Abstract
Background:
Thermal ablation is an accepted therapy for selected hepatic malignancies. However, the reliability of thermal ablation is limited by the inability to accurately monitor and confirm completeness of tumour destruction in real time. We investigated the ability of ultrasound elasticity imaging (USEI) to monitor thermal ablation.
Objectives:
Capitalizing on the known increased stiffness that occurs with protein denaturation and dehydration during thermal therapy, we sought to investigate the feasibility and accuracy of USEI for monitoring of liver tumour ablation.
Methods:
A model for hepatic tumours was developed and elasticity images of liver ablation were acquired in in vivo animal studies, comparing the elasticity images to gross specimens. A clinical pilot study was conducted using USEI in nine patients undergoing open radiofrequency ablation for hepatic malignancies. The size and shape of thermal lesions on USEI were compared to B-mode ultrasound and post-ablation computed tomography (CT).
Results:
In both in vivo animal studies and in the clinical trial, the boundary of thermal lesions was significantly more conspicuous on USEI when compared with B-mode imaging. Animal studies demonstrated good correlation between the diameter of ablated lesions on USEI and the gross specimen (r = 0.81). Moreover, high-quality strain images were generated in real time during therapy. In patients undergoing tumour ablation, a good size correlation was observed between USEI and post-operative CT (r = 0.80).
Conclusion:
USEI can be a valuable tool for the accurate monitoring and real-time verification of successful thermal ablation of liver tumours.
Keywords: colorectal metastases, hepatocellular carcinoma, palliation, liver cancer, radio frequency, adiological intervention/treatment
Introduction
Interstitial tumour ablation is currently considered a viable alternative to resectional therapy, with similar outcome in some patient groups.1 However, unlike liver resection, tumor ablation is limited by the ability to precisely monitor and document complete margin-negative tumour destruction using current technology. This limitation probably contributes to high local recurrence rates sometimes observed.2 Currently, the intra-operative assessment of the success of thermal ablation is largely based on indirect information such as target temperatures and empiric knowledge such as the predicted size of the ablation zone. B-mode ultrasound is often used to target and guide hepatic thermal ablation, including radiofrequency ablation and microwave ablation. While this modality is widely available, including in operative suites, and easily allows for real-time imaging during targeting and treatment, visualization of the zone of ablation is difficult using standard B-mode imaging technology. Tissue heating can result in outgassing and micro-bubble formation in the targeted region but this feature cannot readily distinguish whether the thermal zone has fully encompassed the tumour boundaries. Various studies have shown that there is only limited correlation between the zone of ablated tissue on the B-mode US imaging when compared with the gross specimen.3,4 Post-ablation cross-sectional imaging with either computed tomography (CT) or magnetic resonance imaging (MRI) has been found to more reliably determine completeness of tumour destruction.5,6 Yet, post-procedural, non-real-time assessment limits the ability to adjust or modify the therapy during the procedure.
Advances in US imaging technology afford the opportunity to improve the capability of US to monitor thermal ablation. Specifically, ultrasound elasticity imaging (USEI) has emerged as a potentially useful augmentation to conventional B-mode ultrasound imaging. USEI relies on two important observations: (i) different tissues may have significant differences in their mechanical properties and (ii) the information encoded in the coherent scattering (i.e. speckle) may be sufficient to calculate these differences after a mechanical stimulus or compression.7 During thermal ablation, protein denaturation and tissue dehydration increase the relative stiffness of the tissue with increasing ablation temperatures.8 Application of USEI to achieve real-time visualization of the boundary between ablated and unablated tissue may offer the opportunity to improve the efficacy and safety of thermal ablation of liver tumours. While this novel technique is still under investigation and its clinical applicability has yet to be established, we sought to investigate the ability of this technique to visualize the zone of thermal ablation in vivo in order to guide future work. Therefore, the objectives of the present study were (i) to investigate the accuracy of free-hand intra-operative USEI to visualize the zone of thermal ablation in an in vivo tumour model using off-line reconstructed elasticity images and (ii) to evaluate USEI in a clinical pilot study in order to determine its capability to visualize the ablation zone in patients undergoing radiofrequency ablation (RFA) of liver tumours, both with off-line reconstructed images as well as in real time.
