Abstract
Objectives:
The purpose of this study was to evaluate two electrosurgical vessel-sealing devices in biliary surgery.
Methods:
Porcine common bile ducts (CBDs) were sealed with two electrosurgical devices, an electrothermal bipolar vessel-sealing device (EBVS) and ultrasonic coagulation shears. Acute study animals underwent surgical bile duct sealing followed by immediate burst pressure testing. Chronic study animals were maintained for 1 week postoperatively and then tested.
Results:
The seal failure rate in the acute study was 50% for both the EBVS device and shears, and 0% for the laparoscopic surgical clip device used as a control. The latter had significantly higher burst pressures (646.2 ± 281.8 mmHg; P = 0.006) than the EBVS device (97.6 ± 86.6 mmHg) and shears (71.7 ± 89.3 mmHg). No significant difference in burst pressures was noted between the EBVS device and shears (97.6 ± 86.6 mmHg vs. 71.7 ± 89.3 mmHg). In the chronic study, obvious bile leaks occurred in one of four pigs (25%) in the EBVS device subgroup and two of four pigs (50%) in the shears subgroup. The average proximal CBD pressure in seven pigs was 16.1 ± 4.1 mmHg. The average chronic burst pressure in the control subgroup was 1088.0 ± 922.6 mmHg.
Conclusions:
Given the high rates of failure of the EBVS device and the shears in consistently sealing biliary ducts, we do not recommend their routine use in biliary surgery.
Keywords: electrothermal bipolar vessel-sealing device, ultrasonic shears, biliary surgery
Introduction
Hepatobiliary surgery has witnessed significant advances in technology and surgical techniques in the last decade, leading to the safer and wider applicability of these procedures. A major breakthrough in minimally invasive surgery has involved the novel energy sources developed to help achieve haemostasis via vessel sealing. These energy sources are reported to be safe and efficacious1–3 and have been applied in many surgical disciplines, including colorectal,4,5 endocrine,6 urological7 and gastrointestinal surgery.8,9 Despite the publication of case reports and series using novel electrosurgical devices in laparoscopic cholecystectomy,10,11 little is known about the efficacy of using these devices in the biliary tract, specifically for sealing the cystic duct, and little objective evidence has emerged to support their use.
The importance and clinical relevance of these devices for use in biliary tract surgery, specifically laparoscopic cholecystectomy, refer to the issue of whether these devices can adequately seal the cystic duct; if they can, this would allow the surgeon to use one instrument for sealing the cystic duct and cystic artery, and for dissecting the gallbladder from the liver bed, therefore making the surgeon more efficient and possibly decreasing operating room time.12 The purpose of our study was to evaluate the use of two commonly used electrosurgical devices, an electrothermal bipolar vessel-sealing device (EBVS) and ultrasonic coagulation shears, in biliary tract surgery.
Materials and methods
The experimental protocol was approved by the Carolinas Medical Center Institutional Animal Care and Use Committee. Female Yorkshire pigs (Baux Mountain Farm, Germanton, NC, USA) weighing 40–50 kg were used in the experiments. Porcine common bile ducts (CBDs) were sealed with one of two electrosurgical devices: (i) a 5-mm laparoscopic EBVS device (LigaSure Advance™; Valleylab, Inc., Boulder, CO, USA), and (ii) 5-mm laparoscopic ultrasonic coagulation Harmonic ACE® shears (HS) (Ethicon Endo-Surgery, Inc., Cincinnati, OH, USA). A 10-mm diameter laparoscopic surgical clip applier (LC) (Ligaclip® ERCA; Ethicon Endo-Surgery, Inc.) was used as control. We chose the CBD to test the sealing effect of the electrosurgical devices in order to subject the resultant seals to the maximum conceivable burst load in the form of largest accessible biliary structure and continued postoperative stress load caused by bile secretion. We believe that if seals created by these instruments were shown to handle this level of stress load, this would indicate that the instruments were more than adequate for safe cholecystectomy and hepatic resection.
