Abstract
Background:
Patients with hilar cholangiocarcinoma or hepatolithiasis often develop segmental cholangitis (SC), but it is unclear whether hepatectomy for patients with SC can be performed safely.
Methods:
Rats were subjected to segmental bile duct ligation (SBDL) with LPS (SC group) or a saline (Sham group) infusion into the bile duct of the ligated lobes. The rats were sacrificed at 3, 24 and 48 h after the SBDL. For another experiment, the rats were subjected to partial hepatectomy (PHx) for the ligated lobes. Hepatic regeneration rates and the expression of regeneration-associated genes were evaluated.
Results:
In the SC group, severe parenchymal damage was observed in the acute phase (3 h). Altered gene expression in the liver in response to biliary infection occurred not only in the infected lobes but also in the non-infected lobes. In the rats of the SC group, both the hepatic regeneration rate and serum HGF levels were significantly lower than in the Sham group.
Conclusion:
These results clearly demonstrate that SC impairs the regeneration capacity of the contralateral remnant liver. Therefore, hepatectomy should be avoided for patients with SC even if it occurs in the part of the liver to be resected.
Keywords: segmental bile duct ligation, α-SMA, hepatic regeneration rate, hepatic stellate cells, hepatocyte growth factor
Introduction
Patients with cholangiocarcinoma have cholestatic liver injury as a result of a partial or total obstruction of the bile duct. In the case of hilar cholangiocarcinoma, the hepatic duct is often partitioned into several units by the tumour at the hepatic hilum1. Under such a difficult condition, a segmental biliary infection may develop and this condition is called segmental cholangitis (SC). A similar condition is observed in cases of hepatolithiasis which develop in the branch of the intrahepatic bile duct.2
Several studies have reported that the presence of pre-operative cholangitis is a major prognostic factor in the outcome of major hepatic resection for biliary carcinoma.3,4 One clinical study reported that the presence of SC is a negative prognostic factor for post-hepatectomy mortality in hilar cholangiocarcinoma.5 The leading cause of death in the SC group was liver failure.5 However, these reports are retrospective clinical reviews and there is no mechanistic study that has evaluated the effects of SC on the hepatic regeneration capacity after major hepatectomy.
The pathophysiological mechanism of acute obstructive cholangitis (AOC) has been studied with several animal models.6,7 In these models, pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6) are believed to be central to the pathophysiology of organ injury.8 The magnitude of the increase in these pro-inflammatory cytokines strongly correlates with severity of multiple organ failure and mortality.9 Furthermore, it has been shown in animal septic models that the presence of systemic infection delays liver regeneration.10,11 However, whether SC affects the hepatic regeneration capacity after hepatectomy is unknown.
After hepatectomy, several growth factors are reported to play a crucial role in the regulation of regeneration by providing either stimulatory or inhibitory signals to hepatocytes. Hepatocyte growth factor (HGF) and epidermal growth factor (EGF) stimulate DNA synthesis in hepatocytes in vivo and in culture, and are known to be the most powerful mitogens of hepatocytes.12 In the liver, HGF is produced by non-parenchymal cells, mainly by hepatic stellate cells (HSCs).13,14 However, in the injured liver, HSCs are activated and lose HGF productivity.12,13 On the other hand, transforming growth factor-β1 (TGF-β1), which is produced by activated HSCs, is a potent growth inhibitor of hepatocytes.15–17 However, it is not clear how these liver regeneration-associated factors are modulated under the condition of SC.
In the present study, a rat SC model induced by segmental bile duct ligation (SBDL) and lipopolysaccharide (LPS) infusion into the ligated bile duct was established. The regeneration capacity of the liver after partial hepatectomy was evaluated by resecting the lobes subjected to SBDL.
Materials and methods
Materials
All chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA).
Animals
Male Wister rats (250–300 g) were purchased from SLC (Tokyo, Japan). The animals were kept in a temperature- and humidity-controlled environment in a 12-h light/dark cycle. Animals were allowed free access to water and food at all times. All experiments were approved by the Regulations for Animal Experiments in Nagoya University.
Experiment 1 (SC-Induction)
The rats were randomly assigned to one of two groups: the Sham group and the Segmental cholangitis (SC) group. In both groups, all surgical procedures were performed under general anaesthesia by inhalation of the diethyl ether. The abdomen was opened with a median incision. A PE-10 polyethylene catheter (Imamura Co., Tokyo, Japan) was placed and tied with 5–0 silk thread in the bile duct of the left lateral and median lobes (= left hepatic duct), which are equivalent to 70% of the liver (Fig. 1a). A micro syringe was connected to the catheter, and 0.15 ml of saline (for the Sham group) or LPS (purified from Escherichia coli O111:B4; 1 mg/ml) (for the SC group) was slowly injected into the bile duct. The volume of fluid infused into the bile duct was determined based on a previous study showing that infusion of 0.15 ml of contrast material does not cause parenchymography.18 Afterwards, the left hepatic duct was ligated just above the tip of the PE-10 polyethylene catheter. Then, the PE-10 polyethylene catheter was removed and the left hepatic duct was transected between the ligatures. The rats were sacrificed at 3, 24, and 48 h after the segmental bile duct ligation (SBDL, n = 8 at each time point) and blood samples were obtained. The liver tissues were separately harvested from the lobes with or without the SBDL.
