Abstract
Protein engineering has led to a significantly improved understanding of the biophysical properties of proteins and, importantly, of the molecular mechanisms of disease. Moreover, it has enabled scientists to modify the molecular characteristics of peptides and proteins, leading to improved pharmacokinetics and pharmacodynamics of protein therapeutics. Consequently, biopharmaceuticals, such as monoclonal antibodies (mAbs), interferons/cytokines or vaccines, contribute increasingly to clinical practice. Some of these new treatments have dramatically changed the outcome of specific diseases. However, treatment options remain limited in many conditions, particularly in malignant disease, despite a much-improved understanding of the molecular mechanisms underlying cancer. With the successful pre-clinical development of therapeutic biomolecules, the most significant barrier prior to implementation into clinical practice is proof of concept in humans. This is in part addressed by clinical trials that evaluate the toxicology, dose response and efficacy of the molecules. This observational study summarises the current state of biopharmaceuticals in clinical trials and provides a particular focus on oncology trials. It identifies those cancer types that are most likely to benefit from the efforts made in pre-clinical protein science and establishes evidence that engineered proteins and peptides are set to play a growing role in clinical practice.
This study was based on the 95 254 trials registered on the National Institute of Health Clinical Trials Database by 31 August 2010. Of these, 25 525 trials assigned to cancer conditions, including leukaemia and lymphoma, were further analysed, with a particular focus on the 3653 interventional trials that were based on biological interventions. The inclusion criterion for the analysis was registration on the Clinical Trials Database by the above date. No other trials were included.
Biopharmaceuticals were the more prevalent intervention in cancer trials (14%) compared with trials in non-cancer conditions (6%). Further subgroup analysis based on the 20 cancer subtypes with the highest mortality revealed that biological therapeutics comprise 43% in malignant melanoma trials and more than 20% in five other cancer types. Two-thirds of all monoclonal antibody are registered in cancer trials (1033, 4.6% of all cancer trials). The subgroup analysis demonstrated a predominance of lymphoma and leukaemia trials for antibody interventions, with 204 and 163 trials registered, respectively. In non-cancer conditions only 503 (0.9%) trials investigate monoclonal antibody interventions. A retrospective longitudinal analysis of the trials demonstrated that monoclonal antibody trials are increasingly frequently registered in non-cancer as well as cancer conditions. However, biopharmaceutical trials continue to be registered more frequently only in non-cancer conditions, but have come to a plateau in cancers.
This study is limited by analysis of data from one database only. While the NIH Clinical Trials Database used is the most comprehensive and internationally recognised of its kind, it is possible that the results may have been modified if other databases were also included.
Protein engineering has paved the way for biopharmaceutical clinical interventions. A cross-sectional analysis of trials registered on the NIH Clinical Trial Database shows that biological interventions are increasingly entered into clinical trials. While oncological diseases used to lead this effort, biotherapeutic trials in non-cancer conditions have now become more frequent in comparison. Monoclonal antibodies, however, are still mainly investigated in oncological conditions. Haemato-oncological diseases are most frequently investigated for mAb interventions, although they are not among the eight most common causes of cancer mortality. This may reflect the fact that pre-clinical research, understanding of molecular mechanisms and target identification in other malignancies and diseases is less developed.
Keywords: biopharmaceuticals, cancer, clinical trials, cross-sectional analysis, monoclonal antibody
Introduction
Internationally recognised health organisations, such as the National Institute of Health (NIH), have identified translational research as an important scientific priority (Zerhouni, 2005). One central step in the translation of pre-clinical discoveries to patient benefit is clinical trial research that investigates the effect of potential therapeutics in humans. The results of these trials form a body of evidence that is accessible to clinicians, for example by reference to the Cochrane Library (see reference for URL) (The Cochrane Library, 2010), and have given rise to evidence-based medicine. In recognition of the importance for this evidence base, there has been an increasing effort to make clinical trial registration compulsory. This has been driven by governmental organisations such as the Food and Drug Administration (FDA) and by non-governmental bodies such as the International Committee of Medical Journal Editors. As a result the NIH trial database ClinicalTrials.gov (Clinical Trials Database, 2010; see reference for URL) has been established. The CTD contains the majority of current clinical trials with the total count approaching 1 00 000.