All animals underwent a midline laparotomy under general anaesthesia via endotracheal intubation. The first group (n = 11) underwent surgical bile duct ligation followed immediately by burst pressure testing of the proximal CBD stump; the animals were killed after documentation of the data. The remaining group of animals (n = 12) underwent surgical bile duct ligation after which a 15-Fr, round, hubless, silicone Blake® drain (Ethicon, Inc., Piscataway, NJ, USA) connected externally to a 100-ml J-VAC Bulb Suction Reservoir (Ethicon, Inc.) was placed in the proximity of the sealed CBD before closure of the abdomen. These animals were maintained for 1 week or until they developed signs of bile leak, sepsis or failure to thrive. Animals were killed when bile was noted at any time in the postoperative drainage. At the time of procurement, a re-laparotomy was performed to facilitate the examination of the CBD seal for integrity and burst pressure testing. Burst pressure testing on the resected proximal CBD stump was performed using a Cole-Parmer® automated syringe pump (model SW-74900-00; Cole-Parmer Instrument Co., Vernon Hills, IL, USA) attached to a Fluke® pressure calibrator (model 717-100G; Fluke Biomedical Corp., Everett, WA, USA) depicted in Fig. 1. The pressure calibrator determines maximum and minimum pressures in mmHg when saline is injected into the lumen of a vessel or duct fixed to a pre-designed fixation device using an iris clamp around the injection port (Fig. 2). Proximal bile duct pressures were measured at necropsy using a Spacelabs 514 pressure monitor (Spacelabs Healthcare, Inc., Issaquah, WA, USA) and transducer connected via a 22-gauge needle.
Figure 1.

Instrumentation used for measuring common bile duct burst pressure
Figure 2.
Resected proximal bile duct in burst pressure testing device
Statistical analysis was carried out using sas Version 9.1 (SAS Institute, Inc., Cary, NC, USA). Means and standard deviations were calculated for measurement variables and comparisons were made using analysis of variance (anova) followed by Tukey tests when appropriate. A P-value <0.05 was considered statistically significant.
Results
In total, 23 pigs underwent surgical bile duct sealing. The acute study included 11 pigs with bile ducts sealed by: the LC (n = 3); the EBVS device (n = 4), and the HS device (n = 4). Common bile duct burst pressures in the acute study are listed in Table 1. There were no seal failures for the surgical clip (LC) subgroup, in which the average burst pressure was 646.2 ± 281.8 mmHg. Two of the four (50%) seals made using the EBVS device and two of the four made using the HS in the acute study failed with a burst pressure of <50 mmHg; average burst pressures were 97.6 ± 86.6 mmHg for EBVS seals and 71.7 ± 89.3 mmHg for HS seals. The LC was associated with significantly higher burst pressures than the EBVS device (646.2 ± 281.8 mmHg vs. 97.6 ± 86.6 mmHg; P = 0.006) and the HS device (646.2 ± 281.8 mmHg vs. 71.7 ± 89.3 mmHg; P = 0.006), but no difference in burst pressure was observed between seals made with the EBVS and HS devices (97.6 ± 86.6 mmHg vs. 71.7 ± 89.3 mmHg; P = 0.720), as depicted in Fig. 3.
Table 1.
Acute study: common bile duct burst pressures in 11 pigs
| Sealing device | CBD burst pressure, mmHg | Mean ± SD, mmHg | P-value |
|---|---|---|---|
| Ligaclip® ERCA (LC) | 690.8 | 646.2 ± 281.8 | 0.006 |
| 903.0 | |||
| 344.8 | |||
| LigaSure Advance™ (EBVS) | 123.4 | 97.6 ± 86.6 | 0.720 |
| 0.0 | |||
| 1.0 | |||
| 168.3 | |||
| Harmonic ACE® (HS) | 90.4 | 71.7 ± 89.3 | |
| 190.4 | |||
| 4.8 | |||
| 1.0 |
CBD, common bile duct; SD, standard deviation
Figure 3.
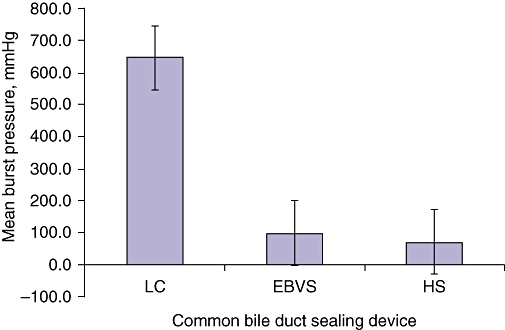
Common bile duct burst pressures in acute study subgroups. LC, Ligaclip® ERCA; EBVS, LigaSure Advance™; HS, Harmonic ACE®. LC, P = 0.006; EBVS, P = 0.720; HS, P = 0.720
The chronic study included 12 pigs in which bile ducts were sealed by the LC (n = 4), the EBVS device (n = 4) and the HS device (n = 4). Outcomes and CBD pressures in the chronic study are listed in Table 2. In the LC subgroup, two pigs were killed on postoperative day 2 because of poor ad libitum food intake and general failure to thrive. On exploration, both seals were intact with no disruption. Both animals had grossly distended stomachs with no obvious obstruction. The other two pigs completed the 7-day study. Average CBD burst pressure in the LC subgroup was 1088.0 ± 922.6 mmHg.