Figure 1.

(a) Schema showing the segmental bile duct ligation (SBDL) procedure and the injection of saline or lipopolysaccharide (LPS) (concentration; 1 mg/ml) into the bile duct. (b) Schedules of experiment 1 (SC-Induction) and 2 (Hepatectomy after SC-Induction)
Experiment 2 (Hepatectomy after SC-Induction)
For experiment 2, the rats were subjected to 70% partial hepatectomy (PHx) at 3, 24 and 48 h after the induction of SC. Briefly, a median incision was made and the liver was freed from its ligaments. The left lateral and median lobes (equivalent to 70% of the liver, the lobes subjected to the SBDL) were ligated with 4–0 silk sutures and resected. The rats were sacrificed on day 1 and 7 after the PHx (n = 8 at each time point) and blood and liver tissues from the remnant lobes were harvested at each time point. Schedules for all procedures are shown in Fig. 1b.
Biochemical assays for blood sample
Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using standard laboratory methods.
Determination of hepatic mRNA expression by real-time RT-PCR
To measure changes in gene expression related to liver regeneration, quantitative real time reverse transcription-polymerase chain reaction (RT-PCR) analysis was performed with an Applied Biosystems Prism 7300 sequence detection system (Applied Biosystems Inc., Foster City, CA). Four different liver samples were harvested at each time point (3, 24 and 48 h after the SBDL): (i) samples from the non-SBDL lobes in the Sham group; (ii) samples from the non-SBDL lobes in the SC group; (iii) samples from the SBDL lobes in the Sham group; and (iv) samples from the SBDL lobes in the SC group. Total RNA was isolated from liver tissues using a Qiagen RNeasy mini kit (Qiagen, GmbH, Germany) according to the manufacturer's protocol. The cDNA was generated from the total RNA samples using a SuperScript III reverse transcriptase reagent (Invitrogen, Carlsberg, CA, USA). Each reaction was performed in a 20-µl reaction mixture containing cDNA, each probe and primer set, and a 2× PCR Master Mix (Applied Biosystems, Foster City, CA, USA). TaqMan gene expression assays (Applied Biosystems) for interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), inducible nitric oxide synthase (iNOS), nuclear factor-κB (NF-κB), hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor-β1 (TGF-β1), interleukin-1β (IL-1β), α-smooth muscle actin (α-SMA) and 18S rRNA (endogenous control) were purchased as a probe and primer set (IL-6, Rn00561420_m1; TNF-α, Rn01525860_g1; iNOS, Rn00561646_m1; NF-κB, Rn01399583_m1; HGF, Rn00566673_m1; EGF, Rn00563336_m1; TGF-β1, Rn99999016_m1; IL-1β, Rn00580432_m1; α-SMA, Rn01759928_g1; and 18S rRNA, Hs99999901_s1). The reaction mixture was denatured with one cycle of 10 min at 95°C and incubated for 40 cycles (denaturing for 15 s at 95°C and annealing and extending for 1 min at 60°C). The amplification data were analysed with Applied Biosystems Prism sequence detection software (Applied Biosystems, version 2.1). In each experiment, the relative expression of the gene of interest was normalized to the 18S control using standard curves prepared for each gene and average values were used for quantification. The average of liver samples from the non-SBDL lobes in the Sham group was set as one-fold induction, and other data were adjusted to that baseline.
Evalualtion of hepatic regeneration after PHx
The restitution of the liver weight was determined as the percentage of regenerated liver mass and calculated by the following equation as described before: hepatic regeneration rate (%) = 100 × [C − (A − B)]/A; in which A is the estimated total liver weight at the time of the partial hepatectomy, B is the excised liver weight and C is the weight of the regenerated liver at the final resection.19 The mitotic index was determined in paraffin-embedded liver samples stained with haematoxylin and eosin. The mitotic index was expressed as the percentage of mitotic hepatocytes per the total number of hepatocytes in high-power fields (HPF).19 Six fields from one specimen were evaluated for eight animals in each group.