The data available on this registry lend themselves to analysis and provide interesting insights into the current state of clinical trial research. The observations can be compared with pre-clinical scientific discovery. The evidence so far demonstrates that the benefit of research in therapeutics can be disappointing. Consequently, the number of newly licensed drugs for non-cancer or cancer treatment is not proportionally reflecting the efforts made in pre-clinical research. (Hughes, 2009) One explanation for this might be that in some diseases proof of mechanism and proof of concept are not sufficiently researched prior to embarking on clinical trials (Janowitz and Menon, 2010).
This tendency remains prevalent in cancer and non-cancer conditions despite cancer research having, as a whole, attracted particular scientific attention in the last decades. This attention has resulted in identification of a multitude of potential drug targets and a detailed understanding of the causative molecular pathways in some tumours. The identification of these molecular targets has been successfully exploited by development of biopharmaceutical interventions that have made a significant impact on patient survival and disease prophylaxis. This new group of therapeutics is set to play a growing role and has now found its way into being licensed for cancer and non-cancer conditions. (Hughes, 2009) For example, engineered recombinant vaccines have been used to prevent human papilloma virus infection, which is closely linked to cervical cancer in women (Munoz et al., 2010). Monoclonal antibodies (mAbs) have changed the treatment options available in haemato-oncological diseases such as non-Hodgkin lymphoma, and have improved the prognosis for patients suffering from this condition (Maloney et al., 1997).
This study investigates the current state of clinical translational efforts from protein and peptide science to biological therapeutic interventions. This is done by means of a cross-sectional, observational summary of the current clinical trial landscape, with a particular focus on oncological trials and mAbs in cancer. The results could provide a foundation for future trends in biopharmaceutical development and clinical practice.
Methods
This study is an observational, cross-sectional study of all clinical trials in TBI registered on the NIH CTD (Clinical Trials Database, 2010). It does not include data from other registries. All trials registered by 31 August 2010 were included. For analysis, a combination of the trial site custom software and manual review was used. Manual review was required for the analysis of the start dates as these occasionally deviated substantially from the date of first registration. The data were not edited. Therefore, if the registered trial authority did not provide information on one category, the study was excluded from the respective part of the analysis. Counts of excluded trials are provided where relevant. It is important to note that some trials are registered for more than one characterised category. In these cases, the trial was counted once each for each subgroup. Totals of subgroups can, therefore, be greater than the actual total number provided.
Results
Overview of the current trial landscape
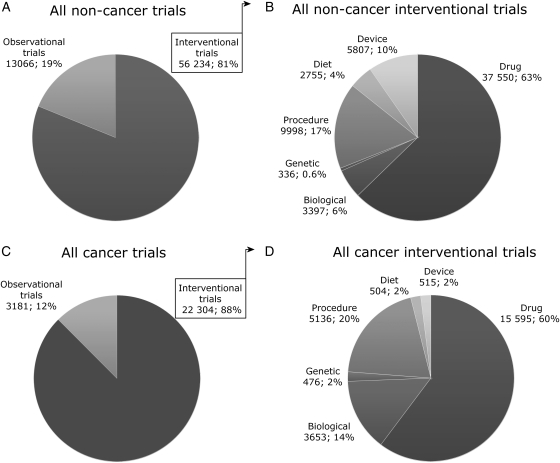
By 31 August 2010, there were 95 254 trials registered on the NIH CTD. A significant proportion of these (25 525, 26.8%) were trials on cancers, including leukaemias and lymphoma. Fig. 1 provides a subgroup analysis of the trials in cancer and non-cancer conditions. The large majority of trials in both of these groups is interventional trials, i.e. trials that evaluate an effect of an intervention on health outcome (cancer 88%, non-cancer 81%; Fig. 1A and C). Fig. 1B and D provide an overview of the respective interventions. Small molecule drug administration is most frequently investigated (cancer 60%, non-cancer 63%), followed by procedural interventions (cancer 20%, non-cancer 17%). The latter subgroup contains all radiotherapy trials. Biological interventions that include administration of engineered proteins for vaccination, treatment with mAbs, interferons/cytokines, enzymes, hormones or clotting factors, account for 14% of the interventions under investigation in cancer trials and make up the third biggest subgroup, with a total of 3653 registrations. The total count for biological interventions for non-cancer conditions is 3397, which corresponds to only 6% of all interventional trials.
