Abstract
Background:
Tumour-infiltrating lymphocytes (TILs) have been shown to predict survival in numerous malignancies. The importance of TILs in primary pancreatic neuroendocrine tumours (NETs) and NET liver metastases (NETLMs) has not been defined.
Methods:
We identified 87 patients with NETs and 39 with NETLMs who had undergone resection. Immunohistochemistry was performed to determine TIL counts. Recurrence-free survival (RFS) and overall survival (OS) were determined using the log-rank test.
Results:
The median follow-up time was 62 months in NET patients and 48 months in NETLM patients. Vascular invasion and histologic grade were the only independent predictors of outcome for NETs and NETLMs, respectively. Analysis of intermediate-grade NETs indicated that a dense T cell (CD3+) infiltrate was associated with a median RFS of 128 months compared with 61 months for those with low levels of intratumoral T cells (P = 0.05, univariate analysis). Examination of NETLMs revealed that a low level of infiltrating regulatory T cells (Treg, FoxP3+) was a predictor of prolonged survival (P < 0.01, univariate analysis).
Conclusions:
A robust T cell infiltrate is associated with improved RFS following resection of intermediate-grade NETs, whereas the presence of more Treg correlated with shorter OS after treatment of NETLMs. Further study of the immune response to intermediate-grade NETs and NETLMs is warranted.
Keywords: pancreatic tumour, neuroendocrine tumour, liver metastasis, T cell
Introduction
Well-differentiated pancreatic neuroendocrine tumours (NETs) are rare and manifest with a broad spectrum of biologic behaviour. Annual incidence in the USA amounts to four cases per million people and survival rates at 5 years have been reported to range from 44% to 71%.1–4 Neuroendocrine tumour liver metastases (NETLMs) are a frequent cause of death in patients with pancreatic or gastrointestinal NETs.5 Despite median survival times of 81–96 months in patients selected for surgical resection of NETLMs, the majority suffer from recurrent or progressive disease.6–8 Numerous correlates of outcome following resection of NETs and NETLMs, including histologic grade, surgical margin status, vascular invasion, tumour size and the presence of metastases, have been described.9–14 Among prognostic factors for NETs and NETLMs, histologic grade, as determined by the mitotic rate and presence of necrosis, has been shown to be a powerful surrogate for tumour biology and patient outcome.6,10,12 Despite the identification of important prognostic factors for NETs and NETLMs, coping with the marked degree of biologic heterogeneity in attempting to determine prognosis or optimal treatment remains challenging.15
Tumour-infiltrating lymphocytes (TILs) have been shown to predict outcome in patients with hepatocellular carcinoma,16,17 colorectal cancer,18–21 and ovarian cancer.22 Although the prognostic importance of TILs in numerous human malignancies has been documented, the utility of TILs for risk stratifying individuals with neuroendocrine neoplasia is unclear. The rarity of NETs and the generally favourable prognosis for those patients with low-grade tumours are likely explanations for the paucity of data with respect to the impact of TILs on this disease. Yet, prognoses for patients with intermediate-grade NETs or those with NETLMs vary considerably. Study of the host immune response may enable the identification of patients at elevated risk for recurrence following treatment and the selection of patients most likely to experience prolonged survival following aggressive intervention. The present study was designed to determine the potential prognostic importance of TILs in NETs and NETLMs.
Materials and methods
Patients and clinical variables
With the approval of the institutional review board and in accordance with Health Insurance Portability and Accountability Act regulations, a prospectively maintained database was used. We identified 87 patients with NETs and 39 with NETLMs who underwent resection at the Memorial Sloan-Kettering Cancer Center (MSKCC), New York, during 1992–2004 and from whom tissue adequate for analysis was available. Clinicopathologic data on the patients in this study have been previously reported.10,12 Only three patients overlapped between the NET and NETLM datasets. Given the rarity of NETs and NETLMs, we included NETLMs from various primary sites.
