Abstract
Heart failure with preserved ejection fraction (HFPEF) is increasingly recognized as a major public health problem worldwide. Significant advances have been made in our understanding of the epidemiology of HFPEF over the past two decades, with the publication of numerous population-based epidemiological studies, large heart failure registries, and randomized clinical trials. These recent studies have provided detailed characterization of larger numbers of patients with HFPEF than ever before. This review summarizes the state of current knowledge with regards to the disease burden, patient characteristics, clinical course, and outcomes of HFPEF. Despite the wealth of available data, substantive gaps in knowledge were identified. These gaps represent opportunities for further research in HFPEF, a syndrome that is clearly a rising societal burden and that is associated with substantial morbidity and mortality.
Keywords: Heart failure with preserved ejection fraction, Epidemiology
See page 11 for the editorial comment on this article (doi:10.1093/eurjhf/hfq215)
Introduction
Heart failure (HF) affects about 2% of the western population, with the prevalence increasing sharply from 1% in 40-year-old individuals to 10% above the age of 75 years. It is the most common cause of hospitalization in patients over 65 years of age.1–3 Heart failure is defined as a syndrome characterized by an impaired ability of the heart to fill with and/or to eject blood commensurate with the metabolic needs of the body, resulting in a classic constellation of signs or symptoms of pulmonary and systemic venous congestion.1
While traditionally associated with the concept of ‘pump failure’ or reduced left ventricular (LV) ejection fraction (EF), it has become widely recognized that HF can occur even when EF is preserved, constituting the syndrome of HF with preserved ejection fraction (HFPEF). Several criteria have been proposed to define the syndrome of HFPEF,2,4,5 the most comprehensive of which are the guidelines by the Echocardiography and Heart Failure Associations of the European Society of Cardiology.2 In general, these diagnostic criteria share three features in common: (i) clinical signs or symptoms of HF; (ii) evidence of normal LV systolic function; and (iii) evidence of abnormal LV diastolic dysfunction.
Prevalence
The reported prevalence of preserved LVEF among patients with HF varied widely from 13 to 74% in early studies,6 depending partly on sample inclusion criteria (including the choice of a ‘normal’ EF cut-point) and clinical settings. These selection biases were addressed in recent population-based echocardiographic investigations performed in large community-based samples in the USA (Olmsted County Study,7 Cardiovascular Health Study,8 Strong Heart Study9), Portugal (EPICA Study10), the Netherlands (Rotterdam Study11), UK,12 Sweden (Vasteras Study13), Finland (the Helsinki Aging Study14), and Spain (Asturias Study15). Together, these recent studies provided a more refined estimate of the prevalence of HFPEF among patients with HF, which averaged 54%, with a range from 40 to 71%.16 Inherent difficulties in making an accurate diagnosis of HFPEF, the lack of standardization of diagnostic criteria, and the potential for misdiagnosis in these often elderly, overweight, or deconditioned patients limit the precision of these estimates.17 Nonetheless, the ‘true’ overall prevalence of HFPEF in the community has been estimated at 1.1–5.5% of the general population.16
Of note, the prevalence of HFPEF in the community increased with advancing age, and was higher in women; the reported age- and sex-specific prevalence rose from 0 (men) and 1% (women) in the age group of 25–49 years to about 4–6% in men and 8–10% in women for individuals 80 years and older.10 Further, the relative prevalence of HFPEF among all HF patients increased over time in a large hospital-based study in Olmsted County, MN, rising from 38 to 54% (of all HF cases) between 1987 and 2001.18 This temporal trend for increasing HFPEF occurred in association with increases in the prevalence of hypertension, diabetes, and atrial fibrillation, but without a corresponding increase in the relative prevalence of HF with reduced ejection fraction (HFREF). In the same time frame, survival was noted to improve in patients with HFREF, but not in those with HFPEF. These secular trends underscore the importance of HFPEF as a major and growing public health problem.
Incidence
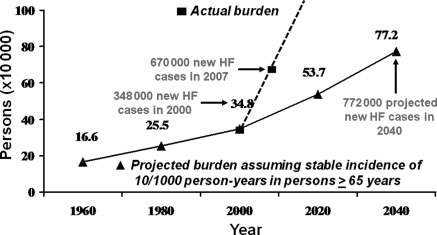
Few population-based studies have examined the temporal trends in the incidence of all HF in the community, regardless of ejection fraction, aetiology, or clinical setting. In the Framingham Heart Study,19 the incidence of HF remained unchanged in men but declined in women between 1950 and 1999. In Olmsted County, MN,20 the incidence of HF did not change between 1979 and 2000 among either men or women. In both samples, the survival after onset of HF improved over time in both men and women. With the ageing of the population and improved survival after HF onset, we can expect a dramatic increase in cases of HF (prevalence) in spite of the stable incidence rates (Figure 1). In fact, recent statistical data from the American Heart Association21 indicate that the annual actual caseload of HF may have exceeded this projected ‘epidemic’. To date, no study has looked specifically at trends in incidence of HFPEF in the community. However, extrapolating from the observations in all HF patients, and assuming that half the caseload of HF consists of HFPEF, one can project an equal, if not greater, increase in HFPEF burden in the future.
Figure 1.
Burden of heart failure. The actual annual incidence of heart failure reported in the USA (squares and dotted line) exceeded the projected annual incidence (triangles and solid line) calculated based on a stable incidence of 10 per 1000 person-years in persons aged ≥65 years. Reproduced from reference16 with permission from Elsevier.
Demographic features and risk factors
Recent large epidemiological studies characterizing more than 57 000 HF patients have helped to confirm observations from previous smaller studies of selected patients,6 and more clearly define the demographic features of patients with HFPEF (Table 1). In general, these patients are older women with a history of hypertension. The prevalence of other cardiovascular risk factors varies depending on the study setting and the diagnostic criteria for the condition. Although not uniformly reported, cardiovascular risk factors are highly prevalent in HFPEF in population-based studies and registries, and include obesity in 41–46%, coronary artery disease in 20–76%, diabetes mellitus in 13–70%, atrial fibrillation (AF) in 15–41%, and hyperlipidaemia in 16–77%. In studies that included both HFPEF and HFREF,18,22–27 patients with HFPEF were consistently found to be older, more often female, more predominantly hypertensive, and have a higher prevalence of atrial fibrillation but a lower prevalence of coronary artery disease compared with those with HFREF. Notably, non-cardiovascular co-morbidities also appear to be highly prevalent in HFPEF, consistent with an elderly population, and include renal impairment, chronic lung diseases, anaemia, cancer, liver disease, peptic ulcer disease, and hypothyroidism. The Charlson index,28 a weighted prognostic score of co-morbidity, was reported in two studies indicating high co-existing disease burden (mean score = 2.829 and score ≥3 in 70% of HFPEF patients23). Controlled clinical trials have, to date, included more than 10 000 HFPEF patients; the demographic characteristics and risk factor profiles of these individuals more closely resemble that of population-based studies in the more recently completed trials (I-PRESERVE, SENIORS, HK DHF, PEP-CHF) (Table 1).