Methods
In vivo animal study: tumour ablation model
In order to determine the accuracy of USEI for visualization of liver ablation compared with the actual specimen examination, a model of in vivo pseudotumor therapy in a porcine model was utilized. The present study was approved by the institutional Animal Care and Use Committee. Specifically, six pseudotumours were created in livers of three 70- to 80-lb female pigs, a model which has been previously described for other purposes.9 Using a midline laparotomy, pseudotumours were created through the intrahepatic injection of 1–2 cc of dental alginate mixture (Kromopran, Lascot, Florence, Italy) under US guidance. This technique reliably results in spherical lesions within the liver parenchyma measuring approximately 1.0–1.5 cm in diameter which are visible both on US and CT. Moreover, these targets are of a consistency that needle-based ablation probes easily penetrate through and into the surrounding liver. These pseudotumours can be thermally ablated without comprise of lesion integrity or effect on thermal conduction, and upon liver sectioning, the pseudotumours are easily visible as white material within the visualized ablated region. US and contrast-enhanced CT imaging was performed in all animals before ablation in order to confirm adequacy of pseudo tumour size and location.
Ablation of these targets was performed either on the same day (n = 1) or 4 days (n = 2) after insertion using a RITA-XL multi-tine RFA probe (Angiodynamics, Queensbury, NY, USA) Model 1500X generator. Different time points were chosen to assess for the elastographic utility of established pseudotumours vs. newly created pseudotumours. Probe placement within each pseudotumour was performed using B-mode US applied directly to the liver surface.
In vivo animal study: image collection and analysis
B-mode and USEI images were collected using a Siemens Antares US scanner with a 4-cm 7.27-MHz linear array probe (Siemens Medical Solutions USA, Inc. Ultrasound Division, Issaquah, WA, USA) or an Ultrasonix US System (Ultrasonix, Richmond, Canada), both equipped with an ultrasound research interface to access raw US data. Conventional B-mode and raw US data were collected in both transverse and sagittal planes directly before and after ablation. Tissue strain was generated by gently compressing and decompressing the region of interest with the US-probe while applied on the liver surface, usually with <1-cm excursion. After collection of all imaging data, animals were sacrificed and livers were harvested. The ablated pseudotumours were serially sectioned transversely, measured and photographed. For this part of the study, elasticity images were generated off-line using previously described algorithms.10 In short, consecutive compressed and uncompressed ultrasound images are automatically compared and processed to provide a map of local displacement measures which can then be used to determine the elastic properties of the tissue. These local elastic properties can then be used to generate a user interpretable image. Pre- and post-ablation diameter measurements from real-time and off-line USEI were compared with B-mode US, CT and gross tissue measurements at the greatest transverse plane.
Clinical study
Between March 2008 and May 2010, US elasticity images were collected in nine patients undergoing RFA for hepatic malignancies at the Johns Hopkins Hospital. Institutional Review Board approval was obtained and all participants provided informed consent. Five patients had metastatic colorectal cancer, two patients had neuroendocrine liver metastases, one patient had metastatic lung cancer and one patient had a recurrent hepatocellular carcinoma (HCC) lesion in the liver. Ablation was performed using a RITA-XL RFA probe and Model 1500X RF generator (Angiodynamics). Tumour sizes ranged from 7 to 19 mm and all ablations were performed with curative intent with a planned diameter 2-cm larger than the tumour. Adequate targeting, array deployment and target temperatures were achieved in all cases. Ultrasound image data (raw US and B-mode data) were captured and recorded during intra-operative US imaging as conducted standardly. US images were acquired before and after RFA therapy using a Siemens Antares US scanner (Siemens Medical Solutions USA, Inc.) with URI in order to access raw US data. The US probe was a 7.27-MHz Siemens VF 10-5 linear array. In four patients, raw data were collected for off-line reconstruction of USEI as described above;11,12 in five patients, real-time elasticity images were obtained using the onboard elasticity imaging module on the Siemens Antares ultrasound machine, which allows for high-resolution real-time elasticity images that are projected over the B-mode ultrasound image. Elasticity images were captured during manual cycled intermittent compression of the region of interest using the hand-held US probe, with typical excursion of < 1 cm. All US images were collected in a transversal plane, to allow for comparison with post-operative CT images. All patients underwent contrast-enhanced CT scanning of the liver 3–4 days post-ablation as done as part of our routine. Ablation lesion diameter by CT was determined based on the size of the non-enhancing hypodense coagulation region.13 Maximum lesion diameter measured using USEI, B-mode US and CT was compared.