Enzyme-linked immunosorbent assay (ELISA) for hepatic regeneration-associated factors after PHx
HGF is well known as the most powerful mitogen for the hepatocytes. On the other hand, IL-1β is known as a strong inhibitor of liver regeneration.20 Therefore, serum HGF and IL-1β levels were determined at 3, 24 and 48 h after the SBDL and on day 1 after the PHx using a commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kit (HGF; Institution of Immunology, Tokyo, Japan: IL-1β; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's protocol.
Immunohistochemistry for α-smooth muscle actin (α-SMA)
To detect the expression of α-SMA, the automated slide preparation system Discovery XT (Ventana Medical Systems, Inc., Tucson, AZ) was used. Before staining, paraffin sections were heated at 65°C for 30 min in a paraffin oven and were blocked with 1% non-fat milk. The staining procedure was carried out according to the manufacturer's protocol (Ventana Medical Systems, Inc.). Anti-α-SMA antibody (Dako, Glostrup, Denmark) was diluted in Discovery® Ab diluent (Ventana Medical Systems, Inc.).
Statistical analysis
There were six to eight animals in each group. The results are presented as the mean ± standard error (SE). The Student's t-test was used to compare the Sham and the SC group. The survival data were analysed by Kaplan–Meier survival analysis with log rank test using SPSSII (SPSS Japan Inc, Tokyo, Japan). A P-value of less than 0.05 was considered to indicate significant difference.
Results
Experiment 1 (SC-Induction)
Serum parameters for liver function
The total serum AST and ALT levels in the SC group were significantly higher compared with those in the Sham group in only the acute phase (3 h) after the SBDL (Fig. 2). However, in the late phase (24 and 48 h) these levels in the SC group were not different from those in the Sham group (Fig. 2). These results indicate that our SC model induces severe parenchymal damage in the acute phase (3 h) and the damage is quickly recovered in the late phase (24 and 48 h).
Figure 2.
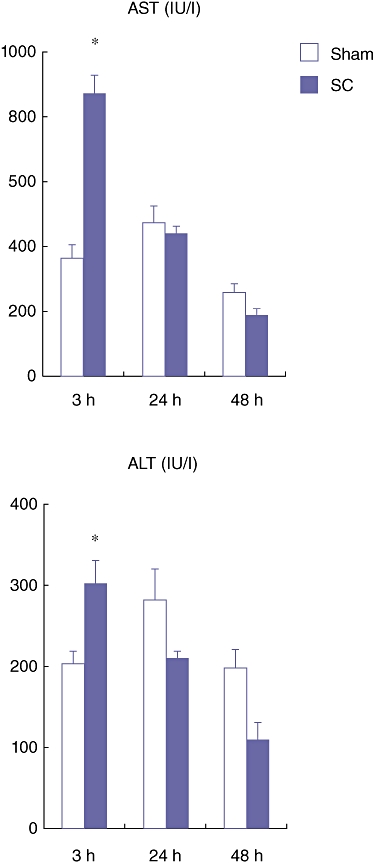
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels at 3, 24 and 48 h after the segmental bile duct ligation (SBDL). Blood samples were obtained from the abdominal aorta. Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test
Real-time RT-PCR for inflammation- and liver regeneration-associated factors after SBDL
Both in the SBDL and non-SBDL lobes, the gene expression of inflammation-associated factors at 3, 24, and 48 h after the SBDL were examined using real-time RT-PCR. At 3 h after the SBDL, the expression of IL-6, TNF-α, iNOS and NF-κB mRNA in the SBDL lobes was markedly increased in the SC group compared with in the Sham group (Fig. 3). However, this difference disappeared at 24 and 48 h after the SBDL. Interestingly, a similar trend was observed in the non-SBDL lobes, in which the liver tissue was not directly exposed to LPS.
Figure 3.

The gene expression of inflammation-associated factors (IL-6, TNF-α, iNOS, and NF-κB) in the livers from both the segmental bile duct ligation (SBDL) lobes and the non-SBDL lobes at 3, 24, and 48 h after the SBDL. Liver samples were harvested at each time point (3, 24 and 48 h) from both the non-SBDL lobes and the SBDL lobes. The average expression level of the non-SBDL lobes in the Sham group at each time point was set to 1.0, and other data were adjusted to this baseline. Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test. IL-6, interleukin-6; TNF-α, tumour necrosis factor-α; iNOS, inducible nitric oxide synthase; NF-κB, nuclear factor-κB
These results strongly imply that in the SC model, altered gene expression in the infected lobes (= the SBDL lobes) parallels that in the non-infected lobes (= the non-SBDL lobes). In a previous clinical review, it was shown that the presence of pre-operative intra-hepatic SC was a major prognostic factor for post-hepatectomy mortality.5 However, it is still unclear whether the resection of the liver lobes can be safely performed after the attack of segmental cholangitis. Thus, in the next series of experiment, the remnant liver function after resecting the SBDL lobes was examined.