Table 1.
Demographic characteristics and risk factors in patients with heart failure with preserved ejection fraction from recent studies
| Study (reference) | Setting | N with HF-PEF | Age | %Women | %Obesity (or mean BMI/weight) | %Hypertension | %Coronary artery disease | %Diabetes mellitus | %Atrial fibrillation | %Renal impairmenta (or mean creatinine) | %Hyperlipidaemiaa (or mean cholesterol) | Non-cardiovascular comorbidity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Population-based studies | ||||||||||||
| Lee et al.22 | Framingham Heart Study, Framingham MA, USA | 220 | 80 | 65 | BMI = 27 kg/m2 | 59 | 37 | 22 | 29 | – | TC = 218 mg/dL | |
| Bursi et al.23 | Rochester Epidemiology Project, Olmsted County MN, USA | 308 | 77 | 57 | BMI = 29.6 kg/m2 | 86 | 36 | 36 | 31 | 11% with severe renal dysfunction (creatinine clearance = 54.2 mL/min) | 77 | 38% chronic obstructive pulmonary disease; 53% anaemia; 70% Charlson index ≥3 |
| Owan et al.18 | Olmsted County MN, USA | 2167 | 74 | 56 | 41 (BMI = 29.7 kg/m2) | 63 | 53 | 33 | 41 | Creatinine = 1.6 mg/dL | – | Mean Hb = 11.8 g/dL |
| Bhatia et al.24 | EFFECT Study, Ontario, Canada | 880 | 75 | 66 | – | 55 | 36 | 32 | 32 | 22% with creatinine >150 mmol/L; 1% on dialysis | 16 | 12% cancer; 18% chronic obstructive pulmonary disease; 8% peptic ulcer disease; 2% hepatitis/cirrhosis; 21% anaemia; 24% hyponatremia |
| Gottdiener et al.55 | Cardiovascular Health Study, Multicenter, USA | 170 | 75 | 56 | – | 59 | 58 | 27 | 15 | Creatinine = 1.2 mg/dL | TC = 197 mg/dL | FEV1 = 1.75 L/min |
| Devereux et al.9 | Strong Heart Study, American Indian reservations, USA | 50 | 64 | 84 | BMI = 33.1 kg/m2 | 76 | 20 | 70 | – | Creatinine = 2.3 mg/dL | LDL = 103 mg/dL | – |
| Yip et al.73 | Hong Kong, SAR, China | 132 | 73 (including non-HFPEF) | 55 | – | 57 | 39 | 35 | – | 9% end-stage renal failure | – | – |
| HF registries | ||||||||||||
| OPTIMIZE-HF, Fonarow et al.25 | Acute HF from 259 hospitals across the USA | 21 149 | 75 | 62 | Weight = 78.9 kg | 76 | 38 | 38 | 33 | Creatinine = 1.3 mg/dL | 32 | – |
| ADHERE, Yancy et al.26 | Acute HF from >274 hospitals across the USA | 26 322 | 74 | 62 | – | 77 | 50 | 45 | 21 | 26 | – | 31% chronic obstructive pulmonary disease or asthma |
| EuroHeart Failure Survey, Lenzen et al.27 | Acute HF from 115 hospitals in 24 European countries | 3148 | 71 | 55 | – | 59 | 59 | 26 | 25 | 5 | – | – |
| New York HF Registry, Klapholz et al.41 | HF hospitalizations from 17 centres in metropolitan New York, USA | 619 | 72 | 73 | 46 (BMI = 30.6 kg/m2) | 78 | 43 | 46 | 23 | 4.5% dialysis (GFR = 50.8 ml/min) | 25% chronic obstructive pulmonary disease or asthma; 10% hypothyroidism; mean Hb = 11.8 mg/dL | |
| UK-HEART, MacCarthy et al.74 | Chronic HF from 4 centres in the UK | 163 | 63 | 28 | – | 6 | 76 | – | – | – | – | – |
| DIAMOND-CHF, Gustafsson et al.75 | Hospital-based multicentre trial screening registry, Denmark | 2218 | 73 | 49 | BMI = 26.4 kg/m2 | 25 | 49 | 13 | 26 | 2, 24, and 34% with creatinine clearance <20, 21–40, and 41–60 mL/min, respectively | – | 26% chronic obstructive pulmonary disease |
| MISCHF, Philbin et al.29 | Acute HF from 10 community hospitals in upstate New York, USA | 312 | 75 | 70 | Weight = 77 kg | 49 | 23 | 33 | 29 | Creatinine = 1.5 mg/dL; creatinine clearance = 57 mL/min) | – | Charlson Index = 2.8 |
| Controlled Clinical Trials | ||||||||||||
| SENIORS, van Veldhuisen et al.76 | 11 countries in Europe | 752 | 76 | 50 | 78 | 77 | 24 | 36 | Excluded significant renal dysfunction | 47 | – | |
| I-PRESERVE, Massie et al.50 | 25 countries in Europe, America, South Africa, Australia | 4128 | 72 | 60 | 41 | 88 | 25 | 27 | 29 | 30% with GFR <60 mL/min/1.73 m2 | – | 12% anaemia |
| HK DHF, Yip et al.77 | Hong Kong SAR, China | 150 | 74 | 62 | BMI ∼ 27 kg/m2 | 82 | 15 | 20 | 16 | – | 9 | – |
| PEP-CHF, Cleland et al.49 | 53 centres in Bulgaria, Czech Republic, Hungary, Ireland, Poland, Russia, Slovakia, UK | 850 | 76 | 55 | BMI ∼ 27.5 kg/m2 | 79 | 27 | 21 | 20 | Creatinine = 97 µmol/L | – | – |
| Ancillary DIG, Ahmed et al.56 | 302 centres in the USA and Canada | 988 | 67 | 41 | BMI = 29 kg/m2 | 62 | 50 | 27 | Excluded | 48% with GFR <60 mL/min/1.73 m2 | – | |
| SWEDIC, Bergstrom et al.78 | 12 hospitals in Sweden | 113 | 67 | 43 | Weight = 58–125 kg | 66 | 11 | 14 | – | – | – | – |
| CHARM-Preserved, Yusuf et al.51 | 618 centres in 26 countries | 3023 | 67 | 40 | – | 64 | 44 | 28 | 29 | Excluded creatinine ≥3 mg/dL (265 mmol/L) | – | 7% cancer |
aVariously defined as detailed below.