Fig. 1.
Subgroup analysis of all trials registered on the NIH Clinical Trials Database by 31 August 2010. The fig. provides data for all non-cancer conditions (A and B) and all cancers, including leukaemia and lymphoma (C and D). There are more than 2.5 times as many trials registered for non-cancer conditions compared with cancers (69300 vs. 25525); however, there are more biological interventions under investigation for all cancers (3653 vs. 3397). Forty open access studies of cancer trials were not included in the analysis. A number of trials are registered for two interventions and the total in B and D, therefore, deviates from the numbers provided in A and C.
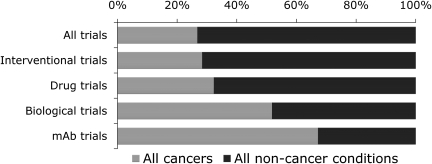
A more specific analysis of the fractional contribution of cancer trials to respective subgroups is provided in Fig. 2 and Table I: 28.6% of all trials relate to cancers, however the fraction is greater in the interventional trials (28.4%), and increases further when analysing drug trials (32.3%). The majority of biological trials are registered in cancer conditions (51.8%) and it should be noted that particularly mAbs are under investigation for cancer treatment (62.3%). The next paragraph provides a retrospective longitudinal analysis of the reasons for this distribution.
Fig. 2.
Illustration of the proportional contribution of non-cancer conditions and cancer trials to different trial subgroups. 26.8% of all trials are cancer trials; however, the proportion increases over different subgroups to 62.3% of all trials that investigate mAbs (data provided in Table I.)
Table I.
Trial counts and fractions for non-cancer conditions and cancers
| All trials |
Interventional trials |
Drug trials |
Biological trials |
mAb trials |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | n | % | |
| Non-cancer conditions | 69 652 | 73.2 | 56 208 | 71.6 | 38 568 | 67.7 | 3396 | 48.8 | 503 | 32.7 |
| Cancers | 25512 | 26.8 | 22302 | 28.4 | 18431 | 32.3 | 3653 | 51.8 | 1033 | 62.3 |
Longitudinal analysis of trial counts
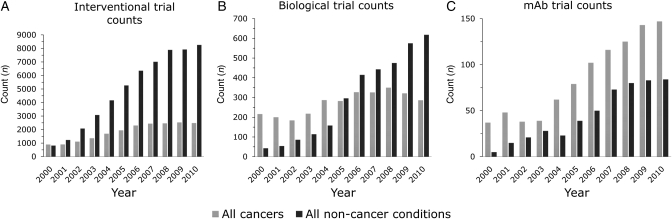
The evolution of the above-listed fractions can be appreciated by analysis of the annual trends in trial counts (Fig. 3 and Table II). For non-cancer conditions (dark grey) there is an almost universal increase of registered trial numbers across interventional, biological and mAb trials (Fig. 3A–C). This trend contrasts with the trial registration in cancer conditions (light grey); between 2006 and 2010 the counts for interventional cancer trials fall into the bracket of 2480 ± 50. Equally, the trials registered for biological interventions in cancers this year will, in all likelihood, not number more than in 2004 and have varied only a little around the 300 mark over the past 6 years. As a consequence, non-cancer conditions have since 2005 more biological interventions under investigation than cancer conditions. Despite this apparent lack of further initiative in cancer trials, mAbs continue to be increasingly investigated for cancer treatment. The count of mAbs trials in cancers has consistently outnumbered that for non-cancer conditions and has more than tripled between 2002 and 2010.
