.60, 2.83 |
| Plasma to Blood Lead Ratio | ||||
| Trimester 1 | 69 | −2.26 | 0.16 | −5.46, 0.94 |
| Trimester 3 | 69 | −0.84 | 0.64 | −4.41, 2.72 |
Abbreviation: N, number; β, beta coefficient; CI, confidence interval.
Coefficients are mean change in gestational age (days) per increase of 1 SD in centered log lead concentration.
Each model is adjusted for sex, maternal age, maternal education, history of adverse birth outcome, cigarette smoking, and parity.
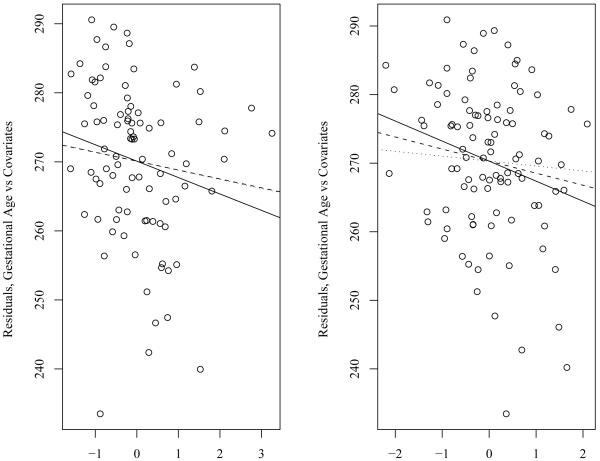
We also performed a subset analysis upon those participants that had either all three blood (N=83) or plasma (N=70) lead measurements. Linear regression plots of gestational age vs. trimester specific levels of blood lead or plasma lead were constructed using the residuals of gestational age controlling for covariates of interest (sex, maternal age, maternal education, history of adverse birth outcome, cigarette smoking, and parity) for the y-axis and the residuals of the trimester specific blood lead levels controlling for the same covariates of interest (Figure 1). In these trimester-specific blood lead models, the same general negative relationship with gestational age was observed, with the strongest, most significant associations occurring in the 1st (standardized coefficient, −2.53 days; 95%CI: −4.73, −0.33) and 2nd (standardized coefficient, −2.67 days; 95%CI: −4.83, −0.49) trimesters. Although the same general negative relationship with gestational length was observed with trimester-specific plasma lead, none of these associations reached statistical significance at p < 0.05.
Figure 1.
Linear Regression Plots of Gestational Age vs. Trimester Specific Levels of Blood Lead, Mexico City, Mexico, 1997-1999.
Graphs were constructed using the residuals of gestational age controlling for covariates of interest (sex, maternal age, maternal education, history of adverse birth outcome, cigarette smoking, and parity) for the Y Axis and the residuals of the trimester specific blood lead levels controlling for the same covariates of interest.
Solid Line=Trimester 1 Blood/Plasma Lead; Dashed Line=Trimester 2 Blood/Plasma Lead; Dotted Line=Trimester 3 Blood/Plasma Lead.
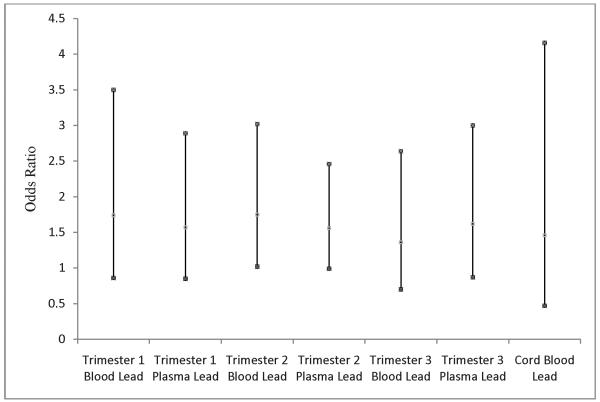
Logistic regression models were fitted to explore the relationship between the trimester-specific biomarkers of lead exposure and odds of prematurity (Figure 2). The odds ratios of premature delivery in relation to lead exposure were consistently positive and the strongest association was found for 2nd trimester blood lead: 1.75 (95%CI: 1.02, 3.02)
Figure 2.