BMI, body mass index; TC, total cholesterol; Hb, haemoglobin; GFR, glomerular filtration rate; LDL, low density lipoprotein.
Echocardiographic and haemodynamic features
In the most recent set of diagnostic criteria proposed by the European Society of Cardiology,2 echocardiographic and haemodynamic features are key components for the diagnosis of HFPEF. After first establishing the presence of signs or symptoms of HF, the presence of an EF >50% and a LV end-diastolic volume index <97 mL/m2 is the second essential criterion for the diagnosis.2 The third criterion is the presence of LV diastolic dysfunction, which can be demonstrated by Doppler echocardiography, cardiac catheterization, or blood natriuretic peptide measurements. Using Doppler echocardiography, a ratio of mitral early diastolic inflow velocity to mitral early annular lengthening velocity (E/e′) exceeding 15 provides evidence for raised LV filling pressures. If the E/e′ ratio is ≤8, then LV filling pressures are probably ‘normal’. If the E/e′ ratio is intermediate (>8 to <15), it may be necessary to consider a multi-parametric approach using ‘second line’ indices: the left atrial volume (>40 mL/m2), LV mass index (>122 g/m2 in women and >149 g/m2 in men), mitral inflow Doppler (ratio of early to late mitral inflow velocity <0.5 and deceleration time >280 ms), pulmonary venous flow velocity patterns (duration of pulmonary venous A-wave reversal >30 ms longer than duration of mitral A-wave), or the presence of AF.
The utility of these ‘second line’ indices was evaluated in a retrospective study of patients referred to a tertiary echocardiography laboratory,30 where left atrial enlargement was shown to distinguish patients with E/e′ > 15 from those with E/e′ < 8 with better diagnostic accuracy than LV mass index or Doppler measurements. However, prospective evaluation is still needed in patients with confirmed clinical HF and E/e′ in the intermediate range of 8–15.31 Recognizing that advanced age and hypertension may be associated with changes in echocardiographic diastolic indices even in the absence of HF, patients with HFPEF (HF by Framingham criteria and EF >50%) were compared with elderly hypertensive and healthy controls without HF from the general community in Olmsted County, MN.32 While the extent of LV hypertrophy was similar in HFPEF and hypertensive controls, there was greater left atrial enlargement and higher estimated LV filling pressures (based on E/e′ ratio) in HFPEF compared with both control groups, adjusting for age and sex. The E/e′ ratio distinguished HFPEF from hypertensive controls without HF with better accuracy than left atrial volume index,33 but the best diagnostic utility was observed with Doppler-estimated pulmonary artery systolic pressure in the Olmsted County cohort. Further, increasing pulmonary artery systolic pressure was associated with increasing mortality in HFPEF.33 Similarly, recognizing that age, sex, co-morbidities, and LV structural remodelling can all affect circulating natriuretic peptide levels, plasma B-type natriuretic peptide (BNP) concentrations were compared between HFPEF and controls without HF in the former Olmsted County population-based study, adjusting for these covariates.32 Plasma BNP concentrations were found to be elevated in HFPEF, consistent with findings in the large patient sample of the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial, in which plasma N-terminal(NT)-proBNP levels were also found to be raised in HFPEF.34 The analysis from I-PRESERVE further showed that the elevation of circulating NT-proBNP was related to severity of symptoms/functional status as well as to the baseline characteristics indicative of poorer outcomes in HFPEF.34
Invasive measurements of LV filling pressures remain the gold standard for the diagnosis of HFPEF and should be considered in cases of diagnostic uncertainty. Cardiac catheterization is also useful for the assessment of pulmonary hypertension, which is common in HFPEF patients and may be related to both post-capillary pulmonary venous hypertension35,36 as well as a reactive pre-capillary component of pulmonary arterial hypertension.33 An emerging area of interest is a reduction in the longitudinal component of LV systolic function (relatively easy to measure by echocardiography, Figure 2).37 The reduction in longitudinal component of LV systolic function is compensated by a preserved/robust radial, circumferential, and twist components that are necessary to maintain a normal LVEF.38,39 Whether this can aid the diagnosis of HFPEF warrants validation in larger prospective studies of patients with suspected HFPEF. The potential contribution of mechanical asynchrony to the pathophysiology of HFPEF is also currently being evaluated.40
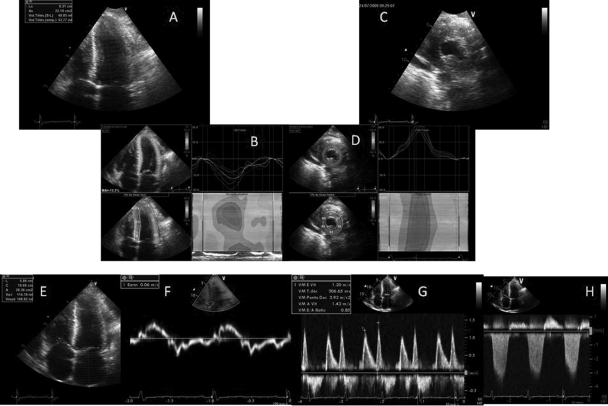
Figure 2.
A patient diagnosed for a heart failure with preserved ejection fraction. (A) Apical four-chamber view: left ventricular concentric hypertrophy with a small end-systolic volume. (B) Deformation imaging performed from this apical view to assess the longitudinal systolic function: the global longitudinal strain is depressed (−12.3%; normal −20%) despite the fact that the left ventricular ejection fraction is 55 ± 5%. (C) Parasternal short-axis view. (D) Radial strain assessment from this parasternal view: the radial strain is increased (60%, normal value 40%) to compensate for the decrease of the longitudinal one. (E) Apical four-chamber view: the left atrium is enlarged with a left atrial value greater than 38 mL/M2. (F) Pulse tissue Doppler demonstrating the e′ is blunted (6 cm/s) as s′ is (other demonstration of the decrease in the left ventricular longitudinal function). (G) Mitral inflow: delayed relaxation pattern with E/e′ >13. (H) Tricuspid regurgitation with an estimated systolic pulmonary arterial pressure of ∼55 mmHg.
In summary, non-invasive haemodynamic assessment by comprehensive echocardiographic evaluation is recommended in patients with suspected HFPEF. Plasma biomarker measurement (natriuretic peptides) may aid the diagnosis but in equivocal cases, invasive assessment should be considered.