Pathologic variables and immunohistochemistry
After review of the archived primary tumour haematoxylin and eosin (H&E) slides and diagnostic confirmation, sections were deparaffinized, rehydrated in graded alcohol, and processed as previously described.23 Monoclonal antibodies were used to detect CD3 (F7.2.38; Dako North America, Inc., Carpinteria, CA, USA), CD8 (c8/144B; Dako North America, Inc.), FoxP3 (236A/E7; Abcam, Inc., Cambridge, MA, USA) and CD4 (polyclonal goat; R&D Systems, Inc., Minneapolis, MN, USA). Appropriate positive controls were used. Samples were then incubated with biotinylated anti-mouse immunoglobulins at a dilution of 1:500 (Vector Laboratories, Inc., Burlingame, CA, USA), followed by avidin–biotin peroxidase complexes (1:25; Vector Laboratories, Inc.) for 30 min. Diaminobenzidine was used as the chromagen and haematoxylin was used as the nuclear counter stain.
Tumours were assigned a histologic grade on the basis of mitotic figure count and degree of tumour necrosis.10,12 Tumour-infiltrating lymphocytes were counted and scored by a single pathologist (LHT) on a scale of 0–3 based on cell density within the tumours. Scores were based on the following criteria: grade 0 = < 10 cells per 10 highpower fields (hpf); grade 1 = < 1% or 10–20 cells per 10 hpf; grade 2 = 1–5% or 21–50 cells per 10 hpf, and grade 3 = > 5% or > 50 cells per 10 hpf (Fig. 1). Cut-off points for analyses were assigned based upon the median score for each marker. We counted intratumoral T cells only, as representative sections of the interface between tumour and normal tissue were not uniformly present, an approach that had been previously validated.18
Figure 1.
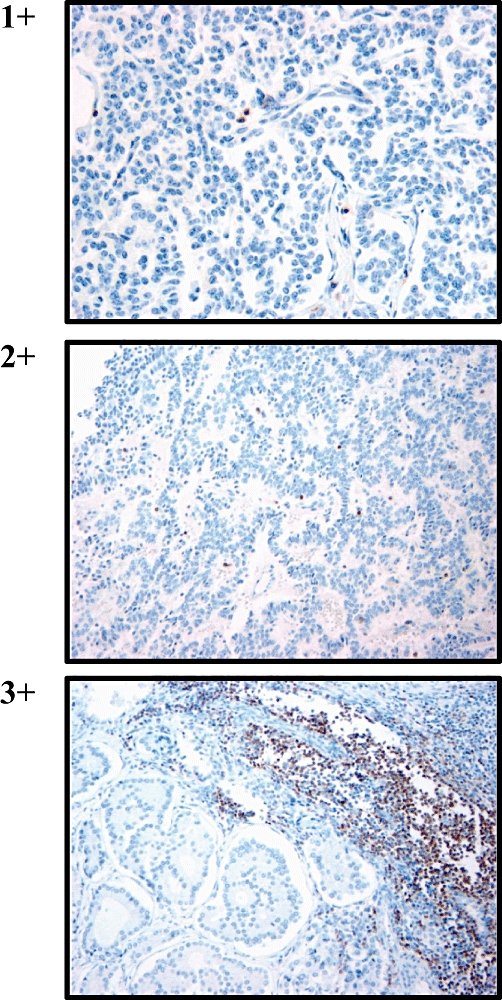
Neuroendocrine tumours were stained with anti-CD3 to identify infiltrating T cells. Grading levels were assigned according to the following criteria per 10 highpower fields: grade 0 = < 10 cells; grade 1 = < 1% or 10–20 cells; grade 2 = 1–5% or 21–50 cells, and grade 3 = > 5% or >50 cells. (Haematoxylin and eosin stain; original magnification 400×)
Statistical analysis
A two-sample t-test was used to compare the distribution of continuous variables across groups when the distribution was reasonably symmetric. When the variables to be compared had skewed distributions, the Mann–Whitney U-test was used. The chi-squared test was used for categorical variables. The date of disease recurrence was defined as the date of earliest radiographic evidence of new tumour formation. Overall survival (OS) and recurrence-free survival (RFS) probabilities were estimated using the Kaplan–Meier method. Univariate associations were assessed using the log-rank test; variables with P ≤ 0.05 on univariate analysis were included by multivariate analysis using a Cox model. Statistical analyses were performed using spss Version 15.0 (SPSS, Inc., Chicago, IL, USA).