Fig. 3.
Trends of trial count start dates registered over the last decade. (A) There are an increasing number of trials registered in non-cancer conditions. The number of trials registered for cancers has been almost static since 2006. (B) Biological trial registrations have increased annually in non-cancer conditions, but have fallen since 2008 in cancers and are now at the level of 2004. (C) mAbs have consistently been more relevant to trials in cancers compared with non-cancer conditions. Counts for mAb trials have been rising since 2004 for both subgroups. Table II summarises all data. The counts for 2010 are extrapolated from the counts available by the end of August. There were no start dates available for 73 cancer and 211 non-cancer trials.
Table II.
Counts of trials registered from 2000 to 2010
| Before 2000 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Ext. 2010 | Aug 2010 | 2011 | N/A | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interventional trials | Non-cancer | 2052 | 815 | 1233 | 2076 | 3078 | 4162 | 5265 | 6352 | 7013 | 7891 | 7924 | 8258 | 5505 | 81 | 2821 |
| Cancers | 2359 | 894 | 900 | 1109 | 1359 | 1695 | 1943 | 2298 | 2439 | 2461 | 2527 | 2480 | 1653 | 25 | 695 | |
| Biological trials | Non-cancer | 81 | 43 | 54 | 86 | 114 | 158 | 296 | 415 | 443 | 475 | 575 | 618 | 412 | 4 | 211 |
| Cancers | 677 | 216 | 200 | 184 | 218 | 287 | 282 | 327 | 326 | 350 | 321 | 286 | 191 | 1 | 73 | |
| mAb trials | Non-cancer | 11 | 5 | 15 | 21 | 28 | 23 | 39 | 50 | 73 | 80 | 83 | 84 | 56 | 3 | 16 |
| Cancers | 112 | 37 | 48 | 38 | 39 | 62 | 79 | 102 | 116 | 125 | 143 | 147 | 98 | 1 | 33 |
The counts for 2010 are extrapolated (ext. 2010) from data available up to August 2010 (Aug 2010). For a number of trials the start date was not registered (N/A) and those were excluded from the data analysis at this step. Data are plotted in Fig. 3.
Comparative analysis of biological and mAb trials for the 20 cancers with the highest UK mortality
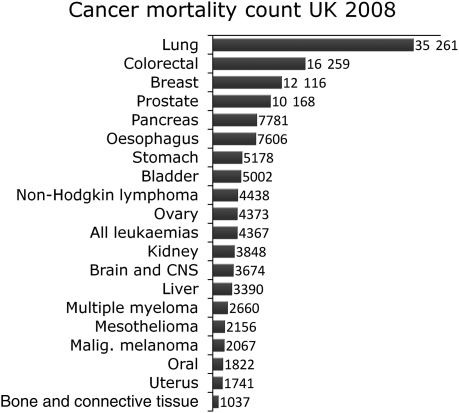
In order to understand the contribution of site-specific cancers to these statistics, the data were finally analysed with regard to the 20 most common causes of cancer death. This analysis was based on the 2008 UK cancer mortalities provided by Cancer Research UK (Fig. 4; see reference for URL). Lung, colorectal, breast, prostate and pancreas cancers remain the main cause of mortality. Together they account for more than 50% of all cancer deaths with lung cancer accounting for 22.5% alone.
Fig. 4.
Cancer mortality in the UK in 2008. Data from Cancer Research UK.