Clinical course
Large prospective national registries have consistently demonstrated that 46–51% of hospitalized acute HF patients have a preserved LV ejection fraction.25–27 These patients are also just as likely to be re-admitted following discharge as patients with HFREF, with a re-hospitalization rate of 29% within 60–90 days,25 and a median time to re-hospitalization of 29 days.27
The clinical factors precipitating acute decompensation vs. the chronic syndrome of HFPEF have been systematically examined in a few studies.22,41–43 Of the clinical risk factors highly prevalent in HFPEF (discussed under ‘Demographic features and risk factors’ above), a few have been consistently identified in these studies to be associated with episodes of acute decompensation. Uncontrolled hypertension is a frequent presenting feature of acute HFPEF. The role of hypertension is underscored by recent large registries of acutely decompensated HFPEF showing raised admission blood pressure (mean systolic blood pressure 149 mmHg25 and 153 mmHg26) and high proportions of patients with uncontrolled systolic hypertension at presentation (12% with uncontrolled hypertension,25 61% with systolic blood pressure >140 mmHg26). Interestingly, whereas systolic blood pressures were higher, mean diastolic blood pressures in both registries were lower in patients with acute HFPEF compared with patients with HFREF, suggesting the presence of widened pulse pressures and possible arterial stiffening in these patients. Another important potentially reversible precipitating factor for HFPEF is AF. This arrhythmia was found on the initial presenting ECG in 21% of acutely decompensated HFPEF patients in the ADHERE registry.26 Indeed, these findings lend support to treatment guidelines advocating judicious blood pressure and rhythm control in HFPEF. Further, the potential contribution of non-cardiovascular factors (such as lung disease, renal impairment, or sepsis41,42) to acute HFPEF decompensation deserves mention. This observation is consistent with the high prevalence of co-morbid conditions in these elderly patients (Table 1).
Overall mortality rates in heart failure with preserved ejection fraction
Several studies have evaluated the short- and long-term mortality of HFPEF, compared these mortality patterns with that of HFREF, and assessed the prognostic factors that determine mortality risk in patients with HFPEF. In general, mortality rates have varied substantially across studies of HFPEF in part because of the heterogeneity in the diagnosis of the condition44 (variability in EF cut-points used, the requirement for demonstrating the presence of diastolic dysfunction or meeting recent criteria for HFPEF advocated by the European Society of Cardiology2), differing sampling strategies and study designs (observational cohort vs. clinical trial vs. hospital-based registries), biases introduced by exclusion of HF patients with missing EF,45 and possible temporal trends in mortality patterns.46 Nonetheless, most studies have consistently demonstrated higher mortality rates in HFPEF patients compared with age- and sex-matched controls without HF in the community.
Heart failure with preserved ejection fraction is associated with high in-hospital, short-term, and long-term mortality rates. In studies that have evaluated mortality during the peri-hospitalization period, the in-hospital mortality rates have ranged from 3 to 6.5% during the index hospitalization.25,47,48 Short-term (30–90-day) mortality also is high, ranging typically between 5 and 9.5%.24,25 The long-term mortality rates seem more variable in the reported literature. Thus, annualized mortality rates ranged from about 3.5 to 6% in 3 of the large randomized clinical trials49–51 to about 15% in the observational community-based Framingham Study.22 The lower mortality of HFPEF patients in clinical trials likely reflects a selection bias favouring relatively younger, more compliant individuals with less co-morbidities. A recent meta-analysis of 7688 patients with HFPEF followed for about 4 years found an overall mortality of 32% (about an 8% annual mortality rate). The longer term (5 years) mortality rates across observational studies and registries evaluating prevalence cohorts of HFPEF are consistently high, although absolute rates have varied considerably from about 5546,52 to 74%.22
Comparison of mortality rates with heart failure with reduced ejection fraction
Numerous investigations have compared long-term mortality rates in patients with HFPEF and HFREF. Several of the observational epidemiological cohort studies have consistently reported similar mortality rates in HFPEF and HFREF.22,46 On the other hand, clinical trials that included both kinds of HF patients have typically reported lower mortality in HFPEF compared with HFREF.51,53,54 More recently, Somaratne et al.45 published the largest systematic meta-analytic comparison of death rates in the two kinds of HF; the investigators compared mortality in 7688 HFPEF patients with 16 831 HFREF patients from 17 studies, and noted a 50% lower hazard for mortality in HFPEF compared with HFREF.45 The strengths of this meta-analysis were that it included only studies where all HF patients had an EF measured; as noted above, missing EF is an important source of bias when one compares mortality rates in HFPEF vs. HFREF.45 It is worth noting that notwithstanding the reported higher mortality of HFREF, given the ageing of the population and the preponderance of HFPEF in the elderly, the overall absolute number of deaths in the community attributable to HFPEF is likely higher than the number of deaths attributable to HFREF.55
Patterns of mortality in heart failure with preserved ejection fraction: cardiovascular vs. non-cardiovascular mortality
As noted above, there is a general consensus that patients with HFPEF have high co-morbidity burden due to their elderly nature. The proportion of deaths attributed to cardiovascular vs. non-cardiovascular causes in HFPEF varies with study design, mode of death ascertainment, and time period of the studies (Table 2).46,50–52,56,57 Thus, a recent report from the Mayo Clinic46 (that was community-based, and in which the cause of death was adjudicated by a coroner) underscored that nearly half of HFPEF patients succumbed to non-cardiovascular diseases, and there has been a temporal trend for higher non-cardiovascular mortality in HFPEF in the most recent decade (late 1990s–early 2000). Overall, community-based studies46,52,57 demonstrate a higher proportion of non-cardiovascular deaths, and clinical trials50,51,56,58 report a higher per cent of cardiovascular deaths (Table 2). This pattern may reflect the enrolment of healthier patients with fewer co-morbidities in controlled clinical trials. Cardiovascular causes of death in HFPEF patients include sudden death, refractory HF (pump failure), myocardial infarction, and other cardiovascular disease (stroke or coronary disease).46,50–52,56–58 When cause-specific mortality patterns are compared between HFPEF and HFREF, the latter has a higher burden of cardiovascular-related death compared with the former.46
Table 2.
Proportions of deaths due to cardiovascular vs. non-cardiovascular mortality in heart failure with preserved ejection fraction patients according to study design
| Study (reference) | Design | % Non-cardiovascular deaths | % Cardiovascular deaths |
|---|---|---|---|
| Henkel et al.46 | Community-based cohort | 49 | 51 |
| Tribouilloy et al.52 | Population-based, hospitalized patients | 41 | 59 |
| Grigorian-Shamagian et al.57 | Single tertiary care hospital | 20 | 80 |
| Yusuf et al.51 | Clinical trial | 28 | 72 |
| Ahmed et al.56 | Clinical trial | 30 | 70 |
| Massie et al.50 | Clinical trial | 30 | 70 |
| Zile et al.58 | Clinical trial | 30 | 60a |
aCardiovascular deaths including 26% sudden death, 14% heart failure, 5% myocardial infarction, and 9% stroke; unknown mode of death in 10% in this trial.