Table 2.
Chronic study: outcomes and common bile duct burst pressures in 12 pigs
| Sealing device | CBD burst pressure, mmHg | Proximal CBD stump pressure, mmHg | Outcomes |
|---|---|---|---|
| Ligaclip® ERCA (LC) | 58.6 | 19.0 | Mean CBD burst pressure 1088.0 ± 922.6 mmHg and mean proximal CBD pressure 16.1 ± 4.1 mmHg; no bile leaks Two of four animals killed on POD 2 for failure to thrive |
| 1675.4 | 20.0 | ||
| 584.0 | 13.0 | ||
| 2034.0 | 10.0 | ||
| LigaSure Advance™ (EBVS) | N/A | 18.0 | One animal killed on POD 1 for bile leak; other three showed no gross bile leak, but CBD seals disrupted during exploration |
| N/A | 20.0 | ||
| N/A | N/A | ||
| N/A | N/A | ||
| Harmonic ACE® (HS) | N/A | 13.0 | Two of four animals killed on POD 3 for bile leak; other two showed no gross bile leak, but CBD seals disrupted during exploration |
| N/A | N/A | ||
| N/A | N/A | ||
| N/A | N/A |
CBD, common bile duct; N/A, not able to be tested; POD, postoperative day
One pig in the EBVS device subgroup (n = 4) was killed on postoperative day 1 because of an obvious bile leak; a proximal bile duct disruption was identified on exploration. The remaining three pigs completed the 7-day study without gross leakage; however, bile ducts were disrupted upon minimal dissection and we were unable to obtain any burst pressures.
In the HS subgroup, two pigs were killed on postoperative day 3 because of obvious bile leaks in the drain; leaks at the proximal CBD were identified on exploration. The other two pigs completed the 7-day study without a gross leak; however, bile ducts were disrupted upon minimal dissection and we were unable to obtain burst pressures.
In the chronic study, we were able to obtain proximal bile duct pressures measured on postoperative day 7 in seven pigs (four in the LC, two in the EBVS and one in the HS subgroups). The average proximal CBD pressure was 16.1 ± 4.1 mmHg.
Discussion
The ability to obtain haemostasis using electrosurgical vessel-sealing devices has represented a great advance in the fields of both open and laparoscopic hepatobiliary surgery. Establishing haemostasis is critical to improving clinical outcomes and providing a clear field for adequate visualization. Although these energy sources have a proven safety and efficacy profile for haemostasis in appropriately sized arteries and veins, their efficacy in sealing bile ducts is unproven and currently surgical clips, which are associated with a low complication rate, remain the most commonly used device to ligate the cystic duct. Secondary to this, clips served as the control in this experiment.
There are several theoretical attractions of using a single electrosurgical device to seal blood vessels as well as biliary ducts in hepatobiliary surgical procedures. Firstly, the use of a single instrument may potentially decrease operative time and costs because it decreases the number of instrument exchanges in laparoscopic surgery and allows one instrument to be used throughout the entire procedure in a fast and efficient manner for multiple applications, including the ligation of appropriately sized blood vessels and biliary structures, the parenchymal transection of liver and the dissection of the gallbladder. Secondly, newer electrothermal surgical devices are associated with a potential reduction in ‘thermal spread’ compared with older monopolar and bipolar instruments used during laparoscopic cholecystectomy.
Two commonly used energy sources for laparoscopic surgery are the EBVS and HS devices. This study may be limited by its use of only two electrosurgical devices because several other instruments with different mechanisms of action that could potentially be used for sealing the cystic duct are available. The rationale behind using these two electrosurgical devices, which have different mechanisms of action, was that these are the two products cited in the literature as being most commonly used for sealing cystic ducts during laparoscopic cholecystectomy.13,14 When evaluating these products, it is important to understand the indications for their use and their mechanisms of action. The EBVS device is a feedback-controlled, high-current (4 amps), low-voltage (<200 volts) bipolar instrument.15 The high current denatures the collagen and elastin in the tissue bundles and vessels, which then quickly reform to create a ‘plastic-like’ seal zone following each cycle of activation of the device. The LigaSure Advance™ is a new-generation EBVS device designed for increased speed of transection and is approved to seal vessels up to 7 mm in diameter. It also has a monopolar tip on one of the jaws to assist with dissection.