Statistical analysis
All statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Mean tumour diameters and their respective standard deviations were calculated and the existence of a linear correlation between the diameters of ablated lesions on different imaging modalities was investigated by calculating Pearson's product moment correlation coefficient.
Results
Animal studies
All six alginate pseudotumours were readily visible on conventional B-mode US and CT imaging as well-demarcated spheroid masses ranging in size from 9–19 mm (mean 13.6 mm) as measured on CT-images. On US, the lesions were hyperechoic lesions whereas on CT, lesions were attenuated compared with the surrounding liver (Fig. 1a–b). The pseudotumours were found to be somewhat stiffer than the hepatic parenchyma, reflected as conspicuous darker mass on USEI (Fig. 1c). The size and shape of the alginate tumours correlated with high precision between CT and USEI (r = 0.87; P < 0.001).
Figure 1.
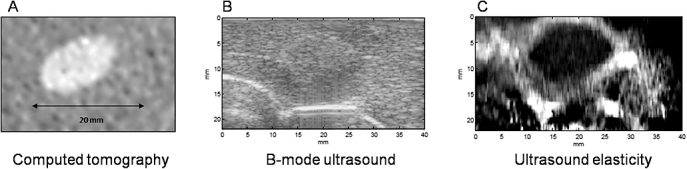
Pre-ablation imaging of in vivo porcine pseudotumours. (a) Computed tomography (CT) scan, (b) B-mode ultrasound and (c) ultrasound elasticity image
After ablation of the pseudotumours, the zone of thermal coagulation around the pseudotumour was only poorly visualized by conventional B-mode US, primarily characterized by patchy regions of hyperechoic and hypoechoic changes associated with gas formation and oedema (Fig. 2a). Within 20–30 s, the dissipation of the gas resulted in almost complete failure to observe any difference between pre- and post-ablation appearance on B-mode US. In contrast, the zone of thermal ablation was highly visible on USEI (Fig. 2b). Moreover, the imaging characteristics of the ablation zone on USEI remained unchanged, probably because of the irreversibility of the elasticity properties of ablated tissue. On these images, the zone of thermal ablation around the pseudotumours was characterized by increased tissue stiffness, exceeding that of the pseudotumour itself (24 vs. 14 mm in mean transverse diameter). Once the ablation region exceeded the tumour volume, the pseudotumour no longer could be visualized within the ablation zone. Importantly, there was a good correlation between the diameter of the ablated pseudotumours on elasticity images when compared with the gross specimen (r = 0.81; P < 0.001) (Fig. 3).
Figure 2.

Post-ablation imaging of in vivo porcine pseudotumours. (a) B-mode ultrasound, (b) ultrasound elasticity image and (c) sectioned specimen
Figure 3.