Heart failure with preserved ejection fraction prognostic factors for mortality risk
Several studies have examined the factors influencing mortality risk in HFPEF. Thus, in one of the larger series from Canada24 that systematically investigated the impact of prognostic factors, the following factors increased mortality risk: older age, associated co-morbidities (presence of peripheral vascular disease, dementia, or cancer each doubled mortality risk), worse clinical profile at presentation as reflected by anaemia (Hb <10 g/dL), higher serum creatinine (>150 µmol/L), hyponatraemia (<136 mmol/L), each of which increased mortality risk by 50%, and a lower systolic BP. Some other studies have emphasized a worse prognosis in men with HFPEF (compared with women),59 those with diabetes,60 chronic obstructive lung disease,61 atrial fibrillation,62 and a restrictive filling pattern.63 The presence of diabetes increases the likelihood of cardiovascular-related death in HFPEF.60
Some recent investigations have evaluated if the paradigm of reverse epidemiology observed in HFREF is also evident in HFPEF. These studies have reported that lower BMI, lower SBP, and lower total cholesterol are all markers of increased mortality risk in HFPEF, thereby extending the reverse epidemiology concept beyond HFREF.64,65 The impact of aetiology of HFPEF on mortality risk is less clear, with conflicting reports in the literature; a recent report noted similar mortality risk in HFPEF due to valve disease, hypertension or ischaemic heart disease,52 whereas another study22 highlighted a worse prognosis in those with coronary disease as the basis of HFPEF.
In summary, HFPEF has a high mortality risk, on an average lower than HFREF, a higher likelihood of non-cardiovascular death, and a range of prognostic factors that are generally similar to those noted for HFREF.
Future directions
Several gaps exist in our knowledge of the epidemiology of HFPEF and represent potential areas for future study (Table 3). The diagnostic cut-points that define a normal LVEF differ across the various studies of HFPEF, with ESC guidelines advocating a threshold of 50%.2 However, this threshold remains arbitrary, and individuals with a LVEF in the range 50–54% may also potentially have systolic dysfunction.66 Use of a higher cut-point for defining normal LVEF (55%) would lower the prevalence of HFPEF. Additional investigations describing the natural history of individuals with borderline LVEF (50–54%) may help resolve this controversy. On a parallel note, the ESC guidelines advocate cut-points for circulating BNP and pro-BNP of 200 and 220 pg/mL, respectively, for substantiating a diagnosis of HF in patients with suspected HFPEF who have a normal LVEF but an equivocal E/e′.2 However, given that women and elderly have higher BNP/proBNP levels, these cut-points likely have a greater negative than positive predictive value.67 Further studies are warranted to identify optimal cut-points for natriuretic peptides to aid the diagnosis of HFPEF in equivocal cases.
Table 3.
Unresolved issues in heart failure with preserved ejection fraction epidemiology: future directions for research
| 1. Definition and diagnosis |
| Define optimal cut-point for normal left ventricular ejection fraction |
| Characterize epidemiology based on stricter adherence to diagnostic guidelines2,44 |
| Better characterize varying subsets of disease with different underlying pathophysiology |
| Better identify cut-points for natriuretic peptides to diagnose HF in patients with equivocal diagnostic criteria |
| Better define role of newer imaging metrics like long-axis function, strain rate |
| Identify role of exercise testing in unmasking symptoms, signs, and imaging features in patients with suspected HFPEF with equivocal rest studies |
| 2. Demographic and other clinical features/risk factors |
| Better data on incidence, prevalence, trends in the same, across regions, and by ethnicity |
| Clarify pathophysiological basis for preponderance in women and elderly, including contributions of multiple non-cardiac organ systems dysfunction, family history, metabolic risk factors (including the metabolic syndrome) |
| Delineate the role of risk factors such as atrial fibrillation, hypertensive crises in the natural progression of HFPEF |
| 3. Mortality patterns |
| Define mortality patterns in studies without selection bias and without missing echocardiograms on patients |
| Delineate the contribution of cardiovascular vs. non-cardiovascular deaths in patients with HFPEF |
Traditionally, HFPEF has been diagnosed based on a normal LVEF, but recent studies have noted the potential importance of abnormalities of the long-axis LV function, LV strain and strain rate, torsion and asynchrony in addition to left atrial systolic and diastolic function.38 Of note, measurement of global strain rate during the isovolumic relaxation period of the cardiac cycle has been advocated as a key diagnostic parameter in individuals with suspected HFPEF but non-diagnostic E/e′ ratios.38 Future prospective studies are needed to validate these newer measures against invasive gold standards and determine their impact on outcomes in HFPEF.40,68
It is also noteworthy that well-compensated HFPEF patients may be asymptomatic at rest but may be prone to exercise-induced exacerbations of HF symptoms and elevations of LV filling pressures. The role of exercise testing for provocation of symptoms and/or diastolic (and systolic) dysfunction in suspected HFPEF patients needs to be better defined.69 On a separate note, several investigators have questioned the need for the demonstration of abnormal LV diastolic function for a diagnosis of HFPEF. Several non-diastolic mechanisms for HFPEF have been reviewed70 and include volume expansion, venoconstriction (altered venous capacitance), increased vascular and ventricular stiffness indices, and chronotropic incompetence. This raises the notion that there are likely several distinct pathophysiological entities encompassed by the syndrome of HFPEF. Thus, describing the principal underlying substrates [diastolic dysfunction vs. non-diastolic cardiac mechanisms; or systemic (non-cardiac) mechanisms; or a combination of factors] may be an important component of the diagnostic strategy. Indeed, Paulus and van Ballegoji44 have recently opined that strict adherence to ESC diagnostic criteria for HFPEF may facilitate the characterization of specific homogeneous subgroups such as those with HF, concentric hypertrophy, and arterial hypertension.
Other gaps in knowledge pertain to the world-wide prevalence of HFPEF (beyond USA and Europe) and variation in the burden of HFPEF according to ethnicity. Recent data indicate a potentially greater burden of diastolic dysfunction in Africans of Caribbean descent,71 highlighting the need for future studies of multi-ethnic samples. Given some suggestion of a rising incidence of HFPEF, longitudinal studies are needed to prospectively monitor incidence and prevalence of HFPEF, including assessment of temporal trends.