The HS device converts electrical energy into mechanical vibrations via the transfer of high-frequency ultrasonic waves (55 500 cycles/second [Hz]) to a vibrating blade for tissue division and blood vessel coagulation.16 At relatively low temperatures (50–100 °C), the mechanical vibrations disrupt hydrogen bonds in tissue and vessel protein to form a coagulum. The Harmonic ACE® is a new-generation product approved for use in vessels of ≤5 mm in diameter and is reported to have increased speed of transection compared with previous generations of harmonic scalpels.
Several reports document the safe use of the HS during laparoscopic cholecystectomy in humans for gallbladder removal, but not cystic duct sealing.10,11,17–19 Power et al. used the HS for dissection of the gallbladder in 282 consecutive cases with a mean operating time of 29 min, reporting two bile leaks related to clipping of the cystic duct.19 Fullum et al. used the HS for ‘dome-down dissection’ of the gallbladder and division of the cystic artery, with division of the cystic duct accomplished by using two size 2–0 polydioxanone endoloops.10 These authors reported a mean operating time of 55 min, a conversion rate of 7.6%, and no bile duct injuries. In one of the largest studies reported to date (a prospective non-randomized trial of 461 consecutive patients undergoing laparoscopic cholecystectomy by Hüscher et al.), the entire operation including cystic duct and artery transection was performed in 331 patients using the HS device alone, and in 130 patients using the HS for coagulation and division of the cystic duct and artery, after which the cystic duct stump was secured with an absorbable suture endoloop. The authors reported no significant difference in postoperative mortality, complications and/or cystic duct leaks.18
To date, the safety and efficacy of the EBVS device in biliary tract surgery remain the subject of debate: several authors have reported favourable results20–22 and others discourage its use based on seal failures and increased rates of necrosis of cystic duct stump23,24 in preclinical studies. Despite the previous reports of success using the HS and EBVS devices in biliary tract surgery, our data do not support their use for sealing the cystic duct. Of particular note was the measurement of proximal CBD pressure at 1 week (16.1 ± 4.1 mmHg), a value that represents the pressure the seal must withstand in the postoperative period. Although several of the seals made using the EBVS and HS devices in the acute group withstood pressures well above this level, we cannot recommend these devices because of the frequency of gross seal failures seen in the chronic study, which amounted to 50% of seals made using the HS and 25% of seals made using the EBVS device. The failure with minimal dissection of the remaining seals made with the HS and EBVS devices in the chronic study suggests that these were leaks sealed by omentum or surrounding tissues, and were not detected clinically. This gives an essential seal failure rate of 100% in the chronic study for both instruments.
The major limitation of our study concerns our use of the porcine CBD as a surrogate for the human cystic duct. The diameters of the porcine CBDs ranged from 5.6 mm to 6.8 mm, slightly larger than that of the typical human cystic duct. We chose the porcine CBD because a previous study23 reported sizes comparable with those of the human cystic duct. An additional problem may lie in the fact that dissection of a porcine cystic duct is technically difficult because of its relatively small size and intrahepatic location. As such, sealing the porcine common duct creates a clinically unrealistic pressure because the seal must withstand the intraluminal pressure contributed by the entire hepatic bile secretory output. This pressure is likely to be significantly higher than that occurring in the cystic duct stump or small bile duct ends exposed during hepatic parenchymal transection. The rationale for using the CBD was that if the electrosurgical vessel-sealing devices were able to seal the CBD, with its increased size and presumed increased pressure, we could assume that they would safely and effectively seal smaller biliary structures, including the cystic duct and intrahepatic bile ducts. As such, although our data cannot support the use of these electrosurgical devices alone for sealing biliary structures, our study design represents the most extreme case scenario for human cystic ducts. Further study is needed to specifically test cystic duct seals in cholecystectomy and intrahepatic bile duct seals in liver resection, and to find an appropriate surrogate for the human cystic duct or intrahepatic bile duct. The question of why these electrosurgical devices can adequately seal blood vessels of up to 7 mm in size, yet fail to seal biliary structures of comparable size, remains. One potential explanation is related to the unique properties of the protein matrix in the bile duct wall or the absence of a thrombogenic coagulum that occurs when ligating vascular structures, as hypothesized by Matthews and colleagues.23 In EBVS devices, changes in the power output algorithm may provide improved sealing of non-blood vessel structures. Unfortunately, the histological evaluations in this study were insufficient to allow us to comment on why the seals may have failed, but this will be addressed in future studies. Lastly, this study represents a pilot study to assess clinically and objectively the efficacy of these two electrosurgical devices and therefore is underpowered to draw definitive conclusions on the use of these devices in biliary tract surgery.