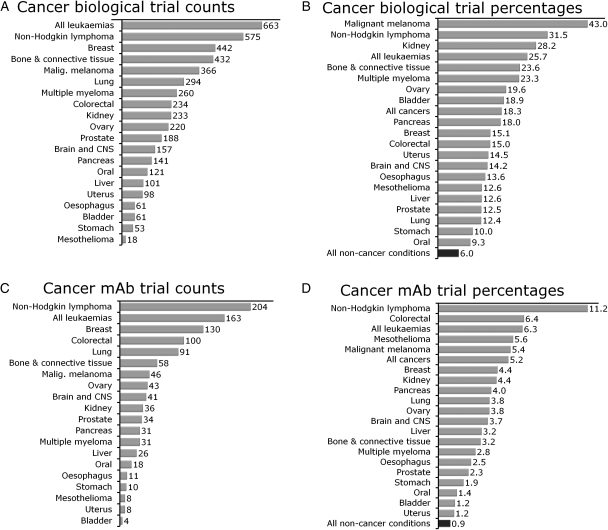
Table III and Fig. 5 specify and illustrate the trial statistics for the 20 cancers in more detail and provide data on non-cancer conditions for comparison. The data in Table III are ordered by mortality. Intriguingly, the clinical relevance of the subtypes is not necessarily mirrored by the efforts in clinical trials. For example, lung cancer trials make up only the third largest proportion of interventional cancer trials, after breast and leukaemia trials. Leukaemias, however, only account for 1 in 30 cancer deaths, whereas lung cancer accounts for almost 1 in 4. The interventional trial count for pancreatic cancer trials ranks only 15 among the 20 conditions. Bone and connective tissue cancers have, for example, more than twice as many interventional trials registered as pancreatic cancer, despite pancreatic cancer having a 7-fold higher impact on mortality in the UK. The disproportional representation of certain cancers in biological and mAb trials is illustrated in Fig. 5A and C. In both categories, leukaemias and non-Hodgkin lymphoma lead the trial counts.
Table III.
Analysis of trial counts and fractions in the 20 cancers with highest UK mortality
| Mortality |
Interventional trials |
Biological trials |
mAb trials |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| All conditions | N/A | N/A | 78510 | 82.5 | 7049 | 9.0 | 1536 | 2.0 |
| All non-cancer conditions | N/A | N/A | 56208 | 80.7 | 3396 | 6.0 | 503 | 0.9 |
| All cancers | 156 723 | 100 | 22 302 | 87.4 | 3653 | 16.4 | 1033 | 4.6 |
| Lung | 35 261 | 22.5 | 2373 | 10.6 | 294 | 8.0 | 91 | 8.8 |
| Colorectal | 16 259 | 10.4 | 1558 | 7.0 | 234 | 6.4 | 100 | 9.7 |
| Breast | 12 116 | 7.7 | 2932 | 13.1 | 442 | 12.1 | 130 | 12.6 |
| Prostate | 10 168 | 6.5 | 1506 | 6.8 | 188 | 5.1 | 34 | 3.3 |
| Pancreas | 7781 | 5.0 | 782 | 3.5 | 141 | 3.9 | 31 | 3.0 |
| Oesophagus | 7606 | 4.9 | 448 | 2.0 | 61 | 1.7 | 11 | 1.1 |
| Stomach | 5178 | 3.3 | 530 | 2.4 | 53 | 1.5 | 10 | 1.0 |
| Bladder | 5002 | 3.2 | 322 | 1.4 | 61 | 1.7 | 4 | 0.4 |
| Non-Hodgkin lymphoma | 4438 | 2.8 | 1825 | 8.2 | 575 | 15.7 | 204 | 19.7 |
| Ovary | 4373 | 2.8 | 1124 | 5.0 | 220 | 6.0 | 43 | 4.2 |
| All leukaemias | 4367 | 2.8 | 2584 | 11.6 | 663 | 18.1 | 163 | 15.8 |
| Kidney | 3848 | 2.5 | 827 | 3.7 | 233 | 6.4 | 36 | 3.5 |
| Brain and CNS | 3674 | 2.3 | 1105 | 5.0 | 157 | 4.3 | 41 | 4.0 |
| Liver | 3390 | 2.2 | 803 | 3.6 | 101 | 2.8 | 26 | 2.5 |
| Multiple myeloma | 2660 | 1.7 | 1116 | 5.0 | 260 | 7.1 | 31 | 3.0 |
| Mesothelioma | 2156 | 1.4 | 143 | 0.6 | 18 | 0.5 | 8 | 0.8 |
| Malignant melanoma | 2067 | 1.3 | 851 | 3.8 | 366 | 10.0 | 46 | 4.5 |
| Oral | 1822 | 1.2 | 1298 | 5.8 | 121 | 3.3 | 18 | 1.7 |
| Uterus | 1741 | 1.1 | 678 | 3.0 | 98 | 2.7 | 8 | 0.8 |
| Bone and connective tissue | 1037 | 0.7 | 1832 | 8.2 | 432 | 11.8 | 58 | 5.6 |
Fig. 5.