Several key clinical factors related to HFPEF merit further study. With a female preponderance for the condition is well known, additional investigations are necessary to identify factors that increase risk for HFPEF in women, including the relative contributions of their greater longevity, the lower burden of coronary disease, sex-related differences in LV remodelling in response to pressure-overload, hormonal factors, and sex-related differences in vascular function, venous capacitance, and susceptibility to volume overload. A family history of HF increases risk of the condition in offspring.72 However, it is unclear whether HFPEF aggregates within families, or if parental HFPEF elevates risk of the condition in the offspring, a premise that should be investigated further.72 Given the high prevalence of obesity, dyslipidaemia, and diabetes mellitus in patients with HFPEF, investigations to elucidate the contribution of metabolic disturbances (including the metabolic syndrome) to the rising burden of HFPEF are warranted.
From a prevention perspective, further investigation of key precipitating factors for HFPEF in well-compensated individuals with LV diastolic dysfunction is critical. For instance, the relations of AF and HF in HFPEF are likely complex; it is unclear in what proportion of individuals AF presages HFPEF, and vice versa. Likewise, given the frequent presence of elevated blood pressure at presentation, studies to evaluate the contribution of exacerbations of pulsatile load on the heart to overt decompensation and to identify potential triggers for these blood pressure escalations are warranted.
The sections above also underscore the current challenges related to describing the mortality patterns in HFPEF. Additional studies without selection bias or missing LVEF data are necessary to fully characterize mortality patterns in HFPEF (overall rates and cardiovascular vs. non-cardiovascular mortality), including comparisons with HFREF and clarifying the impact of aetiology of HFPEF on mortality risk. Well-designed studies are needed to ascertain the exact mode of death in these patients, and to better elucidate the contribution of the HF state itself to non-cardiovascular deaths in HFPEF patients. It is not clear whether diastolic dysfunction or the HF state is a key contributor to likelihood of death due to non-cardiovascular causes.
In conclusion, major advances have been made in our understanding of the epidemiology of HFPEF over the past two decades, but substantive gaps still exist in our knowledge. These gaps present a window of opportunity for additional research delineating these less-studied aspects of HFPEF, a disorder characterized by substantial morbidity and mortality and a rising societal burden.
Conflict of interest: none declared.
Funding
This work was supported in part by National Heart, Lung, and Blood Institute's Framingham Heart Study (Contract No. NO1-HC-25195).
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. doi:10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. doi:10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008 The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur J Heart Fail. 2008;10:933–989. doi: 10.1016/j.ejheart.2008.08.005. doi:10.1016/j.ejheart.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation. 2000;101:2118–2121. doi: 10.1161/01.cir.101.17.2118. [DOI] [PubMed] [Google Scholar]
- 5.Yturralde RF, Gaasch WH. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–319. doi: 10.1016/j.pcad.2005.02.007. doi:10.1016/j.pcad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:1565–1574. doi: 10.1016/0735-1097(95)00381-9. doi:10.1016/0735-1097(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 7.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. doi:10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 8.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. doi:10.1016/S0002-9149(00)01393-X. [DOI] [PubMed] [Google Scholar]
- 9.Devereux RB, Roman MJ, Liu JE, Welty TK, Lee ET, Rodeheffer R, Fabsitz RR, Howard BV. Congestive heart failure despite normal left ventricular systolic function in a population-based sample: the Strong Heart Study. Am J Cardiol. 2000;86:1090–1096. doi: 10.1016/s0002-9149(00)01165-6. doi:10.1016/S0002-9149(00)01165-6. [DOI] [PubMed] [Google Scholar]
- 10.Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A. Prevalence of chronic heart failure in Southwestern Europe: the EPICA study. Eur J Heart Fail. 2002;4:531–539. doi: 10.1016/s1388-9842(02)00034-x. doi:10.1016/S1388-9842(02)00034-X. [DOI] [PubMed] [Google Scholar]
- 11.Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–455. doi:10.1053/euhj.1998.1239. [PubMed] [Google Scholar]
- 12.Morgan S, Smith H, Simpson I, Liddiard GS, Raphael H, Pickering RM, Mant D. Prevalence and clinical characteristics of left ventricular dysfunction among elderly patients in general practice setting: cross sectional survey. BMJ. 1999;318:368–372. doi: 10.1136/bmj.318.7180.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedberg P, Lonnberg I, Jonason T, Nilsson G, Pehrsson K, Ringqvist I. Left ventricular systolic dysfunction in 75-year-old men and women; a population-based study. Eur Heart J. 2001;22:676–683. doi: 10.1053/euhj.2000.2284. doi:10.1053/euhj.2000.2284. [DOI] [PubMed] [Google Scholar]
- 14.Kupari M, Lindroos M, Iivanainen AM, Heikkila J, Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med. 1997;241:387–394. doi: 10.1046/j.1365-2796.1997.129150000.x. doi:10.1046/j.1365-2796.1997.129150000.x. [DOI] [PubMed] [Google Scholar]
- 15.Cortina A, Reguero J, Segovia E, Rodriguez Lambert JL, Cortina R, Arias JC, Vara J, Torre F. Prevalence of heart failure in Asturias (a region in the north of Spain) Am J Cardiol. 2001;87:1417–1419. doi: 10.1016/s0002-9149(01)01568-5. doi:10.1016/S0002-9149(01)01568-5. [DOI] [PubMed] [Google Scholar]
- 16.Owan TE, Redfield MM. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–332. doi: 10.1016/j.pcad.2005.02.010. doi:10.1016/j.pcad.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Caruana L, Petrie MC, Davie AP, McMurray JJ. Do patients with suspected heart failure and preserved left ventricular systolic function suffer from ‘diastolic heart failure’ or from misdiagnosis? A prospective descriptive study. BMJ. 2000;321:215–218. doi: 10.1136/bmj.321.7255.215. doi:10.1136/bmj.321.7255.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. doi:10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 19.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. doi:10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 20.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. doi:10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. doi:10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 22.Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. doi:10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. doi:10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. doi:10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 25.Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. doi:10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. doi:10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Lenzen MJ, Scholte op Reimer WJ, Boersma E, Vantrimpont PJ, Follath F, Swedberg K, Cleland J, Komajda M. Differences between patients with a preserved and a depressed left ventricular function: a report from the EuroHeart Failure Survey. Eur Heart J. 2004;25:1214–1220. doi: 10.1016/j.ehj.2004.06.006. doi:10.1016/j.ehj.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. doi:10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Philbin EF, Rocco TA, Jr, Lindenmuth NW, Ulrich K, Jenkins PL. Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med. 2000;109:605–613. doi: 10.1016/s0002-9343(00)00601-x. doi:10.1016/S0002-9343(00)00601-X. [DOI] [PubMed] [Google Scholar]