In conclusion, the development of the HS and EBVS devices represent a major breakthrough in surgery, facilitating haemostasis in 5–7-mm blood vessels. Despite improvements in the efficiency and vessel-sealing capabilities of these two new-generation electrosurgical devices, we do not recommend the Harmonic ACE® or LigaSure Advance™ alone for use in sealing biliary structures because of the high failure rate associated with the seal. Our study is limited by our use of the porcine CBD, which may not be an ideal surrogate for the human cystic duct or intrahepatic biliary duct. Preclinical and clinical studies evaluating the use of these devices in sealing cystic ducts in cholecystectomy and biliary pedicles in hepatic resections are needed. Further study is needed to determine the mechanisms whereby bile ducts are resistant to sealing using current electrosurgical devices, and to potentially find new sealing algorithms for the electrothermal bipolar vessel-sealing devices to facilitate open and, particularly, laparoscopic hepatobiliary surgery.
Acknowledgments
This study was supported by an unrestricted educational grant from Valleylab, Inc., Boulder, CO, USA.
Conflicts of interest
None declared.
References
- 1.Carbonell AM, Joels CS, Kercher KW, Matthews BD, Sing RF, Heniford BT. A comparison of laparoscopic bipolar vessel sealing devices in the haemostasis of small-, medium-, and large-sized arteries. J Laparoendosc Adv Surg Tech A. 2003;13:377–380. doi: 10.1089/109264203322656441. [DOI] [PubMed] [Google Scholar]
- 2.Diamantis T, Kontos M, Arvelakis A, Syroukis S, Koronarchis D, Papalois A, et al. Comparison of monopolar electrocoagulation, bipolar electrocoagulation, Ultracision, and LigaSure. Surg Today. 2006;36:908–913. doi: 10.1007/s00595-006-3254-1. [DOI] [PubMed] [Google Scholar]
- 3.Harold KL, Pollinger H, Matthews BD, Kercher KW, Sing RF, Heniford BT. Comparison of ultrasonic energy, bipolar thermal energy, and vascular clips for the haemostasis of small-, medium-, and large-sized arteries. Surg Endosc. 2003;17:1228–1230. doi: 10.1007/s00464-002-8833-7. [DOI] [PubMed] [Google Scholar]
- 4.Kwok SY, Chung CC, Tsui KK, Li MK. A double-blind, randomized trial comparing LigaSure and harmonic scalpel haemorrhoidectomy. Dis Colon Rectum. 2005;48:344–348. doi: 10.1007/s10350-004-0845-z. [DOI] [PubMed] [Google Scholar]
- 5.Targarona EM, Balague C, Marin J, Neto RB, Martinez C, Garriga J, et al. Energy sources for laparoscopic colectomy: a prospective randomized comparison of conventional electrosurgery, bipolar computer-controlled electrosurgery and ultrasonic dissection. Operative outcome and costs analysis. Surg Innov. 2005;12:339–344. doi: 10.1177/155335060501200409. [DOI] [PubMed] [Google Scholar]
- 6.Saint Marc O, Cogliandolo A, Piquard A, Fama F, Pidoto RR. LigaSure vs. clamp-and-tie technique to achieve haemostasis in total thyroidectomy for benign multinodular goitre: a prospective randomized study. Arch Surg. 2007;142:150–156. doi: 10.1001/archsurg.142.2.150. discussion 157. [DOI] [PubMed] [Google Scholar]
- 7.Constant DL, Florman SS, Mendez F, Thomas R, Slakey DP. Use of the LigaSure vessel sealing device in laparoscopic living-donor nephrectomy. Transplantation. 2004;78:1661–1664. doi: 10.1097/01.tp.0000144379.29943.ed. [DOI] [PubMed] [Google Scholar]
- 8.Lee WJ, Chen TC, Lai IR, Wang W, Huang MT. Randomized clinical trial of LigaSure versus conventional surgery for extended gastric cancer resection. Br J Surg. 2003;90:1493–1496. doi: 10.1002/bjs.4362. [DOI] [PubMed] [Google Scholar]