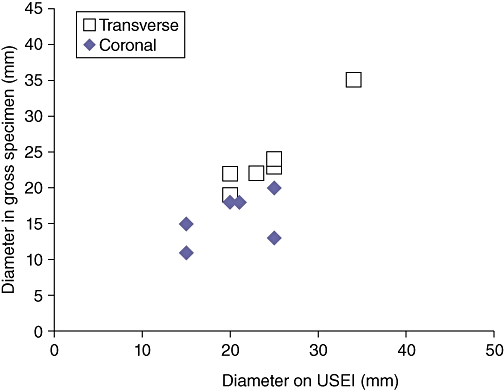
Scatterplot demonstrating the correlation between pseudotumour diameter as measured by elasticity imaging and in the gross specimen (transverse and coronal measurements)
Clinical study
Successful elasticity images were obtained after RFA in all nine patients using free-hand palpation. Figures 4 and 5 provide examples from two patients, comparing post-ablation (i) B-mode US, (ii) real-time USEI and (iii) post-operative CT images. As shown, the region of tissue ablation is poorly visualized on B-mode US compared with real-time USEI. In one patient, multiple overlapping ablations were performed that eventually outsized the size of the ultrasound probe, precluding meaningful correlation with the post-operative CT scan. Among the remaining eight patients, ablation diameter correlated well between USEI and that of post-operative CT (r = 0.80, P < 0.001) (Fig. 6).
Figure 4.
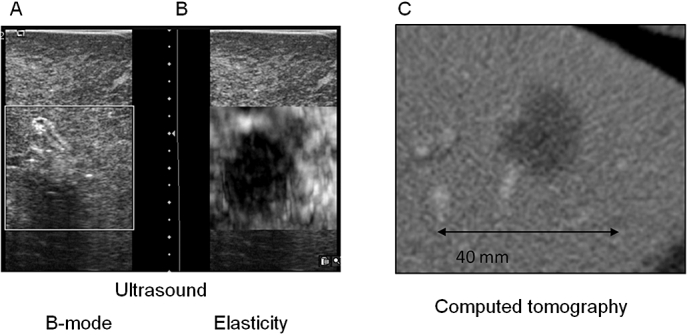
Post-ablation imaging in a patient with a recurrent hepatocellular carcinoma in segment 3 of the liver after undergoing radiofrequency ablation. (a) Real-time B-mode ultrasound, (b) real-time elasticity image and (c) post-operative computed tomography (CT) scan (day 3)
Figure 5.
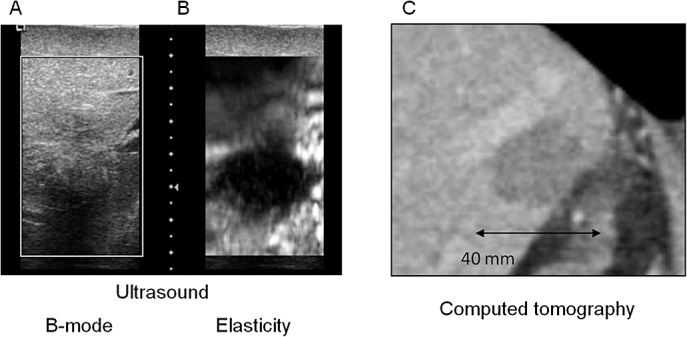
Post-ablation imaging in a patient with a colorectal liver metastasis in the dome of the liver after undergoing radiofrequency ablation. (a) Real-time B-mode ultrasound, (b) real-time elasticity image and (c) post-operative CT scan (day 3)
Figure 6.
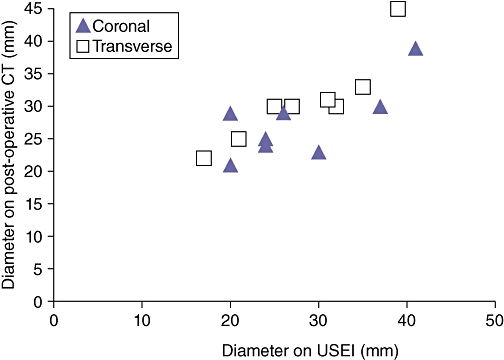
Scatterplot demonstrating the correlation between diameter of the ablation zone in eight patients as measured by elasticity imaging and on the post-operative CT scan (transverse and coronal measurements)
Discussion
Thermal ablation therapies have emerged as a widely available treatment modality for primary and secondary liver cancer over the past decade. However, its efficacy is severely limited by several factors, including the inability to adequately monitor the ablative process and document the margins of ablation. In the present study, we aimed to determine if advanced ultrasound technology in the form of elasticity imaging might offer an opportunity to image and delineate the boundary of thermal ablation in more detail and eventually in real time, potentially allowing for confirmation and adjustment of the ablative process.