Experiment 2 (Hepatectomy after SC-induction)
Serum parameters for hepatic function
At 3, 24 and 48 h after the SBDL, the rats were subjected to 70% PHx of the SBDL lobes. These rats were sacrificed on day 1 and 7 after the PHx (n = 8 at each time point) and blood and liver tissue obtained from the remnant lobes were harvested at each time point. The serum AST and ALT levels in the SC group on day 1 after the PHx were significantly higher than those in the Sham group (Fig. 4). These results indicate that there is more severe hepatic damage after PHx in the SC group compared with in the Sham group.
Figure 4.
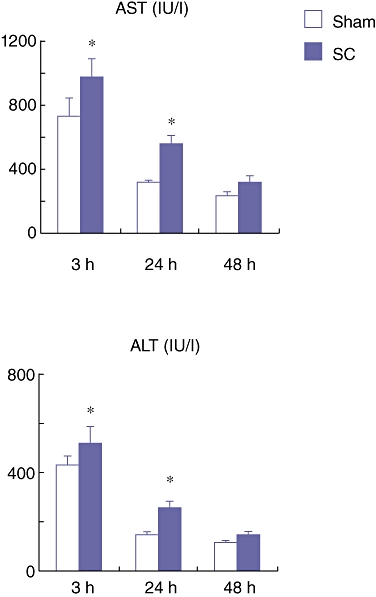
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels on day 1 after partial hepatectomy (PHx) which was performed at 3, 24 and 48 h after the segmental bile duct ligation (SBDL). Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test
Hepatic regeneration rate after PHx
The percentage of newly regenerated liver weight (hepatic regeneration rate) was examined on day 1 and 7 after the PHx. On day 1 after the PHx, the hepatic regeneration rate of the rats in the SC group at 3 h after the SBDL was significantly lower than that in the Sham group, although there was no significant difference between the Sham and the SC group at 24 and 48 h after the SBDL (Fig. 5a). Furthermore, the mortality rate of the rats in the SC group at 3 h after the SBDL was 75% and was significantly higher than in the Sham group (mortality rate; 0%) (Fig. 5b). These results indicate that in the acute phase (3 h) of segmental cholangitis, the liver was placed under severe stress and the onset of hepatic regeneration delayed. Injection of LPS into the peritoneal cavity, with the same dose used for segmental cholangitis, neither induced an impaired hepatic regeneration rate nor lead to mortality, indicating that pathophysiology of SC is different from that of systemic infection (data not shown).
Figure 5.
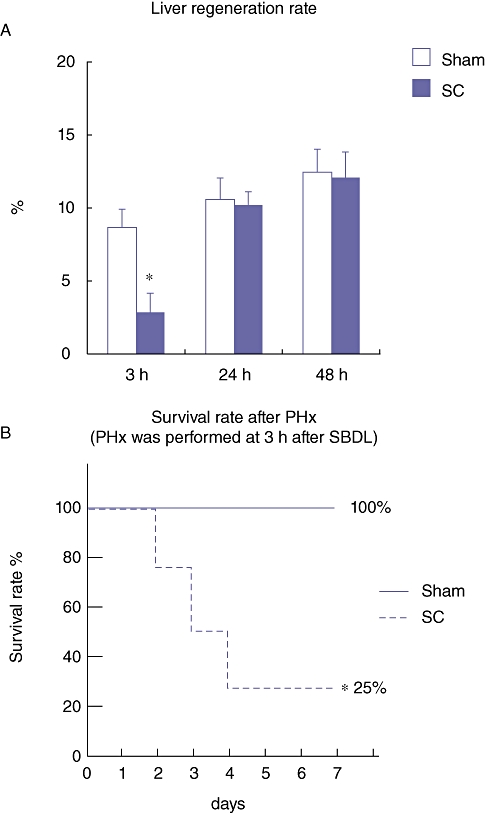
(a) Percentage of the newly regenerated liver weight (hepatic regeneration rate) on day 1 after the partial hepatectomy (PHx), which was performed at 3, 24 and 48 h after the segmental bile duct ligation (SBDL). Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test. (b) Survival rate of rats after the PHx, which was performed at 3 h after the SBDL. Statistical difference between the groups was determined by Kaplan–Meier survival analysis. *P < 0.05 vs. Sham
Mitotic index of the regenerated liver after PHx
The mitotic index in the regenerating liver was also examined on day 1 after the PHx. The mitotic index in the SC group at 3 h after the SBDL was significantly lower than in the Sham group (Fig. 6a–b). These results also imply the delayed onset of hepatic regeneration in the SC rats in the acute phase.
Figure 6.