Results
Primary and metastatic tumour patient characteristics and follow-up
We analysed 87 patients with primary NETs who underwent resection at MSKCC to determine if the degree of T cell infiltration would correlate with either RFS or OS. The median follow-up for the entire group was 62 months and the median RFS was 106 months; the median OS was not reached. The average age of the group was 56 years (range: 19–85 years) and 46% of the patients were male (Table 1). Intermediate- and low-grade tumours were analysed separately to determine the biologic and immunologic differences between the two groups. Those with intermediate-grade tumours presented at a significantly younger age (median age: 53 years) compared with patients with low-grade lesions (median age: 64 years) (P = 0.04). Functional tumours were present in 14% of the patients and tumour function did not correlate with tumour grade. The majority of patients underwent distal pancreatectomy (54%) or pancreaticoduodenectomy (33%), as opposed to enucleation (13%). Intermediate-grade tumours were significantly more likely to be associated with vascular invasion (P = 0.001) or to present with synchronous liver metastases (P = 0.01). Lymph node metastases were more common among patients with intermediate-grade lesions, but this was not significant (P = 0.13). Patients with nodal metastases were significantly more likely to have larger primary tumours (P = 0.04) when only those who underwent lymphadenectomy were considered.
Table 1.
Clinicopathologic variables in primary pancreatic neuroendocrine tumours
| All | Low grade | Intermediate grade | P-value | |
|---|---|---|---|---|
| Age, years | ||||
| Median | 56 | 64 | 53 | 0.04 |
| Range | 19–85 | |||
| Gender | ||||
| Male | 46% | 40% | 56% | 0.12 |
| Female | 54% | 60% | 44% | |
| Tumour sizea | ||||
| ≥4.2 cm | 46% | 42% | 53% | 0.09 |
| <4.2 cm | 54% | 58% | 47% | |
| Surgical margin | ||||
| Positive | 15% | 8% | 23% | 0.07 |
| Negative | 85% | 92% | 77% | |
| Vascular invasion | ||||
| Present | 46% | 31% | 68% | 0.001 |
| Absent | 54% | 69% | 32% | |
| Functional | ||||
| Yes | 14% | 17% | 8% | 0.25 |
| No | 86% | 83% | 92% | |
| Gradeb | ||||
| Intermediate | 43% | – | – | – |
| Low | 54% | |||
| Pancreatic resection | ||||
| Enucleation | 13% | 17% | 8% | 0.41 |
| Distal pancreatectomy | 54% | 50% | 61% | |
| Pancreaticoduodenectomy | 33% | 33% | 31% | |
| Lymph node sampling | ||||
| Yes | 70% | 71% | 72% | 0.84 |
| No | 30% | 29% | 28% | |
| Lymph node metastases | ||||
| Present | 27% | 20% | 39% | 0.13 |
| Absent | 73% | 80% | 61% | |
| Synchronous liver metastases | ||||
| Present | 16% | 6% | 29% | 0.01 |
| Absent | 84% | 94% | 71% | |
| CD3 | ||||
| High (1–3) | 68% | 75% | 64% | 0.19 |
| Low (0) | 32% | 25% | 36% | |
| CD4 | ||||
| High (1–3) | 65% | 64% | 69% | 0.76 |
| Low (0) | 35% | 36% | 31% | |
| CD8 | ||||
| High (0–1) | 45% | 49% | 44% | 0.54 |
| Low (2–3) | 55% | 51% | 56% | |
| FoxP3 | ||||
| High (1–3) | 34% | 33% | 36% | 0.25 |
| Low (0) | 66% | 67% | 64% |
Median tumour size was 4.2 cm
3% were high grade
For the 39 patients with NETLMs, the median follow-up was 48 months and the median OS was 63 months. The average age of patients with NETLMs was 53 years (range: 25–83 years) and 67% were female (Table 2). Most patients were symptomatic, the majority had bilateral NETLMs, and the pancreas was the most common verified primary tumour site. The median liver tumour size was 8.5 cm (range: 1.0–22.3 cm) and the median number of liver metastases was two.
Table 2.