Our animal studies demonstrated that USEI can provide improved delineation between thermally ablated tissue and non-ablated liver tissue with a good correlation between the size of the elasticity images and the actual ablation size and shape. Using an uncomplicated and reproducible porcine pseudotumour model, we found this novel imaging system capable of visualizing an ablation margin around an existing lesion.
In the present study, we found that it was often difficult to visualize the zone of ablation on conventional US imaging. While B-mode ultrasonography is perhaps the most commonly used method to guide and monitor the thermal ablation of liver tumours, its ability to distinguish ablated tissue from non-ablated tissue has proven inadequate to monitor and adequately confirm ablation success.3,4,14 The formation of gas and micro-bubbles cause frequent over- or underestimation of the actual ablation size, leading at times to excessively large or too small ablation volumes. CT imaging and MRI can offer a more accurate estimation of size of thermally ablated lesions, but are less feasible for real-time ablation monitoring as a result of unavailability in most operating suites, radiation exposure and higher costs.
The findings from this limited clinical trial reinforce the potential of ultrasound elasticity imaging for application during thermal ablation therapy. In this early study of elasticity imaging, free-hand compression using the hand-held US probe and 2-D image rendering was used. Yet, even with such a basic system, accurate and useful imaging information was obtained, potentially allowing early introduction of this cost-effective advanced US capability into the clinical arena. Controlled tissue compression systems and 3-D imaging as well as image segmentation could further refine elasticity image capability.
Determination of adequacy (or inadequacy) of an ablation application during the procedure would allow for the ability to control the volume of tissue destruction in a more precise fashion, potentially resulting in better outcomes and improved patient safety. Specifically, repositioning of the ablation probe, adjustment of the ablation time, or change in the extent of probe deployment can be done based on monitoring information. Future ablation systems which allow volume shaping and spatially variable power delivery might be particularly useful when determination of the ablation boundary can be visualized at the time of therapy.
Others investigators have examined application of ultrasound elasticity imaging as a method to monitor thermal ablation. While most reports are limited to ex vivo and in vivo animal studies,15–17 Fahey et al. recently reported on their initial experience of ultrasound elasticity imaging in five patients undergoing hepatic RFA.18 In the previous study, tissue strain (compression) was generated by sending acoustic waves through the tissue to induce tissue movement rather than by direct tissue compression. While this approach is different from ours, their results confirm the feasibility and potential of ultrasound-based elastography to image margins of thermal tumour ablation.
An interest has expanded in recent years regarding the application of elasticity imaging in a variety of clinical settings. In addition to several reports on its application for monitor thermal ablation, ultrasound elasticity imaging has also been described for the characterization of tumours in the breast, thyroid and liver.19–21 This increasing interest is reflected by the fact that many commercially available ultrasound systems are already equipped with on-board real-time elasticity imaging capability similar to that used in our clinical study. While these elasticity imaging modules allow for fast and accurate generation of ultrasound elasticity images, these current algorithms (e.g. cross correlation) are highly susceptible to noise and distortion because of out-of-plane motion and non-linear movement during tissue compression.12 As such, current clinical systems are limited in the quality and usefulness of real-time elastography imaging acquisition, particularly when the region of interest is in a difficult position that cannot be easily reached with the ultrasound probe and axial tissue compression is difficult to achieve (e.g. posterior segments of the liver). These limitations can be addressed using more sophisticated algorithms which account for noise and distortion (e.g. dynamic programming). These experimental systems come at a higher computational price, currently limiting real-time imaging capability. However, efforts are being made by our group to develop rapid, high-resolution real-time elasticity imaging using these more robust algorithms.10,12 Other potential improvements in the technique include the application of real-time 3-D elastography and segmentation of the images.