(a) Mitotic index evaluated by histological examination on day 1 after the partial hepatectomy (PHx), which was performed at 3, 24 and 48 h after the segmental bile duct ligation (SBDL). Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test. (b) Representative micrographs of the remnant liver on day 1 after the PHx, which was performed at 3 h after the SBDL (haematoxylin and eosin staining). Arrows indicate the hepatocytes with mitosis (original magnification × 200). (c) The gene expression of hepatic regeneration-associated factors (hepatic regeneration promoters, HGF and EGF; hepatic regeneration inhibitors, TGF-β and IL-1β) in the liver of the remnant lobes on day 1 after the PHx, which was performed at 3 h after the SBDL. The average expression level of the Sham group's data at each time point was set to 1.0, and other data were adjusted to this baseline. Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test. HGF, hepatocyte growth factor; EGF, epidermal growth factor; TGF-β, transforming growth factor-β; IL-1β, interleukin-1β
Real-time RT-PCR for hepatic regeneration-associated factors after PHx
In the next experiment, the activation of hepatic regeneration-associated factors in the remnant liver was examined on day 1 after the PHx, which was performed at 3 h after the SBDL. The expression of mRNA for hepatic regeneration promoters such as HGF and EGF in the SC group was significantly lower than in the Sham group (Fig. 6c). In contrast, the expression of mRNA for hepatic regeneration inhibitors such as TGF-β1 and IL-1β in the SC group was significantly higher than in the Sham group (Fig. 6c). These results also imply that the presence of SC in the SBDL lobes impairs the regeneration potential of the remnant liver (= non-SBDL lobes).
ELISA for hepatic regeneration-associated factors after PHx
Serum levels of HGF (a promoter of liver regeneration) and IL-1β (an inhibitor of liver regeneration) before the PHx and day 1 after the PHx were examined. Serum HGF levels at 3 h after SBDL in the SC group were significantly lower than in the Sham group (Fig. 7). In sharp contrast, serum IL-1β levels in the SC group were significantly higher than in the Sham group (Fig. 7). These results further indicate that SC suppresses the production of hepato-proliferative factors and increases the production of inhibitors of liver regeneration.
Figure 7.
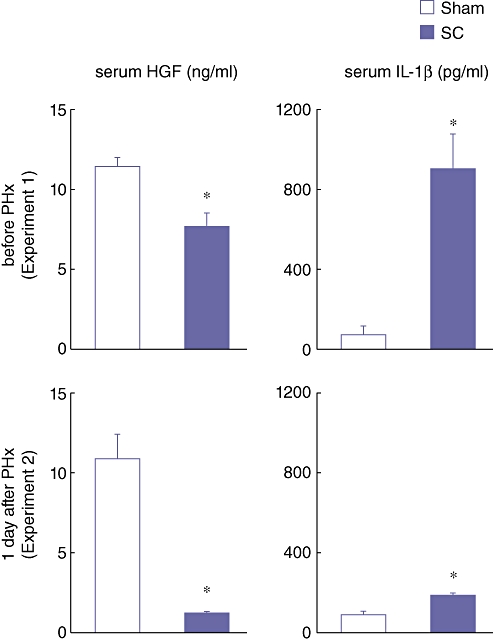
Serum hepatocyte growth factor (HGF) and interleukin-1β (IL-1β) levels at 3 h after the segmental bile duct ligation (SBDL) (before PHx) and on day 1 after the partial hepatectomy (PHx) which was performed at 3 h after the SBDL. Blood samples were obtained from the abdominal aorta. Serum levels of HGF and IL-1β were determined using an enzyme-linked immunosorbent assay (ELISA). Data are means ± SE of eight animals in each group. * P < 0.05 vs. Sham by t-test
Immunohistochemistry and real-time RT-PCR for α-smooth muscle actin (α-SMA)
According to these results, the SC in the SBDL lobes significantly inhibited the production of HGF even in the non-SBDL lobes. However, it is unclear through which mechanism, the production of HGF declined. In the injured liver, non-activated HSCs change to activated HSCs. Under such conditions, HSCs produce more α-smooth muscle actin (α-SMA) and the productivity of HGF is attenuated.12 HSCs are known to be located in the space of Disse, below the sinusoidal endothelial cells lining.21 Therefore, in the next experiment, the expression of α-SMA in both the SBDL and non-SBDL lobes at 3 h after the SBDL and real-time RT-PCR were examined using immunohistochemistry. At 3 h after the SBDL, the expression of α-SMA around the sinusoid was markedly increased in the SC group compared with that in the Sham group both in the SBDL and non-SBDL lobes (Fig. 8a). Furthermore, a similar trend was observed by real-time RT-PCR for α-SMA (Fig. 8b). These results further indicate that SC activates HSCs even in the contralateral liver segment and decreases the productivity of HGF in these cells.
Figure 8.