- 30.Emery WT, Jadavji I, Choy JB, Lawrance RA. Investigating the European Society of Cardiology Diastology Guidelines in a practical scenario. Eur J Echocardiogr. 2008;9:685–691. doi: 10.1093/ejechocard/jen137. doi:10.1093/ejechocard/jen137. [DOI] [PubMed] [Google Scholar]
- 31.Handoko ML, Paulus WJ. Polishing the diastolic dysfunction measurement stick. Eur J Echocardiogr. 2008;9:575–577. doi: 10.1093/ejechocard/jen181. doi:10.1093/ejechocard/jen181. [DOI] [PubMed] [Google Scholar]
- 32.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. doi:10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. doi:10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKelvie RS, Komajda M, McMurray J, Zile M, Ptaszynska A, Donovan M, Carson P, Massie BM. Baseline plasma NT-proBNP and clinical characteristics: results from the irbesartan in heart failure with preserved ejection fraction trial. J Card Fail. 16:128–134. doi: 10.1016/j.cardfail.2009.09.007. doi:10.1016/j.cardfail.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Hoeper MM, Barbera JA, Channick RN, Hassoun PM, Lang IM, Manes A, Martinez FJ, Naeije R, Olschewski H, Pepke-Zaba J, Redfield MM, Robbins IM, Souza R, Torbicki A, McGoon M. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(Suppl. 1):S85–S96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Puwanant S, Priester TC, Mookadam F, Bruce CJ, Redfield MM, Chandrasekaran K. Right ventricular function in patients with preserved and reduced ejection fraction heart failure. Eur J Echocardiogr. 2009;10:733–737. doi: 10.1093/ejechocard/jep052. doi:10.1093/ejechocard/jep052. [DOI] [PubMed] [Google Scholar]
- 37.Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. ‘Pure’ diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail. 2005;7:820–828. doi: 10.1016/j.ejheart.2005.02.003. doi:10.1016/j.ejheart.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation. 2009;119:1146–1157. doi: 10.1161/CIRCULATIONAHA.108.822676. doi:10.1161/CIRCULATIONAHA.108.822676. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29:1283–1289. doi: 10.1093/eurheartj/ehn141. doi:10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 40.Donal E, Lund LH, Linde C, Edner M, Lafitte S, Persson H, Bauer F, Ohrvik J, Ennezat PV, Hage C, Lofman I, Juilliere Y, Logeart D, Derumeaux G, Gueret P, Daubert JC. Rationale and design of the Karolinska-Rennes (KaRen) prospective study of dyssynchrony in heart failure with preserved ejection fraction. Eur J Heart Fail. 2009;11:198–204. doi: 10.1093/eurjhf/hfn025. doi:10.1093/eurjhf/hfn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klapholz M, Maurer M, Lowe AM, Messineo F, Meisner JS, Mitchell J, Kalman J, Phillips RA, Steingart R, Brown EJ, Jr, Berkowitz R, Moskowitz R, Soni A, Mancini D, Bijou R, Sehhat K, Varshneya N, Kukin M, Katz SD, Sleeper LA, Le Jemtel TH. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol. 2004;43:1432–1438. doi: 10.1016/j.jacc.2003.11.040. doi:10.1016/j.jacc.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Chen HH, Lainchbury JG, Senni M, Bailey KR, Redfield MM. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail. 2002;8:279–287. doi: 10.1054/jcaf.2002.128871. doi:10.1054/jcaf.2002.128871. [DOI] [PubMed] [Google Scholar]
- 43.Kramer K, Kirkman P, Kitzman D, Little WC. Flash pulmonary edema: association with hypertension and reoccurrence despite coronary revascularization. Am Heart J. 2000;140:451–455. doi: 10.1067/mhj.2000.108828. doi:10.1067/mhj.2000.108828. [DOI] [PubMed] [Google Scholar]
- 44.Paulus WJ, van Ballegoij JJ. Treatment of heart failure with normal ejection fraction an inconvenient truth! J Am Coll Cardiol. 2010;55:526–537. doi: 10.1016/j.jacc.2009.06.067. doi:10.1016/j.jacc.2009.06.067. [DOI] [PubMed] [Google Scholar]
- 45.Somaratne JB, Berry C, McMurray JJ, Poppe KK, Doughty RN, Whalley GA. The prognostic significance of heart failure with preserved left ventricular ejection fraction: a literature-based meta-analysis. Eur J Heart Fail. 2009;11:855–862. doi: 10.1093/eurjhf/hfp103. doi:10.1093/eurjhf/hfp103. [DOI] [PubMed] [Google Scholar]
- 46.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. doi: 10.1161/CIRCHEARTFAILURE.107.743146. doi:10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez de Isla L, Zamorano J, Hernandez N, Contreras L, Rodrigo JL, Almeria C, Aubele AL, Mataix L, Macaya C. Prognostic factors and predictors of in-hospital mortality of patients with heart failure with preserved left ventricular ejection fraction. J Cardiovasc Med (Hagerstown) 2008;9:1011–1015. doi: 10.2459/JCM.0b013e3282fbca87. [DOI] [PubMed] [Google Scholar]
- 48.Tsuchihashi-Makaya M, Hamaguchi S, Kinugawa S, Yokota T, Goto D, Yokoshiki H, Kato N, Takeshita A, Tsutsui H. Characteristics and outcomes of hospitalized patients with heart failure and reduced vs preserved ejection fraction. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) Circ J. 2009;73:1893–1900. doi: 10.1253/circj.cj-09-0254. doi:10.1253/circj.CJ-09-0254. [DOI] [PubMed] [Google Scholar]
- 49.Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. doi:10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 50.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. doi:10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 51.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. doi:10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 52.Tribouilloy C, Rusinaru D, Mahjoub H, Souliere V, Levy F, Peltier M, Slama M, Massy Z. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–347. doi: 10.1093/eurheartj/ehm554. doi:10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 53.Davis BR, Kostis JB, Simpson LM, Black HR, Cushman WC, Einhorn PT, Farber MA, Ford CE, Levy D, Massie BM, Nawaz S. Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation. 2008;118:2259–2267. doi: 10.1161/CIRCULATIONAHA.107.762229. doi:10.1161/CIRCULATIONAHA.107.762229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Yusuf S, Pocock S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362:759–766. doi: 10.1016/s0140-6736(03)14282-1. doi:10.1016/S0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 55.