Clinicopathologic variables in neuroendocrine tumour liver metastases
| All | |
|---|---|
| Age, years | |
| Median | 53 |
| Range | 25–84 |
| Tumour size, cm | |
| Median | 8.5 |
| Range | 1.0–22.3 |
| Tumour number | |
| Median | 2 |
| Range | 1–11 |
| Gender | |
| Male | 13 (33%) |
| Female | 26 (67%) |
| Margin | |
| R0 | 11 (28%) |
| R1 | 11 (28%) |
| R2 | 15 (39%) |
| Grade | |
| Low | 19 (49%) |
| Intermediate | 13 (33%) |
| High | 7 (18%) |
| Primary site | |
| Pancreas | 11 (28%) |
| Small bowel | 8 (21%) |
| Unknown | 14 (36%) |
| Other | 6 (15%) |
| Symptoms | |
| Present | 28 (72%) |
| Octreotide therapy | |
| Yes | 11 (28%) |
| Chemotherapy | |
| Yes | 10 (26%) |
| Embolization | |
| Yes | 15 (39%) |
| CD3 | |
| High (2–3) | 23 (59%) |
| Low (0–1) | 16 (41%) |
| CD4 | |
| High (3) | 21 (54%) |
| Low (0–2) | 18 (46%) |
| CD8 | |
| High (2–3) | 22 (56%) |
| Low (0–1) | 17 (44%) |
| FoxP3 | |
| High (1–3) | 13 (33%) |
| Low (0) | 26 (67%) |
Degree of primary and metastatic tumour lymphocyte infiltration
Immunohistochemical staining revealed that T cells (CD3+) infiltrated the majority (68%) of NETs (Fig. 1). Only 34% of patients were found to have FoxP3+ TILs, which are regulatory T cells (Treg).24 Tumour grade did not correlate with the degree of lymphocytic infiltration when assessed with CD3, CD4, CD8 or FoxP3 (Table 1). Additionally, the degree of T cell infiltration was not associated with any of the other clinicopathologic variables analysed. The vast majority of patients with NETLMs (97%) had some degree of T cell (CD3+) infiltration and Treg were detected in 33%. Potentially immunosuppressive Treg were present in 55% of intermediate/high-grade tumours, whereas only 16% of low-grade NETLMs demonstrated intratumoral Treg (P = 0.02).
Outcome following resection of primary NETs is predicted by histologic features
In addition to determining the prognostic importance of TILs in NETs, we analysed standard clinicopathologic correlates of outcome in our selected patient group. Larger tumour size, nodal metastases, vascular invasion, higher tumour grade and positive surgical margins were predictors of RFS (Table 3). Among these factors, vascular invasion was an independent predictor of disease recurrence (relative risk [RR] = 6.7, P = 0.05). T cell counts did not correlate with recurrence following resection of NETs when the entire patient group was analysed (Fig. 2).
Table 3.
Predictors of recurrence-free survival in neuroendocrine tumours
| Variables | Median RFS, months | P-value | Multivariate |
|---|---|---|---|
| RR (95%CI), P-value | |||
| Tumour sizea | |||
| ≥4.2 cm | 97 | 0.005 | 0.06 |
| <4.2 cm | 135 | ||
| Lymph node metastasesb | |||
| Present | 67 | 0.006 | 0.46 |
| Absent | 128 | ||
| Vascular invasion | |||
| Present | 72 | <0.001 | 8.2 (1.8–37.0), 0.007 |
| Absent | NR | ||
| Gradec | |||
| Intermediate | 65 | 0.02 | 0.61 |
| Low | 113 | ||
| Surgical margin | |||
| Positive | 66 | 0.007 | 0.50 |
| Negative | 128 | ||
| CD3 | |||
| High | 99 | 0.52 | – |
| Low | 135 | ||
| CD8 | |||
| High | 106 | 0.68 | – |
| Low | 113 | ||
| CD4 | |||
| High | 99 | 0.53 | – |
| Low | 128 | ||
| FoxP3 | |||
| High | 98 | 0.24 | – |
| Low | 116 |
Median tumour size was 4.2 cm
Only patients who underwent lymph node sampling were included
Intermediate grade defined by ≥1 mitotic figure/50 highpower fields
RFS, recurrence-free survival; RR, relative risk; 95% CI, 95% confidence interval; NR, not reported
Figure 2.