Trial counts and fractions of biological and mAbs trials for the 20 most common cancers. (A) Most biological cancer intervention trials are registered for leukaemias and non-Hodgkin lymphoma. Lung cancer, the cancer with the highest mortality, ranks only sixth in this statistic. (B) Biological interventions are registered for 6% of non-cancer trials. This fraction is higher for all of the 20 most common cancers and more than twice as high for 18 out of those 20. The fraction is more than 3 times as high for 10 cancer conditions still. (C) Non-Hodgkin lymphoma and leukaemias also have the highest count of mAb trials among the 20 most common cancers. (D) As for biological interventions, in general mAbs play a more important role in all 20 cancer conditions compared with non-cancer conditions. Table III lists a comprehensive set of data relating to the subgroup counts for the 20 cancers analysed in Figs 4 and 5.
This analysis provides a wealth of information and demonstrates that, for example, cancers that are almost twice as frequently the cause of cancer death (such as oesophagus cancer) have only one-tenth or fewer biological and mAb trials registered of those registered for haemato-oncological conditions.
Finally, based on the mortality ranking for different cancers, this study analysed the contribution of biological and mAb interventions to trial research in site-specific cancers. Fig. 5B and D provide an illustration of the rank-ordered data. They illustrate that malignant melanoma (43%), non-Hodgkin lymphoma (31.5%), kidney cancer (28.2%), leukaemias (25.7%) and bone and connective tissue cancers (23.6%) have a particularly high proportion of biological interventional trials. It is noteworthy that all 20 analysed cancer types have a higher fraction of biological interventions than non-cancer conditions (6%). The same applies to mAb trials that account for less than 1% in non-cancer conditions. Again haemato-oncological conditions rank highest in this statistic. More than 1 in 10 trials (11.2%) in non-Hodgkin lymphoma involved an mAb intervention and trials in non-Hodgkin lymphoma account for about 20% of all mAb clinical trials in cancer. Equally, cancers that are currently difficult to treat such as mesothelioma (5.6%) and malignant melanoma (5.4%) are frequently investigated for treatment benefit from mAb interventions.
Discussion
Translation of pre-clinical science to medical treatment and improved patient care relies on evidence provided by clinical trials. This study provides an overview of the trial landscape and subdivisions of this landscape, with a focus on biopharamaceuticals in oncological trials. The results have to be interpreted with caution as an observational study cannot establish causality. Furthermore, the analysis was limited to trials registered on the NIH CTD only. While this is by far the most comprehensive trial register, the results might have been modified if data from other registries had been included. Despite these two limitations, this study describes some important trends and foci of clinical research.