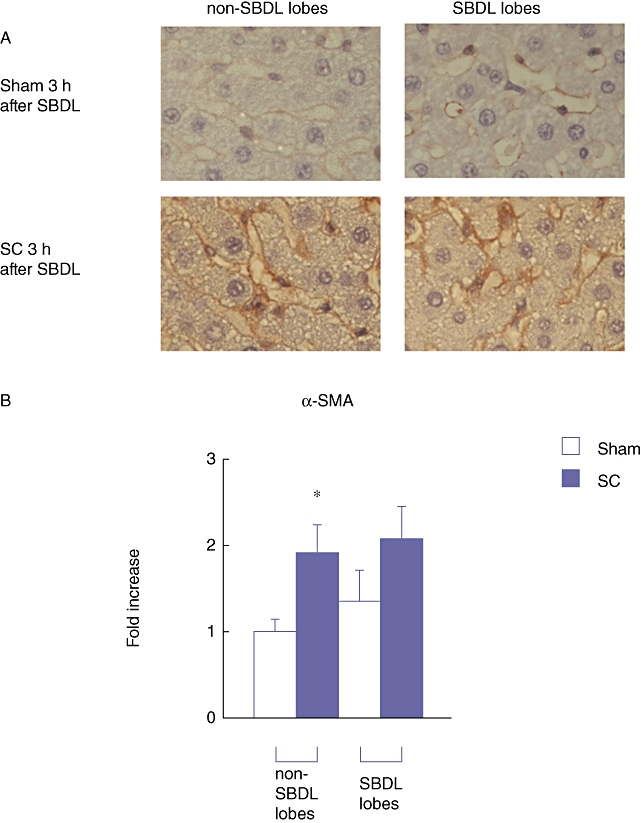
(a) Immunohistochemistry forα-smooth muscle actin (α-SMA) protein in the liver at 3 h after the segmental bile duct ligation (SBDL) (original magnification × 400). (b) The gene expression of α-SMA in the livers from both the SBDL lobes and the non-SBDL lobes 3 h after the SBDL. The average expression level of the non-SBDL lobes in the Sham group at each time point was set to 1.0, and other data were adjusted to this baseline. Data are means ± SE of eight animals in each group. *P < 0.05 vs. Sham by t-test
Discussion
Patients with hilar cholangiocarcinoma or hepatolithiasis occasionally develop SC as a result of partial obstruction of the biliary branch and bacterial contamination. However, it is necessary to perform major hepatectomy and caudate lobectomy with biliary resection for these diseases. In such case, whether SC becomes a negative prognostic factor for major hepatectomy is questionable, because there is no well-controlled clinical study that has evaluated the safety of major hepatectomy for patients with SC. Moreover, no information is available on the hepatic regeneration capacity in patients with SC after major hepatectomy. In this regard, the results of the present study first of all have established and characterized a rat SC model and thus provide useful information.
In the present study, it was demonstrated that in the rats with SC, especially in the acute phase, biochemical assays of liver injury were significantly higher than in the Sham group. Furthermore, in the SC group, the expression of liver regeneration-inhibiting genes was significantly higher than in the Sham group especially in the acute phase (3 h after SBDL). For another experiment, PHx was performed. In the SC group in the acute phase, the regeneration rate after the PHx was significantly lower than in the Sham group. Moreover, the mortality rate was significantly higher in the SC group than in the Sham group. Interestingly, these effects were not observed when LPS was injected into the peritoneal cavity, indicating that the pathophysiology of SC is different from systemic infection. These results also indicate that SC can be a deteriorating factor for liver regeneration and can be a strong negative prognostic factor for major hepatectomy.
It is widely known that endotoxin activates hepatic stellate cells (HSCs)22 and then activated HSCs (identified by positive staining for α-SMA) produce TGF-β1, a potent growth inhibitor of hepatocytes.23,24 Additionally, the activated HSCs have been shown to lose ability to produce HGF,13,25 a major liver regeneration promoting factor. There are other liver regeneration regulating factors including EGF as a liver regeneration promoting factor and IL-1β20 as liver regeneration inhibiting factors. In the present study, we found a significantly lower expression of liver regeneration-promoting genes (HGF and EGF mRNA) and a significantly higher expression of liver regeneration-suppressing genes (TGF-β1 and IL-1β mRNA) in the liver in the SC group than in the Sham group. Moreover, significantly lower serum HGF levels and significantly higher serum IL-1β levels in the SC group than in the Sham group were also observed. Taken together, it is evident that SC, even if it occurs only in the SBDL lobes and those lobes are removed by the partial hepatectomy, impairs the overall regenerating capacity of the remnant liver (the non-SBDL lobes). It could be speculated that this change is what led to a significantly higher mortality rate after partial hepatectomy in the SC group as compared with the Sham group. One of the triggers of the deteriorated liver regeneration in the SC group may be the activation of HSCs; this theory was indirectly confirmed by increased expression of α-SMA around the sinusoidal lining.