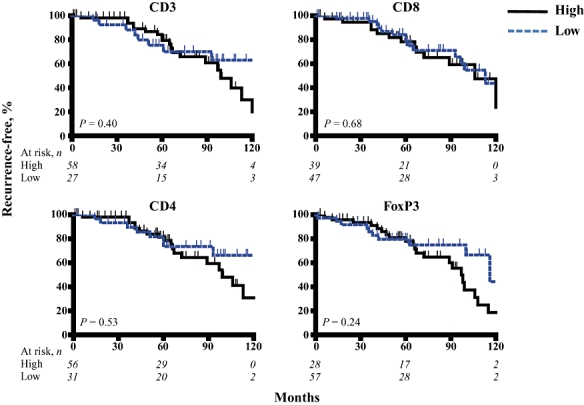
Neuroendocrine tumours were stained with anti-CD3 (all T cells), anti-CD8 (cytotoxic T cells), anti-CD4 (helper T cells), and anti-FoxP3 (regulatory T cells) to quantify the numbers of various T cell subsets within the tumours. Kaplan–Meier analyses were performed to determine differences in recurrence-free survival among patients with high and low levels of T cell infiltration
T cell count predicts recurrence in intermediate-grade NET
As discussed above, NETs encompass a broad spectrum of pathobiology and tumour grade as determined by the mitotic rate and presence of necrosis.10,12 A prior report demonstrated that NETs can be stratified into biologically distinct groups on the basis of grade.12 To determine the biologic relevance of TILs in NETs among patients with more aggressive disease biology, we performed separate analyses for patients with low- and intermediate-grade tumours. Among patients with low-grade NETs, there was no association between T cell infiltrates and outcome. However, when analysing only those with intermediate-grade neoplasms, the T cell infiltrate (CD3+ TILs) was a significant univariate predictor of recurrence following resection (Table 4, Fig. 3).
Table 4.
Predictors of recurrence-free survival in intermediate-grade neuroendocrine tumours
| Variables | Median RFS, months | P-value | Multivariate RR (95%CI), P-value |
|---|---|---|---|
| Tumour sizea | |||
| ≥4.2 cm | 72 | 0.07 | – |
| <4.2 cm | 130 | ||
| Lymph node metastasesb | |||
| Present | 61 | 0.02 | 0.11 |
| Absent | 128 | ||
| Vascular invasion | |||
| Present | 67 | 0.02 | 0.95 |
| Absent | 130 | ||
| Surgical margin | |||
| Positive | 67 | 0.10 | – |
| Negative | 128 | ||
| CD3 | |||
| High | 128 | 0.05 | 0.23 |
| Low | 61 | ||
| CD8 | |||
| High | 128 | 0.27 | – |
| Low | 61 | ||
| CD4 | |||
| High | 106 | 0.19 | – |
| Low | 60 | ||
| FoxP3 | |||
| High | 106 | 0.65 | – |
| Low | 72 |
Median tumour size was 4.2 cm
Only patients who underwent lymph node sampling were included
RFS, recurrence-free survival; RR, relative risk; 95% CI, 95% confidence interval
Figure 3.
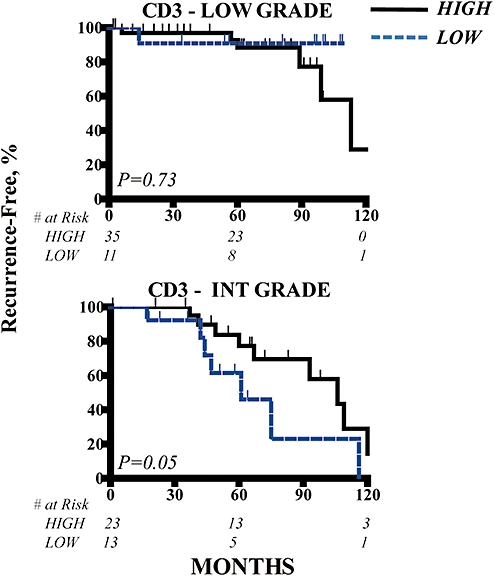
Low- and intermediate-grade neuroendocrine tumours (NETs) were stained with anti-CD3 to quantify the numbers of various T cell subsets within the tumours. Kaplan–Meier analyses were performed to determine differences in recurrence-free survival among patients with high and low levels of T cell infiltration. Thirteen patients without documented grade information were excluded from this analysis
Treg predict outcome following resection of NETLMs
Having found that recurrence following resection of intermediate-grade NETs correlated with the degree of T cell infiltration, we examined TILs in NETLMs, which are more likely to originate from higher-grade primary tumours. Given that many patients with NETLMs underwent debulking procedures for symptoms and that the complete eradication of liver tumours was not possible (Table 2), we chose OS instead of RFS as the endpoint for our analysis of NETLMs. Histologic grade (Table 5) was a significant, independent predictor of OS following resection of NETLMs (P > 0.001). The degree of infiltration by CD3+, CD4+ or CD8+ TILs did not predict OS following treatment of NETLMs. However, the presence of FoxP3+ cells or Treg within NETLMs was significantly correlated with shorter OS times on univariate analysis (P = 0.03).