While the present study was aimed at investigating this technique to monitor radiofrequency ablation in an open surgical situation, its applicability is not necessarily limited to this approach. Specifically, elasticity imaging using probe compression can be achieved using the laparoscopic IOUS probe on the surface of the liver. Even percutaneous ablation can be visualized this way using transabdominal compression or respiratory motion of the liver. In addition, we anticipate the potential application of elasticity imaging with other thermal ablation devices in addition to RFA. In particular, in microwave ablation, while perhaps somewhat more conspicuous on B-mode US than RFA, the ability to precisely delineate the thermal ablation zone in relation to the tumour periphery using elastography could prove exceedingly useful.
One limitation of the present study is the small sample size, and correlation coefficients should therefore be cautiously interpreted. In addition, variability in live animal and clinical imaging datasets without precise image registration limited the ability to be exact regarding the plane in which the various imaging measurements were compared. Yet, correlation was found to be well within acceptable clinical parameters. Furthermore, false estimation of the tumour morphology (e.g. microsatellitosis) might render the imaged margin of ablation less accurate. Therefore, additional studies are warranted to further validate the usefulness of this approach.
In conclusion, ultrasound elasticity imaging may provide a safe and effective method for improving monitoring of thermal ablation, potentially significantly impacting on how patients with solid tumours of the liver and other sites may be treated in the future. Such improvements in real-time imaging capability during tumour ablation may help to further improve outcomes and safety in patients undergoing these therapies.
Acknowledgments
M.A.C., E.M.B. and G.D.H. were supported by Grant Number 1R44CA134169-01A1 from the National Institutes of Health (SBIR). H.R. and P.F. were supported through the U.S. Department of Defense pre-doctoral fellowship program. L.R.A was supported by a research grant from the American Hepato-Pancreato-Biliary Association (2009).
Conflicts of interest
None declared.
References
- 1.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg. 2005;242:158–171. doi: 10.1097/01.sla.0000171032.99149.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha CH, Lee FT, Jr, Gurney JM, Markhardt BK, Warner TF, Kelcz F, et al. CT versus sonography for monitoring radiofrequency ablation in a porcine liver. AJR Am J Roentgenol. 2000;175:705–711. doi: 10.2214/ajr.175.3.1750705. [DOI] [PubMed] [Google Scholar]
- 4.Leyendecker JR, Dodd GD, 3rd, Halff GA, McCoy VA, Napier DH, Hubbard LG, et al. Sonographically observed echogenic response during intraoperative radiofrequency ablation of cirrhotic livers: pathologic correlation. AJR Am J Roentgenol. 2002;178:1147–1151. doi: 10.2214/ajr.178.5.1781147. [DOI] [PubMed] [Google Scholar]
- 5.Lim HS, Jeong YY, Kang HK, Kim JK, Park JG. Imaging features of hepatocellular carcinoma after transcatheter arterial chemoembolization and radiofrequency ablation. AJR Am J Roentgenol. 2006;187:W341–W349. doi: 10.2214/AJR.04.1932. [DOI] [PubMed] [Google Scholar]
- 6.Park MH, Rhim H, Kim YS, Choi D, Lim HK, Lee WJ. Spectrum of CT findings after radiofrequency ablation of hepatic tumors. Radiographics. 2008;28:379–390. doi: 10.1148/rg.282075038. discussion 390–392. [DOI] [PubMed] [Google Scholar]