Although it was unable to determine the mechanism by which SC not only influences the infected lobes but also influences the non-infected lobes, it is not conceivable that LPS infused into the bile duct is directly drained into the systemic circulation as the volume of infusion is very low. Therefore, our SC model is different from that with systemic LPS administration. An inflammatory response of acute obstructive cholangitis can be a major cause of systemic inflammatory response syndrome (SIRS).26,27 In the opposite direction, SIRS from various diseases such as acute pancreatitis, urinary tract infection and peritonitis results in liver failure.28 Therefore, it is conceivable that the infected lobes induced by SC began to release mediators that affect the function of the non-infected lobes. The precise mechanism should be elucidated in future studies.
It could be argued that the infusion of LPS instead of bacteria into the bile duct might fail to fully reproduce clinical cholangitis. While Escherichia coli is the most frequent biliary pathogen isolated in patients with acute cholangitis in both western and eastern countries.29,30 However, other species such as Klebsiella (another Gram-negative rod) are also commonly isolated in patients with acute cholangitis.31 Furthermore, in many animal sepsis models, the LPS models show trends similar to those in models using Escherichia coli.32,33 Therefore, in this study, LPS (a common pathogen found in both Escherichia coli and Klebsiella) was used as a representative antigen responsible for cholangitis.
In summary, the present study clearly demonstrated that segmental cholangitis, especially in the acute phase (3 h), impairs the hepatic regeneration capacity of the remnant liver after partial hepatectomy. Therefore, hepatectomy should be avoided in patients with acute SC even if it occurs on the side of the liver resection.
Conflicts of interest
None declared.
References
- 1.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386–393. doi: 10.1016/j.surg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagino M, Nimura Y, Hayakawa N, Kamiya J, Kondo S, Sasaki R, et al. Logistic regression and discriminant analyses of hepatic failure after liver resection for carcinoma of the biliary tract. World J Surg. 1993;17:250–255. doi: 10.1007/BF01658937. [DOI] [PubMed] [Google Scholar]
- 5.Kanai M, Nimura Y, Kamiya J, Kondo S, Nagino M, Miyachi M, et al. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. doi: 10.1016/s0039-6060(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 6.Gong JP, Wu CX, Liu CA, Li SW, Shi YJ, Li XH, et al. Liver sinusoidal endothelial cell injury by neutrophils in rats with acute obstructive cholangitis. World J Gastroenterol. 2002;8:342–345. doi: 10.3748/wjg.v8.i2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Wu SD. Activation of TLR-4 and liver injury via NF-kappa B in rat with acute cholangitis. Hepatobiliary Pancreat Dis Int. 2008;7:185–191. [PubMed] [Google Scholar]
- 8.Kimmings AN, van Deventer SJ, Obertop H, Rauws EA, Huibregtse K, Gouma DJ. Endotoxin, cytokines, and endotoxin binding proteins in obstructive jaundice and after preoperative biliary drainage. Gut. 2000;46:725–731. doi: 10.1136/gut.46.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calandra T, Baumgartner JD, Grau GE, Wu MM, Lambert PH, Schellekens J, et al. Prognostic values of tumor necrosis factor/cachectin, interleukin-1, interferon-alpha, and interferon-gamma in the serum of patients with septic shock. Swiss-Dutch J5 Immunoglobulin Study Group. J Infect Dis. 1990;161:982–987. doi: 10.1093/infdis/161.5.982. [DOI] [PubMed] [Google Scholar]
- 10.Weiss YG, Bellin L, Kim PK, Andrejko KM, Haaxma CA, Raj N, et al. Compensatory hepatic regeneration after mild, but not fulminant, intraperitoneal sepsis in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280:G968–G973. doi: 10.1152/ajpgi.2001.280.5.G968. [DOI] [PubMed] [Google Scholar]
- 11.Seehofer D, Stockmann M, Schirmeier A, Nussler AK, Cho SY, Rayes N, et al. Intraabdominal bacterial infections significantly alter regeneration and function of the liver in a rat model of major hepatectomy. Langenbecks Arch Surg. 2007;392:273–284. doi: 10.1007/s00423-007-0169-2. [DOI] [PubMed] [Google Scholar]
- 12.Michalopoulos GK, Zarnegav R. Hepatocyte growth factor. Hepatology. 1992;15:149–155. doi: 10.1002/hep.1840150125. [DOI] [PubMed] [Google Scholar]