Table 5.
Predictors of overall survival in neuroendocrine tumour liver metastases
| Variables | Median OS, months | P-value | Multivariate RR (95%CI), P-value |
|---|---|---|---|
| Grade | |||
| High | 6 | <0.001 | 9.5 (3.0–29.9), <0.001 |
| Intermediate | 48 | 5.9 (2.0–17.2), 0.001a | |
| Low | NR | ||
| Blood lossb | |||
| High | 61 | 0.96 | – |
| Low | 67 | ||
| Margins | |||
| Positive | 63 | 0.68 | – |
| Negative | 61 | ||
| Largest tumour size | |||
| ≥8.5 cm | 61 | 0.44 | – |
| <8.5 cm | 67 | ||
| Liver tumour number | |||
| ≥2 | 48 | 0.99 | |
| <2 | 63 | ||
| CD3 | |||
| High | 61 | 0.29 | – |
| Low | 108 | ||
| CD8 | |||
| High | 47 | 0.21 | – |
| Low | 67 | ||
| CD4 | |||
| High | 48 | 0.36 | – |
| Low | 103 | ||
| FoxP3 | |||
| High | 47 | 0.029 | 0.08 |
| Low | 108 |
Separate multivariate model for intermediate or high grade vs. low grade
Median blood loss volume used a cut-off point
OS, overall survival; RR, relative risk; 95% CI, 95% confidence interval; NR, not reported
Discussion
This is the largest study to date to assess the correlation between T cell infiltration and outcome following resection of well-differentiated pancreatic NETs and NETLMs. Consistent with a prior report,25 we have demonstrated the presence of T cells within the majority of NETs. The degree of T cell infiltration as measured by CD3 staining was associated with RFS in patients with intermediate-grade NETs. Although higher levels of CD4 and CD8 T cell infiltration trended toward an association with improved RFS in patients with intermediate-grade tumours, these results were not statistically significant. A higher number of Treg was associated with decreased survival following treatment of NETLMs.
Tumour-infiltrating lymphocytes have been shown to predict outcome in numerous primary human malignancies, including melanoma, pancreatic adenocarcinoma, ovarian carcinoma, hepatocellular carcinoma and colorectal cancer.18,20,21 Ryschich and colleagues reported that a moderate degree of T cell infiltration was present in eight of 11 patients with NETs.25 Our interest in NETLMs TILs was based upon our recent report demonstrating the predictive power of intratumoral T cells following resection of colorectal cancer liver metastases.20 We have confirmed that the majority of NETs and NETLMs are infiltrated by T cells. In the present study, 68% of patients with NETs and 97% of those with NETLMs were found to have T cells within their tumours (Tables 1 and 2). The degree of NET T cell infiltration did not correlate with other variables tested, including grade, metastases, vascular invasion and tumour size. In NETLMs, higher-grade tumours were more likely to demonstrate high levels of infiltration by Treg. A correlation between tumour grade and Treg infiltration has been reported in primary hepatic neoplasms.26
When all patients with NETs were analysed, the degree of T cell infiltration did not correlate with recurrence. In recognition of the distinct biologic behaviour of low- and intermediate-grade NETs,10 we analysed these subgroups separately, as the challenge is to identify those with intermediate-grade tumours who will do poorly. It is reasonable to speculate that the immunologic and biologic impact of infiltrating T cells would be more important in inherently aggressive tumours. The rarity of NETs and the protracted recurrence-free intervals in patients with low-grade NETs are likely to render the impact of infiltrating T cells imperceptible or irrelevant (Fig. 2). However, in patients with intermediate-grade primary NETs and NETLMs, T cell infiltrates were significant predictors of outcome (Figs 3 and 4). Whether the TILs directly affect tumour progression and patient outcome or simply represent another surrogate of tumour biology remains to be determined. It is important to note that TIL counts were not independent predictors of outcome on multivariate analyses. Our small sample size may account for the lack of independent statistical significance in analyses of CD3+ TILs in NETs and FoxP3+ TILs in NETLMs.
Figure 4.