Logistic Regression Analysis of Trimester-Specific Biomarkers of Lead Exposure, Cord Blood Lead, and Odds of Delivering Prematurely, Mexico City, Mexico, 1997-1999. Dots indicate the mean and 95%CI for the odds ratios of delivering prematurely in relation to the trimester-specific biomarkers of lead exposure and cord blood lead.
DISCUSSION
In the present study, we examined the relationship between biomarkers of maternal lead exposure at each trimester and subsequent length of gestation. Our study is the first to assess plasma lead as a biomarker for effects upon gestational length and risk of prematurity. The results of this study indicate that fetal exposure to lead negatively affects length of gestation, but is inconclusive with respect to the risk of delivering prematurely, possibly due to the relatively small number of premature births in this population sample. The negative effects of blood lead were found to be stronger early in pregnancy than in later stages of pregnancy.
There are few prior studies which assess lead exposure at least once during gestation (23-25). Our study is most similar to the results of Jelliffe-Pawlowski et al. (2006). In their study of 262 mother-infant pairs who gave birth between 1996-2002 in California, they found that a significant decrease in length of gestation was associated with second trimester measures of maternal blood lead resulting in a 1.0 day decrease in length of gestation per 1 μg/dl blood lead level over 10 μg/dL (23). In contrast, Sowers et al. (2002) found no significant relationship between risk of preterm delivery and trimester-specific maternal blood lead, though blood lead levels were approximately five times lower in their study when compared to ours (25). Factor-Litvak et al. (1991) also found no significant relationship between length of gestation and maternal blood lead measured at mid-pregnancy in a population in Yugoslavia (24). In a majority of the other epidemiologic studies examining lead exposure and length of gestation, the direction and magnitude of effects were inconsistent (29). Our study suggests that the timing and assessment of exposure with various prenatal biomarkers of lead exposure may have contributed to these inconsistencies.
In this study, we were able to assess plasma lead which is a more reflective biomarker of the circulating fraction of lead (22) and an intermediate biological marker in the pathway between maternal bone lead and fetal lead exposure. Previous studies have documented that substantial changes in plasma lead go unnoticed when lead is measured only in the whole blood fraction, especially at higher blood lead levels (21, 22). We found that as the ratio of plasma-to-blood lead increased, the length of gestation decreased and this relationship was most prominent with exposures measured in the first trimester of pregnancy.
Preterm delivery is a complex condition with a multifactorial etiology. Potential mechanisms of how lead exposure may impact preterm delivery are unclear, but several recent studies provide evidence to support a role of lead in altering the hypothalamic-pituitary-adrenal (HPA) axis. Activation of the fetal HPA axis has been shown to be one of the major events in eliciting the activation of parturition through fetal hypothalamus and/or the placenta increase in secretion of corticotropin-releasing hormone (CRH) ultimately leading to the uterine contraction, cervical ripening and decidual/fetal membrane activation (11). Altering the trajectory of CRH release during pregnancy has been suggested as one plausible mechanism which can lead to delivering an infant prematurely (30-33). Heightened maternal stress is a known risk factor in preterm delivery which is thought to act through the maternal/fetal HPA pathway by altering CRH release (34). Lead exposure may play an important role by increasing the overall baseline level of corticosterone (the biologic equivalent to cortisol in humans) in rats and heightening the response to acute stressors (35, 36). It is then plausible to speculate that increased lead exposure early in gestation may alter CRH release alone or in concert with heightened maternal stress responses.
Our study has several limitations. Gestational length was estimated by date of last maternally-recalled menstrual period which may be an unreliable measure, varying as much as ±7-21 days, depending on a host of factors including nutrition, physical activity, smoking, alcohol consumption, stress, and inter-pregnancy interval (37-40). Unfortunately, it is not standard practice in Mexico to assess gestational length through ultrasound for non-high risk pregnancies, but there are no indications that our population differs significantly from other lower/middle income pregnant women residing in Mexico City. Since the aims of the parent study related to children’s neurocognitive development, and not prematurity, another limitation is the small number of preterm births, which constrained our power for examining this outcome. We had N=22 premature births with regards to available data to assess blood lead during the second and third trimesters, but only N=13 premature births with regards to available data to assess blood lead during the first trimester. Despite these limitations, the strength and direction of our results are consistent with previous research findings in populations with similar maternal blood lead levels and adds new information about timing of exposure and potential critical windows of susceptibility.