- 13.Schirmacher P, Geerts A, Pietrangelo A, Dienes HP, Rogler CE. Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. [DOI] [PubMed] [Google Scholar]
- 14.Maher JJ. Cell-specific expression of hepatocyte growth factor in liver. Upregulation in sinusoidal endothelial cells after carbon tetrachloride. J Clin Invest. 1993;91:2244–2252. doi: 10.1172/JCI116451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- 16.Tucker RF, Shipley GD, Moses HL, Holley RW. Growth inhibitor from BSC-1 cells closely related to platelet type beta transforming growth factor. Science. 1984;226:705–707. doi: 10.1126/science.6093254. [DOI] [PubMed] [Google Scholar]
- 17.Carr BI, Hayashi I, Branum EL, Moses HL. Inhibition of DNA synthesis in rat hepatocytes by platelet-derived type beta transforming growth factor. Cancer Res. 1986;46:2330–2334. [PubMed] [Google Scholar]
- 18.Kyokane T, Nagino M, Oda K, Nimura Y. An experimental study of selective intrahepatic biliary ablation with ethanol. J Surg Res. 2001;96:188–196. doi: 10.1006/jsre.2001.6081. [DOI] [PubMed] [Google Scholar]
- 19.Selzner M, Clavien PA. Failure of regeneration of the steatotic rat liver: disruption at two different levels in the regeneration pathway. Hepatology. 2000;31:35–42. doi: 10.1002/hep.510310108. [DOI] [PubMed] [Google Scholar]
- 20.Furutani M, Arii S, Monden K, Adachi Y, Funaki N, Higashitsuji H, et al. Immunologic activation of hepatic macrophages in septic rats: a possible mechanism of sepsis-associated liver injury. J Lab Clin Med. 1994;123:430–436. [PubMed] [Google Scholar]
- 21.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 22.Quiroz SC, Bucio L, Souza V, Hernandez E, Gonzalez E, Gomez-Quiroz L, et al. Effect of endotoxin pretreatment on hepatic stellate cell response to ethanol and acetaldehyde. J Gastroenterol Hepatol. 2001;16:1267–1273. doi: 10.1046/j.1440-1746.2001.02619.x. [DOI] [PubMed] [Google Scholar]
- 23.Russell WE, Coffey RJ, Jr, Ouellette AJ, Moses HL. Type beta transforming growth factor reversibly inhibits the early proliferative response to partial hepatectomy in the rat. Proc Natl Acad Sci U S A. 1988;85:5126–5130. doi: 10.1073/pnas.85.14.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akita K, Okuno M, Enya M, Imai S, Moriwaki H, Kawada N, et al. Impaired liver regeneration in mice by lipopolysaccharide via TNF-alpha/kallikrein-mediated activation of latent TGF-beta. Gastroenterology. 2002;123:352–364. doi: 10.1053/gast.2002.34234. [DOI] [PubMed] [Google Scholar]
- 25.Corpechot C, Barbu V, Wendum D, Chignard N, Housset C, Poupon R, et al. Hepatocyte growth factor and c-Met inhibition by hepatic cell hypoxia: a potential mechanism for liver regeneration failure in experimental cirrhosis. Am J Pathol. 2002;160:613–620. doi: 10.1016/S0002-9440(10)64881-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimmings AN, van Deventer SJ, Rauws EAJ, Huibregtse K, Gouma DJ. Systemic inflammatory response in acute cholangitis and after subsequent treatment. Eur J Surg. 2000;166:700–705. doi: 10.1080/110241500750008457. [DOI] [PubMed] [Google Scholar]
- 27.Lillemoe KD. Surgical treatment of biliary tract infections. Am Surg. 2000;66:138–144. [PubMed] [Google Scholar]
- 28.Gray KD, Simovic MO, Chapman WC, Blackwell TS, Christman JW, Washington MK, et al. Systemic nf-kappaB activation in a transgenic mouse model of acute pancreatitis. J Surg Res. 2003;110:310–314. doi: 10.1016/s0022-4804(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 29.Jain MK, Jain R. Acute bacterial cholangitis. Curr Treat Options Gastroenterol. 2006;9:113–121. doi: 10.1007/s11938-006-0030-7. [DOI] [PubMed] [Google Scholar]
- 30.Westphal JF, Brogard JM. Biliary tract infections: a guide to drug treatment. Drugs. 1999;57:81–91. doi: 10.2165/00003495-199957010-00007. [DOI] [PubMed] [Google Scholar]
- 31.Lipsett PA, Pitt HA. Acute cholangitis. Surg Clin North Am. 1990;70:1297–1312. doi: 10.1016/s0039-6109(16)45285-0. [DOI] [PubMed] [Google Scholar]
- 32.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An A2A adenosine receptor agonist, ATL313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roger T, Froidevaux C, Le Roy D, Reymond MK, Chanson AL, Mauri D, et al. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc Natl Acad Sci U S A. 2009;106:2348–2352. doi: 10.1073/pnas.0808146106. [DOI] [PMC free article] [PubMed] [Google Scholar]