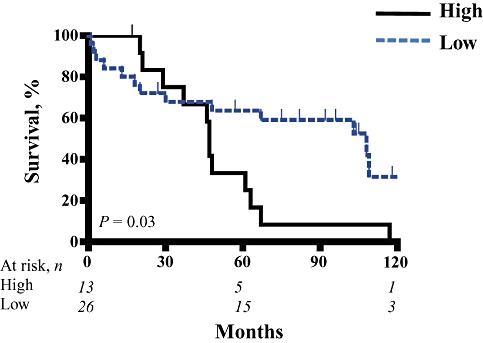
Neuroendocrine tumour liver metastases (NETLMs) were stained with anti-FoxP3 to quantify the numbers of regulatory T cells within the tumours. Kaplan–Meier analyses were performed to determine differences in overall survival among patients with high and low levels of T cell infiltration
Although CD3 and FoxP3 immunoreactivity may represent novel predictors of outcome in patients with NETs and NETLMs, the potential clinical utility of our findings may relate to the ability to identify novel immunotherapeutic targets for patients with biologically aggressive disease. A detailed understanding of NET T cells may allow the application of immunomodulatory therapy in patients with more aggressive primary lesions or metastatic disease. For example, if a low level of T cell infiltration is associated with earlier recurrence of disease, then stimulation of the immune system to increase the anti-tumour T cell response may result in clinical benefit in selected patients with NETs. Likewise, targeted therapy designed to limit the suppressive influence of Treg may be of interest in the management of NETLMs.
Patients with NETLMs from different primary sites were analysed as a single group as a result of previous reports indicating that outcomes in patients with metastatic liver disease are not dependent on the original disease site.8 Our finding that CD3+ cell counts predicted outcome in NETs and not in NETLMs most likely reflects different T cell subset proportions in the different sites. A more prominent role for FoxP3+ cells, which also express CD3, in liver tumours may diminish the positive influence of the overall CD3+ infiltrate. Thus, a greater number of CD3+ cells within NETLMs may confer both favourable and unfavourable biologic effects. Another factor which may affect disparate immune response to primary and metastatic NETs is that patients with liver metastases received additional treatment, including somatostatin and embolization.
Several limitations of these data warrant consideration. The demonstration of T cells within a tumour and a correlation between T cell number and outcome do not definitively prove physiologic relevance. Although the presence of TILs in NETs and NETLMs may represent an epiphenomenon to an unmeasured biologic event, we speculate that an anti-tumour response is associated with improved outcome. Our finding that a higher CD3+ infiltrate among those with intermediate-grade NETs implied less likelihood of recurrence suggests that an immune response confers some advantage. The correlation of higher FoxP3+ cells or Treg with decreased survival following presentation with NETLMs raises the possibility that suppression of the intrahepatic immune response negatively impacts survival. These data resonate well with our current understanding of the immune response to other primary and metastatic tumours.
Another limitation of the current paper is that the utilization of single-marker staining with immunohistochemistry does not allow for the precise cellular characterization achieved with multi-marker flow cytometry. Although immune cells are best identified with a constellation of cell surface markers, we have previously shown that the single-marker immunohistochemistry technique is >70% accurate for identification of T cell subsets.20 The TIL score selected for each T cell marker to distinguish the low and high groups was chosen based upon the median value. Other cut-off values or other scoring systems may also yield statistically significant prognostic data. Whether the methods proposed in this study are optimal will require additional validation. Finally, only a minority of patients with NETLMs received chemotherapy (n = 10) or somatostatin treatment (n = 11). Although we did not find any significant associations between these treatments and TIL counts or outcome, we cannot discount the potential influence of systemic or regional therapy in the immune response to NETLMs.
We have demonstrated that the level of NET T cell infiltration is predictive of RFS in intermediate-grade NETs and NETLMs on univariate analysis. Whether TIL analysis in NETs and NETLMs is a clinically useful marker for risk stratification will require further validation. Although we have not demonstrated TIL counts to be an independent prognostic factor in patients with NETs and NETLMs, the data are suggestive of the biologic importance of the host immune response to neuroendocrine neoplasms.
Acknowledgments
We are grateful for the support by Raymond and Beverly Sackler Research Foundation to LHT.
Conflicts of interest
None declared.