- 7.Ophir J, Cespedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging. 1991;13:111–134. doi: 10.1177/016173469101300201. [DOI] [PubMed] [Google Scholar]
- 8.Kiss MZ, Daniels MJ, Varghese T. Investigation of temperature-dependent viscoelastic properties of thermal lesions in ex vivo animal liver tissue. J Biomech. 2009;42:959–966. doi: 10.1016/j.jbiomech.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eun D, Bhandari A, Boris R, Lyall K, Bhandari M, Menon M, et al. A novel technique for creating solid renal pseudotumors and renal vein-inferior vena caval pseudothrombus in a porcine and cadaveric model. J Urol. 2008;180:1510–1514. doi: 10.1016/j.juro.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Boctor E, deOliveira M, Choti M, Ghanem R, Taylor R, Hager G, et al. Ultrasound monitoring of tissue ablation via deformation model and shape priors. Med Image Comput Comput Assist Interv. 2006;9:405–412. doi: 10.1007/11866763_50. [DOI] [PubMed] [Google Scholar]
- 11.Rivaz H, Fleming I, Assumpcao L, Fichtinger G, Hamper U, Choti M, et al. Ablation monitoring with elastography: 2D in-vivo and 3D ex-vivo studies. Med Image Comput Comput Assist Interv. 2008;11:458–466. doi: 10.1007/978-3-540-85990-1_55. [DOI] [PubMed] [Google Scholar]
- 12.Rivaz H, Boctor E, Foroughi P, Zellars R, Fichtinger G, Hager G. Ultrasound elastography: a dynamic programming approach. IEEE Trans Med Imaging. 2008;27:1373–1377. doi: 10.1109/TMI.2008.917243. [DOI] [PubMed] [Google Scholar]
- 13.Schraml C, Clasen S, Schwenzer NF, Koenigsrainer I, Herberts T, Claussen CD, et al. Diagnostic performance of contrast-enhanced computed tomography in the immediate assessment of radiofrequency ablation success in colorectal liver metastases. Abdom Imaging. 2008;33:643–651. doi: 10.1007/s00261-007-9351-9. [DOI] [PubMed] [Google Scholar]
- 14.Raman SS, Lu DS, Vodopich DJ, Sayre J, Lassman C. Creation of radiofrequency lesions in a porcine model: correlation with sonography, CT, and histopathology. AJR Am J Roentgenol. 2000;175:1253–1258. doi: 10.2214/ajr.175.5.1751253. [DOI] [PubMed] [Google Scholar]
- 15.Bharat S, Varghese T, Madsen EL, Zagzebski JA. Radio-frequency ablation electrode displacement elastography: a phantom study. Med Phys. 2008;35:2432–2442. doi: 10.1118/1.2919763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolokythas O, Gauthier T, Fernandez AT, Xie H, Timm BA, Cuevas C, et al. Ultrasound-based elastography: a novel approach to assess radio frequency ablation of liver masses performed with expandable ablation probes: a feasibility study. J Ultrasound Med. 2008;27:935–946. doi: 10.7863/jum.2008.27.6.935. [DOI] [PubMed] [Google Scholar]
- 17.Zhang M, Castaneda B, Christensen J, Saad WE, Bylund K, Hoyt K, et al. Real-time sonoelastography of hepatic thermal lesions in a swine model. Med Phys. 2008;35:4132–4141. doi: 10.1118/1.2968939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahey BJ, Nelson RC, Hsu SJ, Bradway DP, Dumont DM, Trahey GE. In vivo guidance and assessment of liver radio-frequency ablation with acoustic radiation force elastography. Ultrasound Med Biol. 2008;34:1590–1603. doi: 10.1016/j.ultrasmedbio.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoue Y, Takahashi M, Arita J, Aoki T, Hasegawa K, Beck Y, et al. Intra-operative freehand real-time elastography for small focal liver lesions: ‘visual palpation’ for non-palpable tumors. Surgery. 2010;148:1000–1011. doi: 10.1016/j.surg.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 21.Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, et al. Thyroid gland tumor diagnosis at US elastography. Radiology. 2005;237:202–211. doi: 10.1148/radiol.2363041248. [DOI] [PubMed] [Google Scholar]