In conclusion, our results provide evidence that length of gestation is adversely impacted by prenatal lead exposure and this effect is strongest early in pregnancy. These findings provide further evidence for the prudence of reducing lead exposure sources in the environment to protect pregnant women and their unborn children. In addition, the growing body of literature showing adverse effects of prenatal exposures early in pregnancy may warrant implementing screening for lead exposure in women of reproductive age or pre-pregnancy interventions to prevent exposure, particularly in populations at high risk for exposure.
Acknowledgments
Sources of support:
This study was supported by U.S. National Institute of Environmental Health Sciences (NIEHS) grants R01-ES007821, R01 ES014930, R01 ES013744, P42-ES05947, K23ES000381, and by Consejo Nacional de Ciencia y Tecnología (CONACyT) Grant 4150M9405 and CONSERVA, Department of Federal District, México. Additional support for the interpretation of results and authorship of this publication was made possible by NIEHS P01 ES012874 and a STAR Research Assistance Agreement No. RD-83172501 awarded by the U.S. Environmental Protection Agency (EPA.) The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, or the U.S. EPA.
Footnotes
The authors declare no competing financial interests.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawn JE, Cousens S, Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. Int J Epidemiol. 2006;35:706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- 3.Sesma HW, Georgieff MK. The effect of adverse intrauterine and newborn environments on cognitive development: the experiences of premature delivery and diabetes during pregnancy. Dev Psychopathol. 2003;15:991–1015. doi: 10.1017/s0954579403000488. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007;12:363–373. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Saigal SSB, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108:407–415. doi: 10.1542/peds.108.2.407. [DOI] [PubMed] [Google Scholar]
- 6.Darendeliler FCA, Baş F, Bundak R, Dişçi R, Sükür M, Ince Z, Can G. Catch-up growth in appropriate- or small-for-gestational age preterm infants. Turk J Pediatr. 2008;50:207–213. [PubMed] [Google Scholar]
- 7.Jaakkola JJ, Ahmed P, Ieromnimon A, et al. Preterm delivery and asthma: a systematic review and meta-analysis. The Journal of allergy and clinical immunology. 2006;118:823–830. doi: 10.1016/j.jaci.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Kumar R, Yu Y, Story RE, et al. Prematurity, chorioamnionitis, and the development of recurrent wheezing: a prospective birth cohort study. The Journal of allergy and clinical immunology. 2008;121:878–884. e876. doi: 10.1016/j.jaci.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Mutius E, Nicolai T, Martinez FD. Prematurity as a risk factor for asthma in preadolescent children. J Pediatr. 1993;123:223–229. doi: 10.1016/s0022-3476(05)81692-0. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE. Predictors of asthma and persistent wheeze in a national sample of children in the United States. Association with social class, perinatal events, and race. The American review of respiratory disease. 1990;142:555–562. doi: 10.1164/ajrccm/142.3.555. [DOI] [PubMed] [Google Scholar]
- 11.IOM, editor. Preterm Birth: Causes, Consequences, and Prevention. 2007. [PubMed] [Google Scholar]
- 12.Ahmed K, Ayana G, Engidawork E. Lead exposure study among workers in lead acid battery repair units of transport service enterprises, Addis Ababa, Ethiopia: a cross-sectional study. J Occup Med Toxicol. 2008;3:30. doi: 10.1186/1745-6673-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Shih R, Rothenberg S, Schwartz BS. The epidemiology of lead toxicity in adults: Measuring dose and consideration of other methodologic issues. Environmental Health Perspectives. 2007;115:455–462. doi: 10.1289/ehp.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tellez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, et al. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–330. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- 16.Lanphear BP, Hornung R, Khoury J, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect. 2005;113:894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellinger D, Leviton A, Rabinowitz M, Allred E, Needleman H, Schoenbaum S. Weight gain and maturity in fetuses exposed to low levels of lead. Environmental Research. 1991;54:151–158. doi: 10.1016/s0013-9351(05)80097-0. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Cossio T, Peterson KE, Sanin LH, et al. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- 20.Sanin LH, Gonzalez-Cossio T, Romieu I, et al. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics. 2001;107:1016–1023. doi: 10.1542/peds.107.5.1016. [DOI] [PubMed] [Google Scholar]