References
- 1.Fahy BN, D'Angelica M, DeMatteo RP, Blumgart LH, Weiser MR, Ostrovnaya I, et al. Synchronous hepatic metastases from colon cancer: changing treatment strategies and results of surgical intervention. Ann Surg Oncol. 2009;16:361–370. doi: 10.1245/s10434-008-0217-3. [DOI] [PubMed] [Google Scholar]
- 2.Kulke M. Advances in the treatment of neuroendocrine tumours. Curr Treat Options Oncol. 2005;6:397–409. doi: 10.1007/s11864-005-0043-9. [DOI] [PubMed] [Google Scholar]
- 3.Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumours. Cancer. 1997;79:813–829. doi: 10.1002/(sici)1097-0142(19970215)79:4<813::aid-cncr19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Eriksson B. Medical treatment of neuroendocrine gut and pancreatic tumours. Acta Oncol. 1989;28:425–431. doi: 10.3109/02841868909111217. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190:432–445. doi: 10.1016/s1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 6.Cho CS, Labow DM, Tang L, Klimstra DS, Loeffler AG, Leverson GE, et al. Histologic grade is correlated with outcome after resection of hepatic neuroendocrine neoplasms. Cancer. 2008;113:126–134. doi: 10.1002/cncr.23523. [DOI] [PubMed] [Google Scholar]
- 7.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 8.Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson SD, Pitt HA. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241:776–783. doi: 10.1097/01.sla.0000161981.58631.ab. discussion 783–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilimoria KY, Talamonti MS, Tomlinson JS, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumours: analysis of 3851 patients. Ann Surg. 2008;247:490–500. doi: 10.1097/SLA.0b013e31815b9cae. [DOI] [PubMed] [Google Scholar]
- 10.Ferrone CR, Tang LH, Tomlinson J, Gonen M, Hochwald SN, Brennan MF, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol. 2007;25:5609–5615. doi: 10.1200/JCO.2007.12.9809. [DOI] [PubMed] [Google Scholar]
- 11.Gullo L, Migliori M, Falconi M, Pederzoli P, Bettini R, Casadei R, et al. Non-functioning pancreatic endocrine tumours: a multicentre clinical study. Am J Gastroenterol. 2003;98:2435–2439. doi: 10.1111/j.1572-0241.2003.07704.x. [DOI] [PubMed] [Google Scholar]
- 12.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Phan GQ, Yeo CJ, Hruban RH, Littemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumours: review of 125 patients. J Gastrointest Surg. 1998;2:473–482. doi: 10.1016/S1091-255X(98)80039-5. [DOI] [PubMed] [Google Scholar]
- 14.Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, Thayer SP, et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg. 2007;142:347–354. doi: 10.1001/archsurg.142.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reidy DL, Tang LH, Saltz LB. Treatment of advanced disease in patients with well-differentiated neuroendocrine tumours. Nat Clin Pract Oncol. 2009;6:143–152. doi: 10.1038/ncponc1326. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi N, Hiraoka N, Yamagami W, Ojima H, Kanai Y, Kosuge T, et al. FoxP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin Cancer Res. 2007;13:902–911. doi: 10.1158/1078-0432.CCR-06-2363. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, et al. Prognostic value of tumour-infiltrating FoxP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol. 2008;34:173–179. doi: 10.1016/j.ejso.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumours predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Fridman WH, Pages F. The adaptive immunologic microenvironment in colorectal cancer: a novel perspective. Cancer Res. 2007;67:1883–1886. doi: 10.1158/0008-5472.CAN-06-4806. [DOI] [PubMed] [Google Scholar]
- 20.Katz SC, Pillarisetty V, Bamboat ZM, Shia J, Hedvat C, Gonen M, et al. T cell infiltrate predicts longterm survival following resection of colorectal cancer liver metastases. Ann Surg Oncol. 2009;16:2524–2530. doi: 10.1245/s10434-009-0585-3. [DOI] [PubMed] [Google Scholar]
- 21.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 23.Cohen T, Prus D, Shia J, Abu-Wasel B, Pinto MG, Freund HR, et al. Expression of P53, P27 and KI-67 in colorectal cancer patients of various ethnic origins: clinical and tissue microarray based analysis. J Surg Oncol. 2008;97:416–422. doi: 10.1002/jso.20989. [DOI] [PubMed] [Google Scholar]
- 24.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor FoxP3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 25.Ryschich E, Autschbach F, Eisold S, Klar E, Buchler MW, Schmidt J. Expression of HLA class I/II antigens and T cell immune response in human neuroendocrine tumours of the pancreas. Tissue Antigens. 2003;62:48–54. doi: 10.1034/j.1399-0039.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 26.Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, et al. Increased regulatory T cells correlate with CD8 T cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]