- 9.Yüney E, Höbek A, Keskin M, Yilmaz O, Kamali S, Oktay C, et al. Laparoscopic splenectomy and LigaSure. Surg Laparosc Endosc Percutan Tech. 2005;15:212–215. doi: 10.1097/01.sle.0000174550.94671.30. [DOI] [PubMed] [Google Scholar]
- 10.Fullum TM, Kim S, Dan D, Turner PL. Laparoscopic ‘dome-down’ cholecystectomy with the LCS-5 harmonic scalpel. JSLS. 2005;9:51–57. [PMC free article] [PubMed] [Google Scholar]
- 11.Westervelt J. Clipless cholecystectomy: broadening the role of the harmonic scalpel. JSLS. 2004;8:283–285. [PMC free article] [PubMed] [Google Scholar]
- 12.Gelmini R, Franzoni C, Zona S, Andreotti A, Saviano M. Laparoscopic cholecystectomy with harmonic scalpel. JSLS. 2010;14:14–19. doi: 10.4293/108680810X12674612014301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandil T, El Nakeeb A, El Hefnawy E. Comparative study between clipless laparoscopic cholecystectomy by harmonic scalpel versus conventional method: a prospective randomized study. J Gastrointest Surg. 2008;14:323–328. doi: 10.1007/s11605-009-1039-8. [DOI] [PubMed] [Google Scholar]
- 14.Bessa SS, Al-Fayoumi TA, Katri KM, Awad AT. Clipless laparoscopic cholecystectomy by ultrasonic dissection. J Laparoendosc Adv Surg Tech A. 2008;18:593–598. doi: 10.1089/lap.2007.0227. [DOI] [PubMed] [Google Scholar]
- 15.Heniford BT, Matthews BD. Basic Instrumentation for Laparoscopic Surgery. New York, NY: Springer-Verlag, Inc; 2000. Minimally Invasive Cancer Management; pp. 36–45. [Google Scholar]
- 16.Amaral JF. Ultrasonic dissection. Endosc Surg Allied Technol. 1994;2:181–185. [PubMed] [Google Scholar]
- 17.Tebala GD. Three-port laparoscopic cholecystectomy by harmonic dissection without cystic duct and artery clipping. Am J Surg. 2006;191:718–720. doi: 10.1016/j.amjsurg.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 18.Hüscher CG, Lirici MM, Di Paola M, Crafa F, Napolitano C, Mereu A, et al. Laparoscopic cholecystectomy by ultrasonic dissection without cystic duct and artery ligature. Surg Endosc. 2003;17:442–451. doi: 10.1007/s00464-002-9068-3. [DOI] [PubMed] [Google Scholar]
- 19.Power C, Maguire D, McAnena OJ, Calleary J. Use of the ultrasonic dissecting scalpel in laparoscopic cholecystectomy. Surg Endosc. 2000;14:1070–1073. doi: 10.1007/s004640000034. [DOI] [PubMed] [Google Scholar]
- 20.Constant DL, Slakey DP, Campeau RJ, Dunne JB. Laparoscopic non-anatomic hepatic resection employing the LigaSure device. JSLS. 2005;9:35–38. [PMC free article] [PubMed] [Google Scholar]
- 21.Shamiyeh A, Schrenk P, Tulipan L, Vattay P, Bogner S, Wayand W. A new bipolar feedback-controlled sealing system for closure of the cystic duct and artery. Surg Endosc. 2002;16:812–813. doi: 10.1007/s00464-001-9058-x. [DOI] [PubMed] [Google Scholar]
- 22.Schulze S, Krisitiansen VB, Fischer Hansen B, Rosenberg J. Sealing of cystic duct with bipolar electrocoagulation. Surg Endosc. 2002;16:342–344. doi: 10.1007/s004640090054. [DOI] [PubMed] [Google Scholar]
- 23.Matthews BD, Pratt BL, Backus CL, Kercher KW, Mostafa G, Lentzner A, et al. Effectiveness of the ultrasonic coagulating shears, LigaSure vessel sealer, and surgical clip application in biliary surgery: a comparative analysis. Am Surg. 2001;67:901–906. [PubMed] [Google Scholar]
- 24.Shamiyeh A, Vattay P, Tulipan L, Schrenk P, Bogner S, Danis J, et al. Closure of the cystic duct during laparoscopic cholecystectomy with a new feedback-controlled bipolar sealing system in case of biliary obstruction – an experimental study in pigs. Hepatogastroenterology. 2004;51:931–933. [PubMed] [Google Scholar]