Cancer trials make up about one-quarter of all trials registered on the CTD. This means that cancer is overrepresented in trials if compared with global disease burden and mortality, as it accounted for approximately 12% of deaths worldwide in 2002 and is predicted to cause 17% of deaths by 2030 (Mathers and Loncar, 2006). This might partially be explained by the extensive efforts made in pre-clinical research related to mechanisms of tumour growths and by market forces driving pharmaceutical development, as the proportion represents more closely the burden of disease caused by cancer in western countries (Parkin et al., 2005). The pre-clinical work has improved our understanding of extracellular molecules that mediate tumour growth and provide potential drug targets. Protein engineering has facilitated the discovery of biomolecules that modulate these targets, such as humanised murine mAbs (Winter and Milstein, 1991). These targets are of course not exclusive to cancer. In fact, there is a growing body of evidence for benefit from mAbs in other conditions (Olsen and Stein, 2004; Shah and Mayer 2010). However, the observations of this paper suggest that the predominance of mAb treatment for cancers will continue, as cancer trials remain the most common trials with mAbs. In contrast, for biological pharmaceuticals in general, the results of this study demonstrate that the trend is towards non-cancer conditions. Since 2005 these conditions have been registered more frequently for biological interventions than cancers. Protein engineering has been of relevance here by facilitating the design of drugs with improved pharmacological properties (Krejsa et al., 2006) and has, for example, led to drugs with improved response profiles in non-cancer conditions, such as the engineered consensus interferon α (Melian and Plosker, 2001). The increased effort of pre-clinical and clinical research on biopharmaceuticals is reflected in the increased number of FDA approval applications in the last 3 years (Hughes, 2009).
In addition to the general observations in cancer trials, this paper investigates the state of site-specific cancer trials based on the 20 most common cancer types, as ranked by UK mortality data from 2008. These data were collected by Cancer Research UK for the UK only, while the CTD accepts registration worldwide. The rank order provided in Fig. 4 would be slightly modified for global cancer mortalities. For example, stomach cancer is the second most common cause of cancer mortality internationally, and liver cancer together with colorectal cancer rank third in this statistic (Mathers and Loncar, 2006). However, overall the list of common causes for cancer mortality remains similar irrespective if UK or global statistics are analysed; the data for the UK statistics are more recent and have therefore been chosen for illustration. Equally, the mismatch between relevance of some cancer conditions as assessed by mortality and the effort in clinical trials for the respective conditions remain. For example, lung, colorectal and pancreatic cancer rank much higher in the rank order of mortality than in the rank order for interventional trial research. Conversely, haemato-oncological malignancies and breast cancer rank higher in the rank order for trial activity than in the order for mortality. The reasons for this are almost certainly complex, but will in all likelihood reflect basic research efforts in the respective conditions, as well as public interest in specific cancers.
The subgroup analysis of this work corroborates the above-mentioned link of cancer trials to biological treatments and, in particular, to mAbs. Some cancers that present difficult treatment challenges, such as malignant melanoma and kidney cancer, have a particularly high percentage of biological treatment trials registered. Here, interleukins and protein vaccines play a key role in trials in addition to mAbs. These are most frequently the focus of trials involving haematological malignancies. Non-Hodgkin lymphoma was the first condition treated with mAbs (Maloney et al., 1997). Therefore, this may be due to the long experience with mAbs in pre-clinical research and clinical practice relating to haematological malignancies.
Conclusion and outlook
Protein engineering has been at the forefront for biopharmaceutical development and has made a significant contribution to treatment of malignant diseases. It has also resulted in improved understanding of disease processes central to cancer. mAbs that exploit these insights have been among the most important additions to the therapeutic portfolio over the past ten to fifteen years. This development has been facilitated by ever increasing clinical trial activity relating to these biopharmaceuticals. While the efforts in biopharmaceutical trial research continue to expand in non-cancer conditions, they have reached a plateau in cancers, with the exception of mAbs. New hypotheses and successful pre-clinical studies could reinvigorate the biological trial landscape in cancers. The development of domain antibodies (Holt et al., 2003), small bi-cyclic peptides (Heinis et al., 2009), and engineered peptides that interact with p53 (Issaeva et al., 2003; Friedler et al., 2004) could lay the path for future therapeutics.