- 21.Lamadrid-Figueroa H, Tellez-Rojo MM, Hernandez-Cadena L, et al. Biological markers of fetal lead exposure at each stage of pregnancy. J Toxicol Environ Health A. 2006;69:1781–1796. doi: 10.1080/15287390600630195. [DOI] [PubMed] [Google Scholar]
- 22.Smith D, Hernandez-Avila M, Tellez-Rojo MM, Mercado A, Hu H. The relationship between lead in plasma and whole blood in women. Environ Health Perspect. 2002;110:263–268. doi: 10.1289/ehp.02110263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V. Effect of magnitude and timing of maternal pregnancy blood lead (Pb) levels on birth outcomes. Journal of Perinatology. 2006;26:154–162. doi: 10.1038/sj.jp.7211453. [DOI] [PubMed] [Google Scholar]
- 24.Factor-Litvak P, Graziano JH, Kline JK, et al. A prospective study of birthweight and length of gestation in a population surrounding a lead smelter in Kosovo, Yugoslavia. International Journal of Epidemiology. 1991;20:722–728. doi: 10.1093/ije/20.3.722. [DOI] [PubMed] [Google Scholar]
- 25.Sowers M, Jannausch M, Scholl T, Li W, Kemp FW, Bogden JD. Blood lead concentrations and pregnancy outcomes. Archives of Environmental Health. 2002;57:489–495. doi: 10.1080/00039890209601442. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Tellez-Rojo MM, Bellinger D, et al. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect. 2006;114:1730–1735. doi: 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith DR, Ilustre RP, Osterloh JD. Methodological considerations for the accurate determination of lead in human plasma and serum. Am J Ind Med. 1998;33:430–438. doi: 10.1002/(sici)1097-0274(199805)33:5<430::aid-ajim2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Rosner B. Percentage Points for a Generalized ESD Many-Outlier Procedure. Technometrics. 1983;25:165–172. [Google Scholar]
- 29.Andrews KW, Savitz DA, Hertz-Picciotto I. Prenatal lead exposure in relation to gestational age and birth weight: A review of epidemiologic studies. American Journal of Industrial Medicine. 1994;26:13–32. doi: 10.1002/ajim.4700260103. [DOI] [PubMed] [Google Scholar]
- 30.Hobel CJ, Arora CP, Korst LM. Corticotrophin-releasing hormone and CRH-binding protein. Differences between patients at risk for preterm birth and hypertension. Annals of the New York Academy of Sciences. 1999;897:54–65. doi: 10.1111/j.1749-6632.1999.tb07878.x. [DOI] [PubMed] [Google Scholar]
- 31.Leung TN, Chung TK, Madsen G, et al. Analysis of mid-trimester corticotrophin-releasing hormone and alpha-fetoprotein concentrations for predicting pre-eclampsia. Hum Reprod. 2000;15:1813–1818. doi: 10.1093/humrep/15.8.1813. [DOI] [PubMed] [Google Scholar]
- 32.McLean M, Smith R. Corticotropin-releasing Hormone in Human Pregnancy and Parturition. Trends Endocrinol Metab. 1999;10:174–178. doi: 10.1016/s1043-2760(98)00146-5. [DOI] [PubMed] [Google Scholar]
- 33.McLean M, Smith R. Corticotrophin-releasing hormone and human parturition. Reproduction. 2001;121:493–501. doi: 10.1530/rep.0.1210493. [DOI] [PubMed] [Google Scholar]
- 34.Wadhwa PD, Glynn L, Hobel CJ, et al. Behavioral perinatology: biobehavioral processes in human fetal development. Regul Pept. 2002;108:149–157. doi: 10.1016/s0167-0115(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 35.Cory-Slechta DA, Virgolini MB, Rossi-George A, Thiruchelvam M, Lisek R, Weston D. Lifetime consequences of combined maternal lead and stress. Basic Clin Pharmacol Toxicol. 2008;102:218–227. doi: 10.1111/j.1742-7843.2007.00189.x. [DOI] [PubMed] [Google Scholar]
- 36.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. 2004;112:717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato I, Toniolo P, Koenig KL, et al. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol. 1999;15:809–814. doi: 10.1023/a:1007669430686. [DOI] [PubMed] [Google Scholar]
- 38.Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle--a cross-sectional study from a Danish county. Br J Obstet Gynaecol. 1992;99:422–429. doi: 10.1111/j.1471-0528.1992.tb13762.x. [DOI] [PubMed] [Google Scholar]
- 39.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13:668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160:131–140. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]