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 56.Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, Love TE, Aronow WS, Adams KF, Jr, Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. doi:10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grigorian-Shamagian L, Otero Ravina F, Abu Assi E, Vidal Perez R, Teijeira-Fernandez E, Varela Roman A, Moreira Sayagues L, Gonzalez-Juanatey JR. Why and when do patients with heart failure and normal left ventricular ejection fraction die? Analysis of >600 deaths in a community long-term study. Am Heart J. 2008;156:1184–1190. doi: 10.1016/j.ahj.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez-Sendon J, Teerlink JR, White M, McMurray JJ, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE. Mode of death in patients with heart failure and a preserved ejection fraction: results from the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-Preserve) trial. Circulation. 121:1393–1405. doi: 10.1161/CIRCULATIONAHA.109.909614. doi:10.1161/CIRCULATIONAHA.109.909614. [DOI] [PubMed] [Google Scholar]
- 59.Alla F, Al-Hindi AY, Lee CR, Schwartz TA, Patterson JH, Adams KF., Jr Relation of sex to morbidity and mortality in patients with heart failure and reduced or preserved left ventricular ejection fraction. Am Heart J. 2007;153:1074–1080. doi: 10.1016/j.ahj.2007.03.016. doi:10.1016/j.ahj.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, Solomon SD, Granger CB, Swedberg K, Yusuf S, Pfeffer MA, McMurray JJ. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153. doi:10.1093/eurheartj/ehn153. [DOI] [PubMed] [Google Scholar]
- 61.Rusinaru D, Saaidi I, Godard S, Mahjoub H, Battle C, Tribouilloy C. Impact of chronic obstructive pulmonary disease on long-term outcome of patients hospitalized for heart failure. Am J Cardiol. 2008;101:353–358. doi: 10.1016/j.amjcard.2007.08.046. doi:10.1016/j.amjcard.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 62.Fung JW, Sanderson JE, Yip GW, Zhang Q, Yu CM. Impact of atrial fibrillation in heart failure with normal ejection fraction: a clinical and echocardiographic study. J Card Fail. 2007;13:649–655. doi: 10.1016/j.cardfail.2007.04.014. doi:10.1016/j.cardfail.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Meta-analysis Research Group in Echocardiography (MeRGE) Heart Failure Collaborators. Independence of restrictive filling pattern and LV ejection fraction with mortality in heart failure: an individual patient meta-analysis. Eur J Heart Fail. 2008;10:786–792. doi: 10.1016/j.ejheart.2008.06.005. doi:10.1016/j.ejheart.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 64.Guder G, Frantz S, Bauersachs J, Allolio B, Wanner C, Koller MT, Ertl G, Angermann CE, Stork S. Reverse epidemiology in systolic and nonsystolic heart failure: cumulative prognostic benefit of classical cardiovascular risk factors. Circ Heart Fail. 2009;2:563–571. doi: 10.1161/CIRCHEARTFAILURE.108.825059. doi:10.1161/CIRCHEARTFAILURE.108.825059. [DOI] [PubMed] [Google Scholar]
- 65.Kapoor JR, Heidenreich PA. Obesity and survival in patients with heart failure and preserved systolic function: a U-shaped relationship. Am Heart J. 159:75–80. doi: 10.1016/j.ahj.2009.10.026. doi:10.1016/j.ahj.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Mahadevan G, Davis RC, Frenneaux MP, Hobbs FD, Lip GY, Sanderson JE, Davies MK. Left ventricular ejection fraction: are the revised cut-off points for defining systolic dysfunction sufficiently evidence based? Heart. 2008;94:426–428. doi: 10.1136/hrt.2007.123877. doi:10.1136/hrt.2007.123877. [DOI] [PubMed] [Google Scholar]
- 67.Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. doi:10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 68.Donal E, Lund LH, Oger E, Edner M, Persson H. What are the true prognostic differences between heart failure with preserved and reduced ejection fraction? Eur J Heart Fail. 2010;12 98. [Google Scholar]
- 69.Tan YT, Wenzelburger F, Lee E, Heatlie G, Leyva F, Patel K, Frenneaux M, Sanderson JE. The pathophysiology of heart failure with normal ejection fraction: exercise echocardiography reveals complex abnormalities of both systolic and diastolic ventricular function involving torsion, untwist, and longitudinal motion. J Am Coll Cardiol. 2009;54:36–46. doi: 10.1016/j.jacc.2009.03.037. doi:10.1016/j.jacc.2009.03.037. [DOI] [PubMed] [Google Scholar]
- 70.Bench T, Burkhoff D, O'Connell JB, Costanzo MR, Abraham WT, St John Sutton M, Maurer MS. Heart failure with normal ejection fraction: consideration of mechanisms other than diastolic dysfunction. Curr Heart Fail Rep. 2009;6:57–64. doi: 10.1007/s11897-009-0010-z. doi:10.1007/s11897-009-0010-z. [DOI] [PubMed] [Google Scholar]
- 71.Sharp A, Tapp R, Francis DP, Mc GTSA, Hughes AD, Stanton AV, Zambanini A, Chaturvedi N, Byrd S, Poulter NR, Sever PS, Mayet J. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015–1021. doi: 10.1016/j.jacc.2008.04.065. doi:10.1016/j.jacc.2008.04.065. [DOI] [PubMed] [Google Scholar]
- 72.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O'Donnell CJ, Nam BH, Larson MG, D'Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. doi:10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 73.Yip GW, Ho PP, Woo KS, Sanderson JE. Comparison of frequencies of left ventricular systolic and diastolic heart failure in Chinese living in Hong Kong. Am J Cardiol. 1999;84:563–567. doi: 10.1016/s0002-9149(99)00378-1. doi:10.1016/S0002-9149(99)00378-1. [DOI] [PubMed] [Google Scholar]
- 74.MacCarthy PA, Kearney MT, Nolan J, Lee AJ, Prescott RJ, Shah AM, Brooksby WP, Fox KA. Prognosis in heart failure with preserved left ventricular systolic function: prospective cohort study. BMJ. 2003;327:78–79. doi: 10.1136/bmj.327.7406.78. doi:10.1136/bmj.327.7406.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gustafsson F, Torp-Pedersen C, Brendorp B, Seibaek M, Burchardt H, Kober L. Long-term survival in patients hospitalized with congestive heart failure: relation to preserved and reduced left ventricular systolic function. Eur Heart J. 2003;24:863–870. doi: 10.1016/s0195-668x(02)00845-x. doi:10.1016/S0195-668X(02)00845-X. [DOI] [PubMed] [Google Scholar]
- 76.van Veldhuisen DJ, Cohen-Solal A, Bohm M, Anker SD, Babalis D, Roughton M, Coats AJ, Poole-Wilson PA, Flather MD. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data from SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure) J Am Coll Cardiol. 2009;53:2150–2158. doi: 10.1016/j.jacc.2009.02.046. doi:10.1016/j.jacc.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 77.Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE. The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart. 2008;94:573–580. doi: 10.1136/hrt.2007.117978. doi:10.1136/hrt.2007.117978. [DOI] [PubMed] [Google Scholar]
- 78.Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–461. doi: 10.1016/j.ejheart.2004.02.003. doi:10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]