Funding
This work was supported by a National Institute for Health Research/Wellcome Trust Academic Clinical Fellowship in Translational Medicine and Therapeutics.
Acknowledgements
I would like to thank Professor Sir Alan Fersht for continuous support. Professor Duncan Jodrell and Professor David K. Menon provided helpful comments on the manuscript. Funding by the National Health Research Institute and Wellcome Trust is gratefully acknowledged.
References
- Clinical Trials Database ‘US National Institute of Health Trial Registry’. http://clinicaltrials.gov/ doi:10.1007/BF02672073.
- Friedler A., DeDecker B.S., Freund S.M., Blair C., Rudiger S., Fersht A.R. J. Mol. Biol. 2004;336:187–196. doi: 10.1016/j.jmb.2003.12.005. doi:10.1007/BF00935980. [DOI] [PubMed] [Google Scholar]
- Heinis C., Rutherford T., Freund S., Winter G. Nat. Chem. Biol. 2009;5:502–507. doi: 10.1038/nchembio.184. doi:10.1139/g95-128. [DOI] [PubMed] [Google Scholar]
- Holt L.J., Herring C., Jespers L.S., Woolven B.P., Tomlinson I.M. Trends Biotechnol. 2003;21:484–490. doi: 10.1016/j.tibtech.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Hughes B. Nat. Rev. Drug Discov. 2009;9:89–92. doi: 10.1038/nrd3101. [DOI] [PubMed] [Google Scholar]
- Issaeva N., Friedler A., Bozko P., Wiman K.G., Fersht A.R., Selivanova G. Proc. Natl Acad. Sci. USA. 2003;100:13303–13307. doi: 10.1073/pnas.1835733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowitz T., Menon D.K. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000330. 27rv1 doi:10.1006/anbo.1995.1085. [DOI] [PubMed] [Google Scholar]
- Krejsa C., Rogge M., Sadee W. Nat. Rev. Drug Discov. 2006;5:507–521. doi: 10.1038/nrd2039. [DOI] [PubMed] [Google Scholar]
- Maloney D.G., Grillo-Lopez A.J., White C.A., et al. Blood. 1997;90:2188–2195. doi:10.1006/anbo.1999.1071. [PubMed] [Google Scholar]
- Mathers C.D., Loncar D. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. doi:10.1098/rstb.1976.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melian E.B., Plosker G.L. Drugs. 2001;61:1661–1691. doi: 10.2165/00003495-200161110-00009. doi:10.1023/A:1006344508454. [DOI] [PubMed] [Google Scholar]
- Munoz N., Kjaer S.K., Sigurdsson K., et al. J. Natl Cancer Inst. 2010;102:325–339. doi: 10.1093/jnci/djp534. doi:10.1016/j.pbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Olsen N.J., Stein C.M. N. Engl. J. Med. 2004;350:2167–2179. doi: 10.1056/NEJMra032906. doi:10.1093/aob/mch074. [DOI] [PubMed] [Google Scholar]
- Parkin D.M., Bray F., Ferlay J., Pisani P. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. doi:10.1007/s001220050774. [DOI] [PubMed] [Google Scholar]
- Shah B., Mayer L. Expert Rev. Clin. Immuno. 2010;6:607–620. doi: 10.1586/eci.10.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Library. http://www.thecochranelibrary.com. doi:10.1534/genetics.108.092304.
- Winter G., Milstein C. Nature. 1991;349:293–299. doi: 10.1038/349293a0. [DOI] [PubMed] [Google Scholar]
- Zerhouni E.A. N. Engl. J. Med. 2005;353:1621–1623. doi: 10.1056/NEJMsb053723. doi:10.1111/j.1469-8137.1994.tb03001.x. [DOI] [PubMed] [Google Scholar]